Introduction
A host rarely harbours only a single parasite species. Usually, it is exploited by several parasite species that form an infracommunity (Holmes & Price, Reference Holmes, Price, Kikkawa and Anderson1986). Two types of parasite infracommunities are commonly recognized – interactive and non-interactive infracommunities. An interactive infracommunity is characterized by interspecific interactions and a saturated niche space, whereas a non-interactive infracommunity has an unsaturated niche space and parasite species do not interact (Rohde, Reference Rohde2013).
In fish, a great variety of parasites resides in the gill apparatus because the gills provide ample space for attachment and an unlimited food supply (epithelium, mucus, blood), as well as shelter from predators (Sulman, Reference Sulman1984; Gussev, Reference Gussev1985; Bauer, Reference Bauer1987).
The distribution of gill parasites has been studied frequently. It has been established that parasites occupy gills non-randomly (Rohde, Reference Rohde1979). Two main mechanisms may determine this non-randomness: species-specific microenvironmental preferences and interactions between parasite species. Microhabitat preferences of parasites have been studied for most major taxa inhabiting fish gills (Rohde, Reference Rohde1977; Sutherland & Wittrock, Reference Sutherland and Wittrock1985; Gutierrez & Martorelli, Reference Gutierrez and Martorelli1999; Blažek & Gelnar, Reference Blažek and Gelnar2006). These preferences are often determined by parasite morphology, although host characteristics may also play a role. For example, many studies have analysed the microhabitat preferences of Pseudodactylogyrus anguillae and Pseudodactylogyrus bini within the gill apparatus of eels (Buchmann, Reference Buchmann1988, Reference Buchmann1989; Rodrigues & Saraiva, Reference Rodrigues and Saraiva1996; Dzika, Reference Dzika1999; Woo & Buchmann, Reference Woo and Buchmann2012; Soylu et al., Reference Soylu, Çolak, Erdogan, Erdogan and Tektas2013) and reported that the microhabitat preferences of parasites depended on the morphology of their attachment apparatus and size of the host.
Interactions between parasites have mainly been investigated in closely related species, such as congeneric monogeneans (El Hafidi et al., Reference El Hafidi, Berrada-Rkhami, Benazzou and Gabrion1998; Simková et al., Reference Simková, Desdevises, Gelnar and Morand2000; Buchmann & Lindenstrøm, Reference Buchmann and Lindenstrøm2002; Koskivaara et al., Reference Koskivaara, Valtonen and Vuori2009). For example, Simková et al. (Reference Simková, Desdevises, Gelnar and Morand2000, Reference Simková, Gelnar and Sasal2001) studied the distribution of congeneric Dactylogyrus species and found that interspecific competition played only a minor role in their niche specialization, despite having similar microhabitat and/or feeding preferences. However, parasite abundance might affect the extent of interspecific interactions, niche breadth and overlap. High densities of Dactylogyrus individuals promoted interspecific interactions, expanded niche breadth and overlap, whereas dactylogyrid infracommunities at low densities behaved in a non-interactive fashion (Koskivaara & Valtonen, Reference Koskivaara and Valtonen1992; Koskivaara et al., Reference Koskivaara, Valtonen and Vuori2009). Moreover, distantly related parasites (e.g. species belonging to different major taxa) can also interact if they exploit similar resources. However, such interactions have rarely been studied, due to the aggregation of parasites among hosts where most hosts harbour a few parasite species and only some host species harbour large infracommunities (Shaw & Dobson, Reference Shaw and Dobson1995).
Positive or negative interactions of organisms can be proven only with experimental manipulations. However, manipulating parasites on living hosts is extremely difficult and not always possible. Therefore, census data are often used as an alternative to experiments to infer interactions among parasites. For example, Krasnov et al. (Reference Krasnov, Vinarski, Korallo-Vinarskaya, Mouillot and Poulin2009) used census data to evaluate positive and negative associations among fleas parasitic on small mammals. Census data were also applied to analyse interspecific interactions among fish ecto- and endoparasites (Friggens & Brown, Reference Friggens and Brown2005; Pronkina et al., Reference Pronkina, Dmitrieva and Gerasev2010).
Here, we studied the distribution and co-occurrence of gill parasites belonging to three taxa (Monogenea, Digenea and Copepoda) in infracommunities of the common bream Abramis brama L., 1758. This fish harbours relatively large communities of closely and distantly related gill parasites (Sulman, Reference Sulman1984; Gussev, Reference Gussev1985; Bauer, Reference Bauer1987), with the number of parasite individuals reaching several thousands (Dzika, Reference Dzika2002; Ottová et al., Reference Ottová, Šimková, Jurajda, Dávidová, Ondračková, Pečínková and Gelnar2005; Rückert et al., Reference Rückert, Klimpel and Palm2007; Dzika et al., Reference Dzika, Kuształa and Kazłowski2008). We focused on digenean metacercariae because their microhabitat preferences and relationships with other parasites are poorly known. Their life strategy differs from those of monogeneans, copepods and glochidia. For digeneans, fish are intermediate hosts. Metacercariae encyst in gills and are completely immobile. To complete their life cycle, metacercariae use the existing food chains, with definitive hosts (birds, mammals and predator fishes) predating upon infected fish. Successful predation results in the death of other parasite species, whereas avoidance of predation by fish results in the death of metacercariae (Bauer, Reference Bauer1987). Digenean metacercariae excrete waste products from metabolic processes that may modify the behaviour or phenotype of hosts in a way that increases their susceptibility to predation (Dobson, Reference Dobson1988; Johnson et al., Reference Johnson, Lunde, Ritchie and Launer1999, Seppälä et al., Reference Seppälä, Karvonen and Valtonen2004). Furthermore, infection by any parasite triggers defence mechanisms of a host. Increased mucus production is a non-specific response to gill parasitism (Alvarez-Pellitero, Reference Alvarez-Pellitero2008). Although moderate mucus production facilitates parasite infestation, acting as chemical stimuli and serving as a food source for parasites, an excessive mucus amount might suppress parasite attachment. For example, Buchmann & Bresciani (Reference Buchmann and Bresciani1998) studied the microhabitat selection of Gyrodactylus derjavini on the body surface of rainbow trout and found that some chemical molecules in fish mucus may attract monogeneans to attach on its surface. However, an increasing production of mucus negatively affected the intensity of parasite infestation. The inhibiting effect of abundant mucus production is likely to be stronger on parasites that attach to gills than on encysted metacercariae. Moreover, digenean metacercariae often cause proliferation and hyperplasia of gills, which significantly deforms gill filaments, which become shortened, thickened, or even bent and fused (Blazer & Gratzek, Reference Blazer and Gratzek1985; Olson & Pierce, Reference Olson and Pierce1997; Mitchell et al., Reference Mitchell, Salmon, Huffman, Goodwin and Brandt2000; Shoaibi Omrani et al., Reference Shoaibi Omrani, Ebrahimzadeh Mousavi and Sharifpour2010). Consequently, changes in gills caused by the occurrence of digenean metacercariae may reduce the suitable surface area available for attachment of ectoparasitic species.
Here, we asked whether digenean metacercariae: (1) prefer to encyst at specific patches of the gill apparatus; (2) co-occur in the same patches with monogeneans and copepods within a host individual; and (3) interact with monogeneans and copepods, so that occurrence or high number of metacercariae (or both) reduces success of attachment of monogeneans and copepods in the patches where digenean metacercariae occur. To address these questions, we recorded all parasites on the gill arches of the same host species and used null models (Gotelli, Reference Gotelli2000) to analyse the co-occurrences of monogeneans, digenean metacercariae and copepods. Zero-inflated mixture models (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009) were used to define the preferred patches for those parasites.
Materials and methods
Collection of bream and parasites of gill apparatus
Bream were collected from Lake Lubāns (56°46′N 26°52′E) in the summer, autumn and winter. In total, 60 fish were captured by gill nets. The gill apparatus of each side of each fish was examined. We numbered gill arches from the anterior to the posterior as 1–4 and divided each arch into three sectors from the dorsal to the ventral end as I–III (Zolovs et al., Reference Zolovs, Deksne, Daukšte, Aizups and Kirjušina2016). We recorded all gill parasites of an individual fish in each of these 24 gill parts. For convenience, we further refer to each of these parts as a patch, which may differ from other localizations in its physical, chemical or biological properties. All parasites were counted and identified to species level using microscopy and identification keys (Sulman, Reference Sulman1984; Gussev, Reference Gussev1985; Bauer, Reference Bauer1987). In total, we recorded 15,464 parasite individuals belonging to 13 species (seven monogeneans, three digeneans, one mollusc, one copepod and one branchiuran). In addition, two protist species were found.
Statistical data analysis of microhabitat preference and co-occurrence of parasites
To characterize spatial niche breadth of each parasite species, we calculated standardized Levin's niche breadth ![]() $B_A = (1/\mathop \sum \nolimits {p_{i}^2} - 1)/\left( {n - 1} \right)$ (Krebs, Reference Krebs1998), where p i is a proportion of individuals found in the localization i, and n is a number of possible localizations (24). Standardized Levin's index ranges from 0 to 1, where 0 indicates the absence of a species and 1 indicates that the species occupies all available patches. Pairwise niche overlap between species was measured by Pianka's index:
$B_A = (1/\mathop \sum \nolimits {p_{i}^2} - 1)/\left( {n - 1} \right)$ (Krebs, Reference Krebs1998), where p i is a proportion of individuals found in the localization i, and n is a number of possible localizations (24). Standardized Levin's index ranges from 0 to 1, where 0 indicates the absence of a species and 1 indicates that the species occupies all available patches. Pairwise niche overlap between species was measured by Pianka's index: ![]() $O_{ij} = \sum p_{ij}p_{ik}/\sqrt {({\sum p_{ij}^2} {\sum p_{ik}^2} )} $ (Pianka, Reference Pianka1974), where p ij is the proportion of patches i used by species j, and p ik is the proportion of patches i used by species k. Pianka's index ranges from 0 to 1, where 0 indicates that two species do not share any patch and 1 indicates complete overlap in patch use (Krebs, Reference Krebs1998).
$O_{ij} = \sum p_{ij}p_{ik}/\sqrt {({\sum p_{ij}^2} {\sum p_{ik}^2} )} $ (Pianka, Reference Pianka1974), where p ij is the proportion of patches i used by species j, and p ik is the proportion of patches i used by species k. Pianka's index ranges from 0 to 1, where 0 indicates that two species do not share any patch and 1 indicates complete overlap in patch use (Krebs, Reference Krebs1998).
Parasite counts often have a highly skewed distribution with an excessive number of zeroes (Pilosof et al., Reference Pilosof, Lareschi and Krasnov2012; Barnard et al., Reference Barnard, Krasnov, Goff and Matthee2015). To cope with this, zero-inflated mixture modelling was designed (Ridout et al., Reference Ridout, Demétrio and Hinde1998). Zero counts can arise from two sources: ‘true zeros’ and ‘false zeros’. ‘True zeros’ are generated by real ecological effects, such as when a parasite is absent in a given localization because it is not suitable for this species (Martin et al., Reference Martin, Wintle, Rhodes, Kuhnert, Field, Low-Choy, Tyre and Possingham2005). ‘False zeros’ are generated by study design or observation errors; for example, when host individuals were examined during too short a period or a parasite species was identified incorrectly. In mixture models, count data are represented by two separate components: (1) true zeros and non-zero counts are modelled by Poisson or negative binomial distribution; and (2) false zeros are modelled by the binomial distribution. A more detailed description of the logic and calculation of the models may be found in Zuur et al. (Reference Zuur, Ieno, Walker, Saveliev and Smith2009).
To account for the large number of zeros in our data, we applied zero-inflated Poisson (ZIP) or zero-inflated negative binomial (ZINB) mixture models to test for the effects of fish length, sex, localization and month of host collection on parasite count. Separate datasets were generated for the most common species (six monogeneans, one digenean and two copepods). For each species, we ran two models (negative binomial and Poisson distribution) using the package ‘pscl’ (Jackman, Reference Jackman2015) implemented in R (R Development Core Team, 2016). To select the best model for each dataset we used the Akaike Information Criterion (AIC). The significance of the estimated coefficients was tested against a reference level, which was chosen arbitrarily among four factor levels (fish length, sex, months and patches in gills). The preferred patch of infection for each parasite species was estimated by sequentially selecting the patch as the reference level for each run. The significance of the estimated coefficients of the reference level (intercept of the model) was tested against zero (Pilosof et al., Reference Pilosof, Lareschi and Krasnov2012).
The co-occurrence of parasites in patches within individual host gills was analysed using the null model analyses implemented in the program EcoSim Professional (Entsminger, Reference Entsminger2014). Each infracommunity of parasites was arranged in a presence/absence matrix in which rows represented parasite species and columns represented patches. The co-occurrence of parasite species was tested using the C-score index (the average number of checkerboard units that are found for each pair of species) (Stone & Roberts, Reference Stone and Roberts1990). We calculated the observed C-score (O) index for each presence/absence matrix and compared it with the index calculated for 5000 randomly assembled null matrices (E) (expected C-score). For the sake of biological realism, we used a fixed–equiprobable (FE) algorithm for assembling the null matrices. This algorithm implies that each parasite species occurs in the same number of patches as in the real data (fixed), but the number of parasite species in each patch may vary (equiprobable). An observed C-score significantly larger than expected by chance (O > E) indicates negative co-occurrence of parasite species, while an observed C-score significantly smaller than expected by chance (O < E) indicates positive co-occurrence of parasite species. We calculated the standardized effect size for each matrix. This measures the number of standard deviations that the observed index is above or below the mean index of simulated matrices [(observed index – mean of simulated indexes)/standard deviation of simulated indices] (Gotelli & McCabe, Reference Gotelli and McCabe2002). To test the null hypothesis that the average standardized effect size (SES) across host individuals was zero, we used one-sample t-tests. Assuming a normal distribution of deviations, approximately 95% of the observed SES values are expected to fall between –2.0 and 2.0.
To study pairwise associations of parasite species within the entire matrix, we used Bayes M criterion implemented in the program Pairs (Ulrich, Reference Ulrich2008). This is one of four methods proposed by Gotelli & Ulrich (Reference Gotelli and Ulrich2010). Bayes M criterion is based on an empirical Bayesian approach where the observed frequency distribution of scores is compared with the frequency distribution of scores generated by the null model. This method reduces the frequency of false-positive tests (type I error) more efficiently than the more liberal CL (95% confidence limit) criterion and is less conservative than the Bayes CL criterion. More detailed descriptions and comparisons of all methods may be found in Gotelli & Ulrich (Reference Gotelli and Ulrich2010). We studied the associations of parasite pairs for the most common parasite species (prevalence > 10%).
The chi-square test was used to test whether a parasite prefers certain patches in gills over others. This test was carried out using SPSS Statistics version 22 (IBM Corporation, Chicago, Illinois, USA).
Results
Composition of the parasite community of bream gills
We found 15 parasite species on the gills of bream. Data on prevalence, mean abundance and standardized niche breadth for each parasite species are presented in table 1.
Table 1. Abundance (A; mean ± SE), prevalence (P) and standardized niche breadth (BA) of parasites recorded in gills of bream (Abramis brama). Niche breadth has not been calculated for parasites with low abundance (n/a). Trichodina sp. was counted per 60× magnification view.

Dactylogyrids had a higher prevalence and mean abundance than other parasites, with D. wunderi occurring in almost all fish and its abundance being at least double that of other dactylogyrids. All dactylogyrids, Bucephalus polymorphus and Ergasilus sieboldi occurred on bream throughout the year, whereas the occurrence of other parasites was seasonal (Diplozoon paradoxum in summer and winter, Gyrodactylus elegans and Argulus foliaceus in summer, and Paracoenogonimus ovatus in autumn and winter).
We recorded nine species that attach externally to gill filaments as larvae and four species with larvae encysting in gill tissues. Most of the species with parasitic larvae had low prevalence and abundance (less than 10% and less than 1.0, respectively), except B. polymorphus (83% and 57.1, respectively).
Niche size and overlap
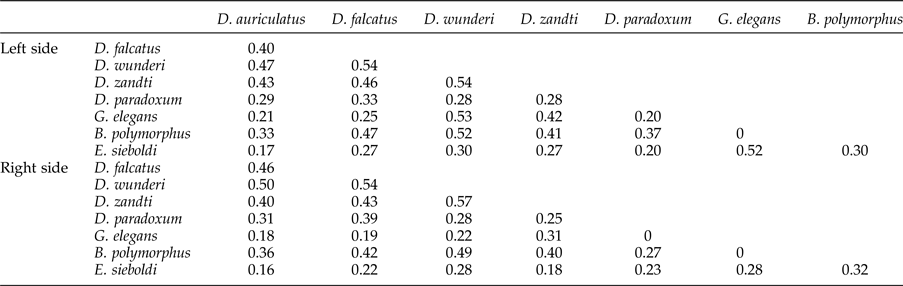
The niche breadth of Dactylogyrus was at least twice the width of other parasite species, except B. polymorphus (table 1). Across all parasites, pairwise niche overlap was less than 60%. Niche overlap between congeners was significantly higher than niche overlap between distantly related species (M = 47.8%, SD = 5.8 versus M = 28.7%, SD = 12.5, respectively; t(54) = 5.07, P < 0.001) (table 2).
Table 2. Niche overlap between parasite species recorded on the left and right sides of the gill apparatus of bream (Abramis brama).
Parasite distribution within gill apparatus
The number of parasite individuals found in a given patch of gills in all examined bream is summarized in table 3. The chi-square test shows that the six most common parasite species preferred a specific patch on the gill apparatus, whereas species of low abundance were randomly distributed within gills. The number of preferred patches varied between 3 and 14 among parasite species, and was greater in species with a higher intensity of infection (table 4).
Table 3. Distribution of parasite species within the gill apparatus of bream (Abramis brama). Number of specimens found in a given gill patch in all examined fish, and chi-square test results indicating non-random distribution of parasites within the gill apparatus.
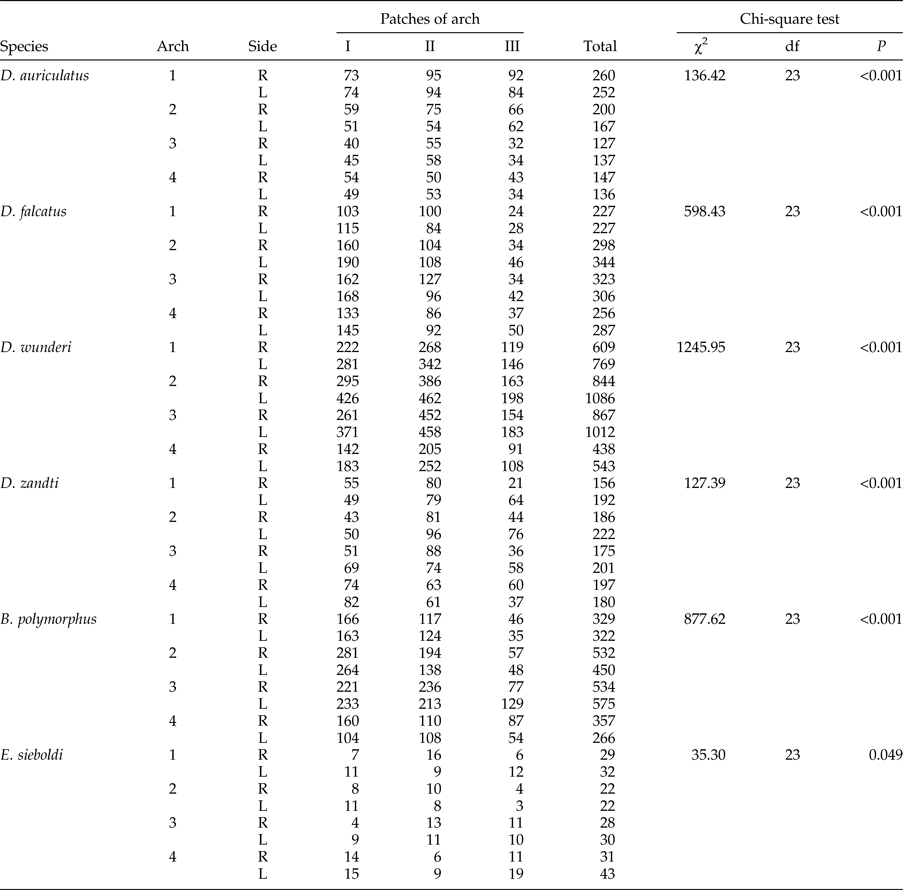
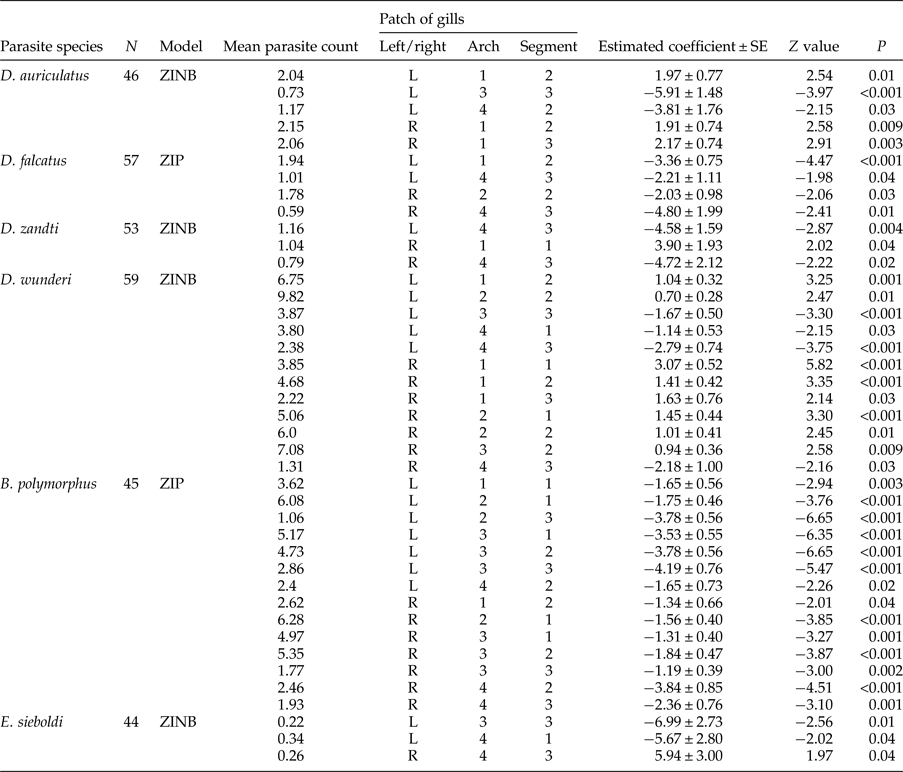
Table 4. Gill patches in which the mean count of a given parasite differed significantly from zero. The preferred patch of infection for each parasite species was estimated by zero-inflated Poisson (ZIP) or zero-inflated negative binomial (ZINB) mixture models (see methods for explanation).
Factors influencing parasite abundance
We found that the abundance of all parasites depends on fish size, sex and season of collection. In addition, an abundance of Dactylogyrus species depended also on the patch of the gill apparatus. Zero-inflated mixture models showed that all four factors might generate false zeros (table 5).
Table 5. Summary of zero-inflated models of factors significantly affecting abundance of parasites in gill apparatus of bream (Abramis brama). ZIP and ZINB are zero-inflated Poisson and zero-inflated negative binomial mixture models, respectively (see methods for explanation). Results are shown only for models with significant coefficients.

Co-occurrence of parasites
The C-score of the observed presence/absence matrices differed significantly from that of simulated matrices in only ten of 60 infracommunities of gill parasites. In these ten infracommunities, the observed C-score was lower than expected by chance. The average SES value for the C-score values was −2.33 ± 0.53 and differed significantly from zero (t(9) = −13.73, P < 0.001) but did not differ significantly from −2 (t(9) = −1.96, P = 0.08). There was a total of 793 unique species pairs in 60 matrices, where Bayes M criterion indicated only one significantly co-occurring pair (D. wunderi with D. zandti).
Discussion
Our results showed that most parasite species: (1) preferred specific patches in the gill apparatus; (2) shared these preferences (i.e. they aggregate in the same patches); and (3) did not interact with each other. In other words, our expectations about interactions between digenean metacercariae, monogeneans and copepods were not supported. These results suggest that aggregation of both closely related and distantly related species is predominantly determined by their microenvironmental preferences. Below, we will discuss factors that might be responsible for (1) selection of preferable gill patches; and (2) patterns of co-occurrence of different parasite species.
Factors responsible for parasites’ preference of occurrence
Various factors responsible for ectoparasite occurrence in a specific patch within and among gills have been demonstrated. First, water flow distributes parasites among gill arches randomly, and then they migrate to a suitable microhabitat within the gill arch (Llewellyn, Reference Llewellyn1956; Wootten, Reference Wootten1974; Dmitrieva, Reference Dmitrieva2000). Second, selection of a suitable microhabitat within a gill arch is determined by the interplay between the morphological properties of gill filaments and the attachment apparatus of the parasite (Wootten, Reference Wootten1974; Buchmann, Reference Buchmann1989; Simková et al., Reference Simková, Desdevises, Gelnar and Morand2000; Woo & Buchmann, Reference Woo and Buchmann2012). The form, size and number of gill filaments vary markedly between fish species. Generally, the length of gill filaments gradually increases along the dorsal third of the gill arch and then gradually decreases to the ventral end. The gill filament lamellae differ in shape and number both within the gill arch and filament (Hoar & Randall, Reference Hoar and Randall1984). Parasites tend to localize in a specific patch within a gill apparatus because of the morphological variation of the gill filaments. For example, P. anguillae, which have long and slender anchors, are usually found on the basal half of the gill filaments of the first sector of the gill arch. In contrast, P. bini, which have short and stout anchors, occur predominantly on the distal halves of the gill filaments of the first and second sectors of the gill arch (Buchmann, Reference Buchmann1988, Reference Buchmann1989; Rodrigues & Saraiva, Reference Rodrigues and Saraiva1996; Dzika, Reference Dzika1999; Matejusová et al., Reference Matejusová, Simková, Sasal and Gelnar2003; Soylu et al., Reference Soylu, Çolak, Erdogan, Erdogan and Tektas2013). However, digenean metacercariae, despite the lack of special attachment apparatus, also prefer specific patches to encyst in the gill filaments. Given that some digeneans penetrate the epithelium during the cercarial stage to encyst in the gill filament, these preferences might be associated with the morphology of the gill filaments (variation in thickness, blood circulation and cell-type composition). Other species penetrate the skin and muscle of a fish and then migrate within its blood system to find a preferred patch in the gills (Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij1998; Olson, Reference Olson2002; Wilson & Laurent, Reference Wilson and Laurent2002). Olson (Reference Olson2002) identified three vascular networks within the gill filaments. Digenean parasites use sensory receptors to navigate within an organism and find the preferred vascular network (Haas, Reference Haas2003; Grabe & Haas, Reference Grabe and Haas2004).
Mechanisms involved in detection of a suitable localization by digenean metacercariae
Digenean sensory receptors have often been investigated (Short & Cartrett, Reference Short and Cartrett1973; Pariselle & Matricon-gondran, Reference Pariselle and Matricon-gondran1985; Krejci & Fried, Reference Krejci and Fried1994; Bogéa & Caira, Reference Bogéa and Caira2001). It was found that cercarial receptors are numerous, highly variable and located in specific parts of their body. The ultrastructure and site specificity of receptors presume that some of the receptors are chemoreceptors, while others are mechanoreceptors (Bogéa & Caira, Reference Bogéa and Caira2001), but the morphological diversity of receptors probably indicates that parasites may respond to many different stimuli to find the preferred patch. In particular, these receptors may respond to various environmental (light, gravity, water currents and temperature) and host-induced stimuli (shadowing, water turbulence, touch and chemical gradient) to disperse in water and find a host (Haas, Reference Haas1994). Ostrowski De Nuñez & Haas (Reference Ostrowski De Nuñez and Haas2009) found that the penetration of the fish skin by cercariae is stimulated by a combination of stimuli, such as mucus proteins and fish-skin fatty acids. They also noted that chemical signals for host identification differ between digenean species. For example, Acanthostomum brauni cercariae penetrate fish skin when they sense protein components of fish mucus that have molecular weights greater than 10,000 Da (Ostrowski De Nuñez & Haas, Reference Ostrowski De Nuñez and Haas2009), whereas Opisthorchis viverrini cercariae penetrate fish skin when they sense the hydrophilic component of fish skin that has a molecular weight of more than 30,000 Da (Haas et al., Reference Haas, Granzer and Brockelman1990). Nevertheless, it is still largely unknown how digeneans navigate to their destinations within a fish. For example, Haas (Reference Haas2003) tried to identify the signals that navigate parasites to their patches and found that digeneans seek preferred blood vessels using d-glucose and l-arginine residues.
Intraspecific aggregation
Our results support the findings of earlier studies that some parasite species are distributed non-randomly within gills (Ives, Reference Ives1988; Morand et al., Reference Morand, Poulin, Rohde and Hayward1999; Simková et al., Reference Simková, Desdevises, Gelnar and Morand2000, Reference Simková, Gelnar and Sasal2001). It has been suggested that parasites aggregate within gills because of intraspecific interactions. Rohde (Reference Rohde, Lewis, Campbell and Sukhdeo2002) argued that mating facilitation could be the main reason for intraspecific aggregation of parasites. However, mating facilitation may explain a non-random distribution of hermaphrodite parasites such as monogeneans or bisexual parasites such as copepods, but not an aggregation of encysted digenean metacercariae. We propose two possible explanations for intraspecific aggregation of digenean metacercariae. First, aggregation of metacercariae increases the chances of infecting a definitive host by damaging the respiratory system of an intermediate host, because the fish lose their fitness and become easy prey for predators. For example, Blazer & Gratzek (Reference Blazer and Gratzek1985) experimentally infected fish with cercariae to study the pathological reaction of gills and found that light infection of the gill filament (one or two metacercariae) did not impair gill function, whereas heavy infection caused destruction of the secondary lamellae, thus resulting in the loss of gill physiological function. Second, assuming that digenean parasites follow the concentration gradient of specific chemical components within the host, they will eventually reach and aggregate in a preferred patch where the concentration of this particular signal is the highest. However, numerous attempts to identify this specific chemical gradient of preferred localization have been unsuccessful (Kemp & Devine, Reference Kemp and Devine1982; Holmes & Prices, Reference Holmes, Price, Kikkawa and Anderson1986; Sukhdeo & Mettrick, Reference Sukhdeo and Mettrick1987). Sukhdeo et al. (Reference Sukhdeo, Sukhdeo and Mettrick1987) found that parasites have non-directional fixed behaviours sequentially triggered by several chemicals, depending on the particular environment. For example, each specific behaviour of Fasciola hepatica is sequentially triggered by CO2, glycocholic acid, deoxycholic acid, duodenal proteins and mesenteric proteins that lead it to its final destination. Sukhdeo (Reference Sukhdeo1990) proposed that a specific chemical gradient at the final location of parasites might lead to intraspecific aggregation, at least for mating facilitation.
Interspecific aggregation
We found no evidence for interaction of digenean metacercariae with either monogeneans or copepods. Rohde (Reference Rohde1991) explained the lack of interaction between gill parasites by their low density and resource richness. In other words, fish gills provide many more resources (space, food, etc.) than parasites of any one taxon could utilize. An additional reason for the lack of interaction between digenean metacercariae, monogeneans and copepods is that digenean metacercariae encyst in gill filaments, whereas monogeneans and copepods attach to gill filaments. In other words, these taxa utilize different spatial resources.
In conclusion, digenean metacercariae prefer specific patches to encyst in the gill apparatus, and their occurrence (even in high numbers) does not reduce the success of monogenean and copepod attachment in the same gill patches.
We propose that digenean metacercariae are a convenient model taxon for the study of factors that lead to microhabitat selection within a host organ or tissues, because: (1) they are completely immobile in their suitable habitat; (2) they encyst when reaching the destination patch; (3) they aggregate in a preferred patch; and (4) their aggregation is not influenced by interspecific interactions.
Acknowledgements
We would like to thank Boris Krasnov for helpful comments and useful suggestions that have helped to improve this paper.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.







