Introduction
Nematodes have evolved to parasitize arthropods, plants and mammals (Blaxter et al., Reference Blaxter, de Ley and Garey1998) but the genetic mechanisms of how parasitism evolved are unknown. Pre-adaptations that are thought to be key for this evolutionary transition include close associations with arthropod hosts (Blaxter & Koutsovoulos, Reference Blaxter and Koutsovoulos2015) and the ability to arrest development, which can aid in coping with stressful conditions such as host enzymes (Poulin, Reference Poulin1998; Weischer & Brown, Reference Weischer and Brown2000). Several parasitic species have been developed as genetic models but they are unsuitable to answer this question as they require intermediate and/or definitive mammal hosts (e.g. Brugia sp.) to complete their life cycle, which can be financially and logistically prohibitive and laborious (Lok & Unnasch, Reference Lok and Unnasch2013). Due to these problems genetic experiments can be difficult. More developed genetic model nematodes are associated with invertebrates. Caenorhabditis elegans is thought to have a loose association with slugs and snails (Petersen et al., Reference Petersen, Hermann, Barg, Schalkowski, Dirksen, Barbosa and Schulenburg2015; Schulenburg & Félix, Reference Schulenburg and Félix2017), and the diplogastrid nematode Pristionchus pacificus has a necromenic relationship (coined by Schulte, Reference Schulte1989) with scarab beetles, whereby it infects the host, waits for it to die and reproduces on its cadaver (Morgan et al., Reference Morgan, MacGaughran, Villate, Herrmann, Witte, Bartelmes, Rochat and Sommer2012). However, neither species is parasitic (Herrmann et al., Reference Herrmann, Mayer and Sommer2006; Rae & Sommer, Reference Rae and Sommer2011), and therefore they provide little information about the underlying evolution of genetic mechanisms that are used to infect, parasitize and even kill their hosts. They are, however, formidable at unravelling genes associated with a plethora of biologically and ecologically important traits (The C. elegans Research Community, 2005; Sommer, Reference Sommer2015). Both of these species are successful as nematode genetic models as they can be isolated easily, kept in culture and grown in large numbers (on nematode growth media (NGM) plates fed Escherichia coli OP50), and they can be mutagenized and mated easily (Brenner, Reference Brenner1974; Sommer et al., Reference Sommer, Carmi and Eizinger2000). Furthermore, as well as routine full genome sequencing (C. elegans Sequencing Consortium, 1998; Dieterich et al., Reference Dieterich, Clifton and Schuster2008), post-genomic tools, such as reverse genetic techniques – first RNAi (Fire et al., Reference Fire, Xu, Montgomery, Kostas, Driver and Mello1998; Cinkornpumin & Hong, Reference Cinkornpumin and Hong2011) and now CRISPR-Cas9 (Lo et al., Reference Lo, Pickle, Lin, Ralston, Gurling, Schartner, Bian, Doudna and Meyer2013; Witte et al., Reference Witte, Moreno, Rodelsperger, Kim, Kim, Streit and Sommer2015) – can be carried out in both species to understand gene function, while transgenic techniques facilitate the analysis of gene expression (Chalfie et al., Reference Chalfie, Tu, Euskirchen, Ward and Prasher1994; Schlager et al., Reference Schlager, Wang, Braach and Sommer2009). Similar techniques can be carried out in mammalian parasites, e.g. Brugia malayi, Nippostrongylus brasiliensis and Ascaris suum, but the efficiency is variable and only a selection of genes can be inhibited (Geldhof et al., Reference Geldhof, Murray, Couthier, Gilleard, McLauchlan, Knox and Britton2006, Reference Geldhof, Visser, Clark, Saunders, Britton, Gilleard, Berriman and Knox2007). A promising genetic model to study nematode parasitism would combine the ease of keeping and growing C. elegans and P. pacificus en masse in the lab with the ability to collect different species and strains easily to facilitate micro and macro evolutionary studies. Also, it would be closely related to other parasitic and necromenic species that would allow genomic comparison of the evolution of potential parasitism genes from different parasitic lifestyles. Furthermore, it could be genetically manipulated, which would facilitate an in-depth analysis of gene function.
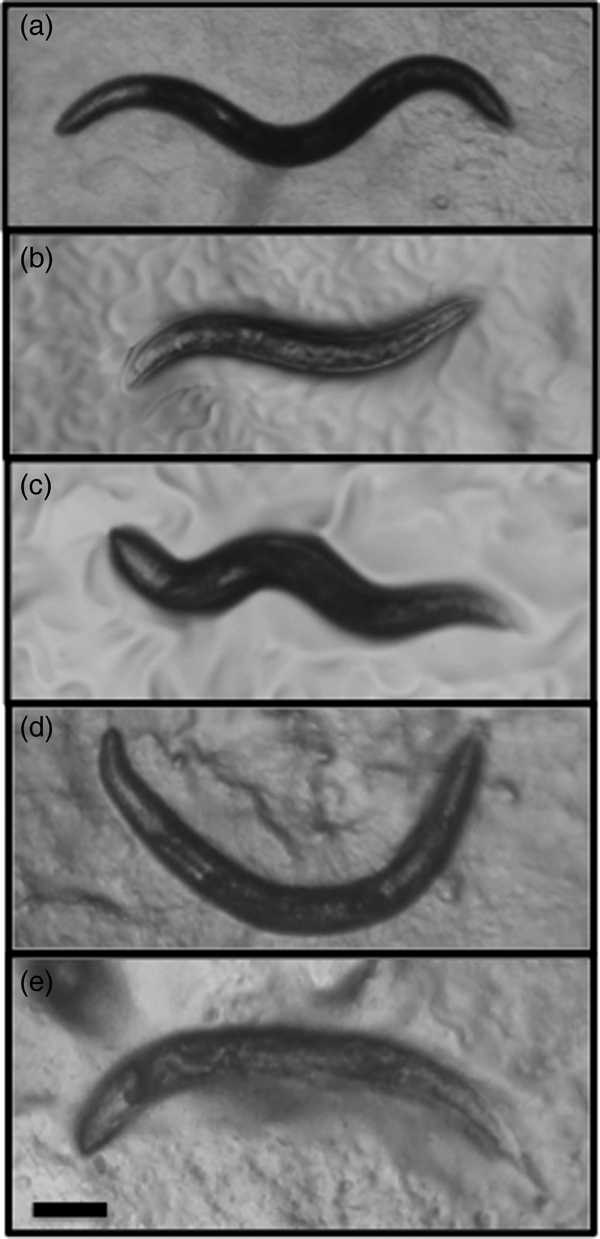
A nematode that theoretically meets all these criteria is the terrestrial gastropod parasite Phasmarhabditis hermaphrodita (fig. 1a). Phasmarhabditis hermaphrodita can complete its life cycle in several ways. First, it can infect and kill several susceptible slug species (e.g. Deroceras reticulatum) (Wilson et al., Reference Wilson, Glen and George1993; Rae et al., Reference Rae, Robertson and Wilson2009). Second, it can infect and remain inside larger slug and snail species, where it waits for the host to die and then reproduces on the decaying cadaver (termed ‘necromeny’) (Rae et al., Reference Rae, Robertson and Wilson2009). Third, it can reproduce on decomposing organic matter such as leaf litter, dead earthworms and slug faeces (Tan & Grewal, Reference Tan and Grewal2001a; MacMillan et al., Reference MacMillan, Haukeland, Rae, Young, Crawford, Hapca and Wilson2009). Therefore, it is not an obligate parasite that requires a host to survive but a bacterivorous nematode that can be grown in the lab without slugs but is still able to retain is pathogenicity towards slugs. Due to its pathogenic potential it has been formulated into a biological control agent (Nemaslug®, BASF, Germany) for farmers and gardeners to control slugs and snails (Rae et al., Reference Rae, Verdun, Grewal, Robertson and Wilson2007). Nematodes are applied to soil, where they actively seek out slugs and infect and kill them 4–21 days later (Wilson et al., Reference Wilson, Glen and George1993; Tan & Grewal, Reference Tan and Grewal2001a). Phasmarhabditis hermaphrodita has been shown to provide protection against slug damage in many agriculturally important crops (Wilson & Rae, Reference Wilson, Rae and Campos Herrera2015). Although P. hermaphrodita has received considerable attention as an agricultural biopesticide, it is also interesting from a fundamental evolutionary perspective and has been proposed as an excellent candidate as a genetic model to elucidate how parasitism has arisen in free-living species (Wilson et al., Reference Wilson, Ivanova and Spiridonov2015; Rae, Reference Rae2017). It was even a potential candidate as Sydney Brenner's nematode of choice instead of C. elegans (Cold Spring Harbor Laboratory Archives, 2017; http://libgallery.cshl.edu/items/show/75709). Phasmarhabditis hermaphrodita is the only nematode of an estimated 1 million (Lambshead, Reference Lambshead1993) that has evolved to parasitize and kill gastropods. There are over 108 species of nematodes that parasitize molluscs, and four of five clades of the Nematoda have members that parasitize gastropods (Blaxter et al., Reference Blaxter, de Ley and Garey1998; Grewal et al., Reference Grewal, Grewal, Tan and Adams2003). Parasitism of gastropods is therefore a very important lifestyle choice amongst nematodes; however, the genes involved in infecting and surviving in these hosts are unknown.
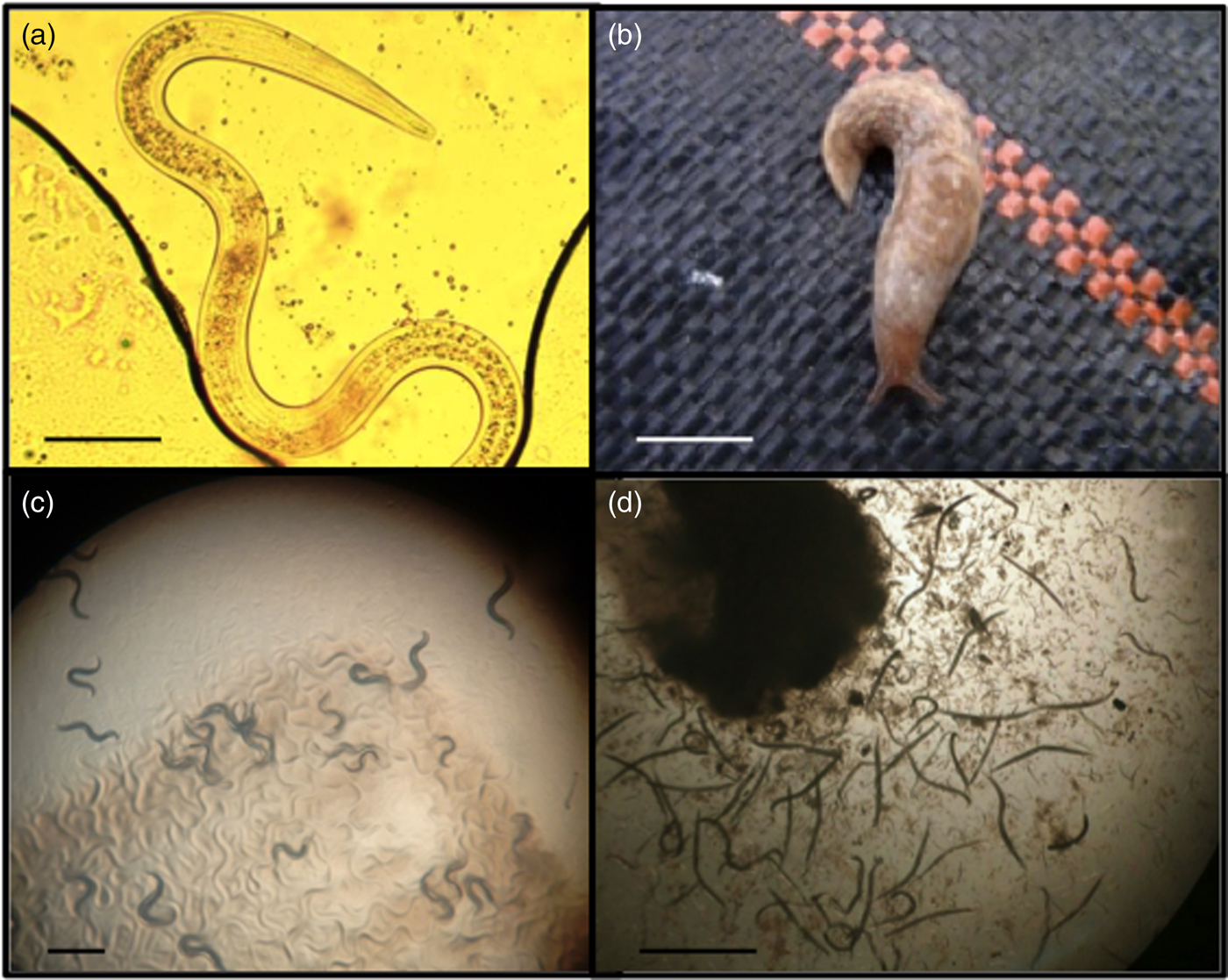
Fig. 1. (a) The nematode Phasmarhabditis hermaphrodita is a parasite of several slug species, including (b) Deroceras reticulatum. It can be kept under lab conditions on NGM agar, feeding on (c) Pseudomonas sp. 1 and on (d) rotting Limax flavus in a White trap. Scale bars: (a) 100 μm; (b) 1 cm; (c, d) 1 mm.
The majority of research on P. hermaphrodita has focused on optimizing application techniques in the field (see Rae et al., Reference Rae, Verdun, Grewal, Robertson and Wilson2007), host range studies (Wilson et al., Reference Wilson, Glen and George1993; Grewal et al., Reference Grewal, Grewal, Tan and Adams2003; Rae et al., Reference Rae, Robertson and Wilson2009), taxonomic descriptions, and surveys charting abundance and diversity of Phasmarhabditis in various countries (Ross et al., Reference Ross, Ivanova, Sirgel, Malan and Wilson2012, Reference Ross, Ivanova, Hatteland, Brurberg and Haukeland2016; Wilson et al., Reference Wilson, Burch, Tourna, Aalders and Barker2012; Tandingan De Ley et al., Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014, Reference Tandingan De Ley, Holovachov, McDonnell, Bert, Paine and De Ley2016; Nermut et al., Reference Nermut, Půža and Mráček2016a, Reference Nermut, Půža, Mekete and Mráčekb, Reference Nermut, Půža, Mekete and Mráček2017). There are few details and no reported methods regarding how to keep this nematode under lab conditions, like C. elegans, and whether it could be amenable to genetic manipulation. There is little information about its life history traits and how they change with bacterial diet or temperature. Also, there have been few experiments investigating natural variation in any ecological traits of the genus Phasmarhabditis; all studies so far have focused on the commercial strain of P. hermaphrodita (DMG0001), which has been in culture for over 20 years (Rae et al., Reference Rae, Verdun, Grewal, Robertson and Wilson2007).
Here we provide information on how to grow, maintain, mutagenize and mate not only P. hermaphrodita but also several other species of the Phasmarhabditis genus under lab conditions. We also provide information on how to isolate, identify and make isogenic lines of P. hermaphrodita. Taken together, these results show that many of these species can be maintained easily under lab conditions and could make excellent candidates as genetic models to understand the evolution of parasitism in the Nematoda.
Materials and methods
Terrestrial gastropod survey and molecular identification of parasitic nematodes
Slugs (Deroceras panormitanum, D. reticulatum (fig. 1b), Arion subfuscus, A. ater, A. hortensis, Limax flavus, L. maximus, Lehmannia valentiana, Milax budapestensis and M. sowerbyi) and snails (Cepaea nemoralis, Cornu aspersum and Oxychilus draparnaudi) were collected from several locations in Liverpool, UK, including Priory Wood near St Michael's station (Grid reference number SJ3673586862) (n = 107), Sefton Park (SJ3787187058) (n = 195) and Otterspool (SJ3707686321) (n = 57). Slugs were also collected from the Cruickshank Botanic Garden at the University of Aberdeen (NJ9376008556) (n = 48) and from Dale, Wales (SM809057) (n = 19). Once collected they were transported back to the lab, where they were chopped in half and placed in a 5 cm Petri dish with a few drops of distilled water and stored at room temperature (Wilson et al., Reference Wilson, Wilson, Aalders and Tourna2016). Over four days the slugs were examined for presence of nematodes. Any nematodes that morphologically resembled Phasmarhabditis nematodes (fig. 1), e.g. hermaphrodites, females or males over 1 mm, were transferred individually to modified White traps (White, Reference White1927) (see below for description) to make isogenic lines. After 21 days, when the food was exhausted and the nematodes had reached the dauer stage, they were present in the surrounding water and were removed and centrifuged at 16,000 rpm to concentrate, and their DNA was extracted using a DNA extraction kit (Qiagen, Hilden, Germany). Using polymerase chain reaction (PCR), three genes were then amplified (ITS1, 18SrRNA and the D2-D3 domain of large subunit (LSU) rDNA) (Blaxter et al., Reference Blaxter, de Ley and Garey1998; Tandingan De Ley et al., Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014) and sequenced in both directions for species identification. For the ITS1 gene the primers used were N93 (5′-TTGAACCGGGTAAAAGTCG-3′) and N94 (5′-TTAGTTTCTTTTCCTCCGCT-3′). The 18SrRNA gene was amplified using 18A (5′-AAAGATTAAGCCATGCATG-3′) and 26R (5′-CATTCTTGGCAAATGCTTTCG-3′). The D2-D3 LSU rDNA primers were D2 (5′-AGCGGAGGAAAAGAAACTAA-3′) and D3 (5′-TCGGAAGGAACCAGCTACTA-3′). As well as these three genes P. hermaphrodita-specific primers were also used, which were based on 150–200 bp of the Cytochrome Oxidase I gene created by Read et al. (Reference Read, Sheppard, Bruford, Glen and Symondson2006), which consisted of Ph-F-1754 (5′-TGGGTGCCCCTGATATAAGAT-3′) and Ph-R-1887 (5′-CGGATGACCAAGGGTACTTAAT-3′). These primers were used to investigate their potential use as a rapid and cheap method for identifying P. hermaphrodita without DNA sequencing, as they have been used previously to determine if P. hermaphrodita was present in mites and collembolans (Read et al., Reference Read, Sheppard, Bruford, Glen and Symondson2006). PCR cycling conditions consisted of the following: 3 minutes at 95°C followed by 35 cycles of 15 s at 95°C, 30 s at 55°C, 1.5 minutes at 72°C and a final step of 8 minutes at 72°C. The PCR products were then purified and sequenced in both forward and reverse directions for each gene (ITS1, 18SrRNA and the D2-D3 domain of LSU rDNA). Gene sequences of nematodes were then compared with National Center for Biotechnology Information (NCBI) database sequences using BLASTN searches, using similarity matches of 99%. For PCR using primers designed by Read et al. (Reference Read, Sheppard, Bruford, Glen and Symondson2006) the 200 bp product was visualized after gel electrophoresis, where a positive band indicated presence of P. hermaphrodita, and no sequencing was carried out.
Semi-natural conditions for growth of Phasmarhabditis species to make isogenic lines and dauer juveniles
Any potential Phasmarhabditis-like nematodes observed growing on the collected rotting slugs and snails were transferred to modified White traps (White, Reference White1927) for maintenance, to make isogenic lines and to quantify the number of dauers that were produced per individual hermaphrodite mother. Modified White traps consisted of a 10 cm Petri dish half filled with tap water. A 5 cm lid of a Petri dish was then placed on top of the water, with a 4.5 cm diameter piece of pre-moistened Whatman No. 1 filter paper (Whatman, Maidstone, UK) inside. A 3 mm slice of L. flavus was added to each White trap as a food source. Limax flavus were collected from Liverpool John Moores University greenhouses and frozen at −80°C for 1 h to kill any nematodes present. Limax flavus was used as food for Phasmarhabditis for several reasons: (1) it is a common slug species that is easily collected; (2) it is a large slug, and therefore many White traps can be made at one time; and (3) from preliminary experiments it consistently produces large numbers of nematodes. Also, a previous study has shown that feeding on this slug species in White traps produces consistently virulent P. hermaphrodita (Rae et al., Reference Rae, Tourna and Wilson2010). A single hermaphrodite mother was then transferred via a worm pick to each White trap, which was then sealed with Parafilm® (Bemis, Neenah, Wisconsin, USA) and stored at 20°C for 21 days, after which dauer stage nematodes were found in the surrounding water. This procedure was repeated for all of the Phasmarhabditis collection to make isogenic lines.
In a separate experiment the number of dauer juveniles produced by one self-fertilizing hermaphrodite mother was quantified for two strains of P. hermaphrodita (the commercial strain DMG0001 and the naturally isolated strain DMG0007) and one strain of P. californica (DMG0017) to understand the brood size of these nematodes. These strains were chosen to investigate if there was a difference between the growth of the commercial strain (P. hermaphrodita DMG0001; Hooper et al., Reference Hooper, Wilson, Rowe and Glen1999) and natural strains of P. hermaphrodita, as the commercial strain has been in culture for over 20 years and fed solely on the monoxenic diet consisting of the bacterium Moraxella osloensis (Wilson et al., Reference Wilson, Glen, George and Pearce1995a, Reference Wilson, Glen, Pearce and Rodgersb). The number of dauer juveniles produced by a single hermaphrodite mother of P. hermaphrodita DMG0001, DMG0007 or P. californica DMG0017 was quantified by setting five White traps containing either 0.025 g or 0.25 g of L. flavus, left for 21 days at 20°C (fig. 1d). The experiment was repeated twice.
Brood size of Phasmarhabditis species exposed to lab and naturally isolated bacteria
We investigated whether Phasmarhabditis nematodes could grow on several different bacteria, including Escherichia coli OP50 (the food of C. elegans and P. pacificus; Brenner, Reference Brenner1974; Sommer et al., Reference Sommer, Carmi and Eizinger2000), E. coli BR (an easily available strain used for cloning in molecular biology) and two naturally isolated bacterial species found to be associated with Pristionchus entomophagus called Pseudomonas sp. 1 and Bacillus sp. 1 (Rae et al., Reference Rae, Riebesell, Dinkelacker, Wang, Herrmann, Weller, Dieterich and Sommer2008) (fig. 1c). Bacteria were grown in nutrient broth at 30°C overnight. The following morning 100 μl of each bacterium was spread onto five 5 cm NGM plates (Hope, Reference Hope1999), which were then incubated at 30°C overnight. An individual dauer stage nematode was transferred to each plate via a worm pick and incubated at 20°C. The numbers of offspring per plate were then recorded after three and six days. The experiment used P. hermaphrodita DMG0001 and DMG0007 and P. californica DMG0017. This experiment was repeated three times with all four bacteria and with all three nematode isolates.
To understand the feeding behaviour of Phasmarhabditis nematodes in more detail we also recorded the pharyngeal pumping rate whilst they were eating. This has been recorded easily in both C. elegans and P. pacificus (Kroetz et al., Reference Kroetz, Srinivasan, Yaghoobian, Sternberg and Hong2012) but never for any Phasmarhabditis species. The pharyngeal pumping rate of an individual of each of three Phasmarhabditis species (P. hermaphrodita DMG0007, P. neopapillosa DMG0012 and DMG0016, and P. californica DMG0017) was counted for 60 s and repeated ten times with different worms.
Investigating the effect of temperature on the brood size of Phasmarhabditis species
To ascertain the optimum temperature for growth of Phasmarhabditis nematodes under laboratory conditions, 15 5-cm NGM plates were seeded with 100 μl of Pseudomonas sp. 1 and then incubated at 30°C overnight. Pseudomonas sp. 1 was chosen from the four bacterial species tested because it resulted in Phasmarhabditis nematodes producing a large number of offspring, which were easy to see in the bacterial lawn because of its translucent nature. A single L4 hermaphrodite was placed onto each NGM plate and groups of five plates were incubated at either 10°C, 15°C or 20°C for six days. On days 3 and 6 the numbers of offspring were recorded. This experiment was repeated three times using the same nematode species and strains as above.
Heat shocking and rate of spontaneous male production of several Phasmarhabditis species
It is imperative that a genetic model nematode can be mated under laboratory conditions and it is unknown how commonly males are produced in P. hermaphrodita collected from the wild. Five NGM plates (5 cm) were spread with 50 μl of Pseudomonas sp. 1 and incubated overnight at 30°C. The following morning dauer juveniles (1000–7000 per strain) were added to each plate and incubated at 20°C for two to three days. The numbers of males present were then recorded. The species and strains used (and numbers of dauer observed) were P. hermaphrodita DMG0001 (n = 4040), DMG0002 (n = 6771), DMG0010 (n = 4581), DMG0009 (n = 3108), DMG0003 (n = 2503) and DMG0007 (n = 3572); P. californica DMG0017 (n = 1098) and DMG0019 (n = 1127); and P. neopapillosa (DMG0012, DMG0015 and DMG0016; n = 750 for each strain).
In C. elegans the number of males can be increased by exposing hermaphrodite mothers to 30°C for 4 h (Hope, Reference Hope1999). We investigated whether the same was true for P. hermaphrodita (DMG0001, DMG0007 and DMG0009). Fifteen to 20 L4 hermaphrodites were added to five separate 5 cm NGM plates seeded with Pseudomonas sp. 1 and placed in a 30°C incubator for 1, 3, 4.5, 5 and 6 h, after which the plates were maintained at 20°C to recover and the number of males in the offspring was recorded after four days. The experiment was repeated three times.
Genetic crosses of Phasmarhabditis species under lab conditions
Some parasitic nematodes are difficult to mate under lab conditions using agar plates, e.g. the free-living generation of Strongyloides ratti (Nemetschke et al., Reference Nemetschke, Eberhardt, Viney and Streit2010), and it is unknown if P. hermaphrodita or any other Phasmarhabditis species can be mated, which is essential to monitor the inheritance of mutations and to facilitate mapping of mutated genes. Therefore, we used methods that are commonly used to mate C. elegans. Specifically, five 5 cm NGM plates with 50 μl of Pseudomonas sp. 1 were incubated at 30°C overnight. One L4 hermaphrodite was added to each plate with two young males and the plates were incubated at 20°C for six days. After two days of mating the males were removed with a worm pick and killed. After six days the sex and number of offspring were recorded. We used P. neopapillosa (DMG0012 and DMG0016), a gonochoristic species that produces almost 50% males, as we had difficulty finding males from P. hermaphrodita even after heat shocking. The experiment was repeated three times.
Natural genetic variation in thermotolerance and pH tolerance of Phasmarhabditis species
To assess whether there was natural genetic variation in the ability of the Phasmarhabditis nematodes to cope with extreme pH and temperatures, the following experiments were carried out. For the thermotolerance experiment, three 1.5 ml Eppendorf tubes containing 4500–6000 nematodes per 1 ml were placed into a heat block set at 33°C, 37°C or 41°C for 15 minutes. At time 0 and after 15 minutes the numbers of nematodes were quantified. The Eppendorf containing the nematodes was vortexed every 2.5 minutes to avoid clumping. Eppendorfs containing the same numbers of nematodes but kept at room temperature were used as the control. The experiment was repeated three times for each temperature. The following species and strains were used: P. hermaphrodita (DMG0001, DMG0007, DMG0010, DMG0006 and DMG0008), P. californica (DMG0017 and DMG0019) and P. neopapillosa (DMG0013, DMG0015 and DMG0016).
To assess natural variation of pH tolerance, 10 individual dauer stage nematodes were added to 80 wells containing 60 μl of water adjusted to pH 4, 5, 6, 7, 8, 9 and 10, as well as a control of distilled water. The correct pH was obtained by addition of either 1 m NaOH or 1 m HCl and confirmed using a pH meter and indicator paper. There were ten wells per pH and the whole experiment was repeated twice. The 96 well plates were then incubated at 20°C and survival was recorded daily for four days. The same Phasmarhabditis species and strains were used as in the thermotolerance experiment.
Formaldehyde mutagenesis of P. hermaphrodita DMG0001
To investigate if P. hermaphrodita can be mutated using formaldehyde mutagenesis (like C. elegans and P. pacificus) we used similar methods to those developed by Johnsen & Baillie (Reference Johnsen and Baillie1988) for C. elegans. P. hermaphrodita DMG0001 (L4 and young adult stage) were grown on several NGM plates with Pseudomonas sp. 1 for four days. They were then washed in distilled water, concentrated to a pellet and exposed to 0.1% formaldehyde for four hours, after which the P0's were washed several times in water to remove any residual formaldehyde and 100 individual mothers were separated out and placed on individual NGM plates seeded with Pseudomonas sp. 1. They were stored at 20°C for three to four days and allowed to produce offspring, and then 300 F1's were separated out (three individuals were picked randomly from each plate of P0 mothers) and the F2's were screened for any morphological abnormalities after five to seven days.
Data analysis
The difference between the numbers of dauers produced by P. hermaphrodita DMG0001 and DMG0007 and P. californica DMG0017 grown on 0.025 g and 0.25 g of L. flavus was analysed using a one-way analysis of variance (ANOVA) with Tukey's post-hoc test. These tests were also used to analyse the data on pumping rate, number of offspring produced on different bacteria and at different temperatures, and the numbers of surviving nematodes exposed to 33, 37 and 41°C and pH 4–10. The body length of P. hermaphrodita DMG0001 WT and sma mutants was compared using a Student's t test. SPSS v. 23 (IBM, Armonk, USA) was used for data analysis.
Results
Phasmarhabditis species can be easily isolated and identified from gastropods
From 426 slugs and snails collected from around the UK we found 12 isolates of P. hermaphrodita, three isolates of P. californica and five isolates of P. neopapillosa (table 1). These isolates were all from separate slugs apart from P. californica, of which three isolates were found in a single O. draparnaudi. We had initially identified many of these Phasmarhabditis species as P. hermaphrodita by using species-specific primers developed by Read et al. (Reference Read, Sheppard, Bruford, Glen and Symondson2006) (supplementary fig. S1). However, we found that these primers amplify not only P. hermaphrodita but also other members of the Phasmarhabditis genus and even diverse insect-associated and free-living species such as Steinernema feltiae, Panagrellus redivivus, Aphelenchus avenae, Pelodera teres and Pristionchus entomophagus (supplementary fig. S1). Hence, they are not suitable for identification of P. hermaphrodita specifically and should be used with caution. We therefore amplified and sequenced three genes (ITS1, 18SrRNA and the D2-D3 domain of LSU rDNA) for species identification. These P. hermaphrodita strains and Phasmarhabditis species are the start of an ongoing effort to make a collection of P. hermaphrodita strains and Phasmarhabditis species to study the genetic evolution of parasitism, and we have categorized them using C. elegans nomenclature (table 1).
Table 1. The Phasmarhabditis species that were isolated from slugs and snails collected from Aberdeen, Liverpool and Pembrokeshire, UK, and codes of isolated Phasmarhabditis species based on C. elegans nomenclature.

Growth of Phasmarhabditis species using semi-natural conditions
We made isogenic lines by growing single hermaphrodites of P. hermaphrodita DMG0001 and DMG0007 and P. californica DMG0017 on 0.025 g (fig. 2a) and 0.25 g of L. flavus (fig. 2b). The numbers of dauer juveniles that were produced by one P. hermaphrodita DMG0001, DMG0007 and P. californica DMG0017 on 0.025 g of L. flavus after 21 days ranged from 43 to 6166 dauers per White trap (fig. 2a) and did not differ significantly (F (2, 36) = 1.369; P = 0.268). The numbers of dauer juveniles that were produced by single mothers of P. hermaphrodita DMG0001 and DMG0007 and P. californica DMG0017 fed on 0.25 g of L. flavus after 21 days ranged from 417 to 27,750 dauers per plate (fig. 2b) and also did not differ significantly (F (2, 38) = 2.832; P = 0.072). Therefore, Phasmarhabditis spp. can be grown easily under semi-natural conditions using L. flavus White traps and in large numbers for experiments.
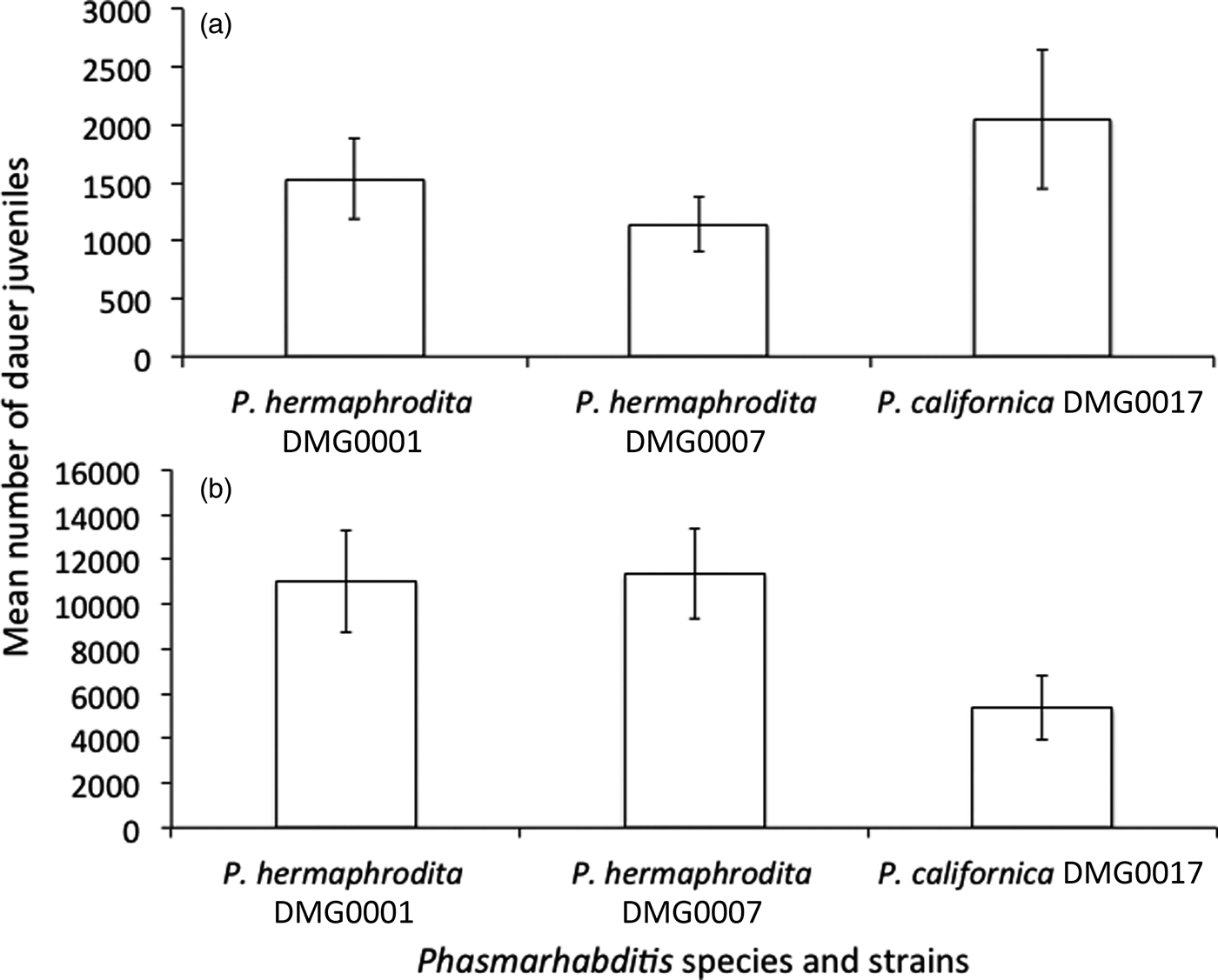
Fig. 2. The mean (± SE) number of offspring that were produced by P. hermaphrodita DMG0001, P. hermaphrodita DMG0007 and P. californica DMG0017 when fed (a) 0.025 g and (b) 0.25 g of L. flavus.
Growth of Phasmarhabditis species on different bacteria at different temperatures
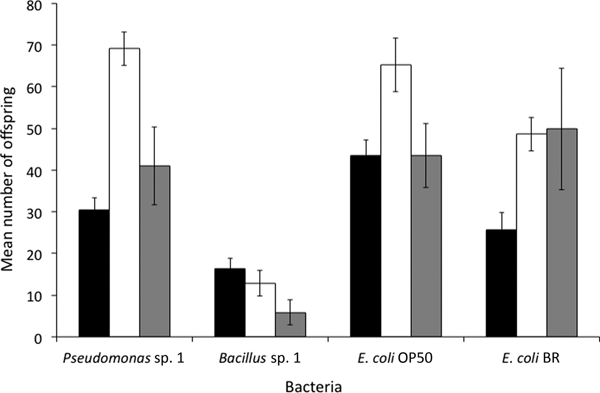
Phasmarhabditis hermaphrodita DMG0001 and DMG0007 and P. californica DMG0017 were fed two nematode-associated bacteria (Pseudomonas sp. 1 and Bacillus sp. 1) and two strains of E. coli that have been used historically in C. elegans culture and molecular biology (E. coli OP50 and E. coli BR, respectively). Over six days both nematode species were able to grow on each of these bacteria, and laid F1 eggs that developed to offspring and were quantified (fig. 3). There was a significant difference between the numbers of offspring produced by P. hermaphrodita DMG0001 when fed Pseudomonas sp. 1, Bacillus sp. 1, E. coli OP50 and E. coli BR (F (3, 29) = 11.101; P = 0.000). Specifically, the numbers of offspring produced by P. hermaphrodita DMG0001 was highest on Pseudomonas sp. 1, E. coli OP50 and E. coli BR, and lowest on Bacillus sp. 1 (P < 0.05). This was also the case for P. hermaphrodita DMG0007 and P. californica DMG0017. Therefore, the laboratory bacteria (E. coli OP50 and E. coli BR) and naturally isolated Pseudomonas sp. 1 can be used for growing Phasmarhabditis species.

Fig. 3. The mean (± SE) number of offspring that were produced by P. hermaphrodita DMG0001 (black bars), P. hermaphrodita DMG0007 (white) and P. californica DMG0017 (grey) when fed Pseudomonas sp. 1, Bacillus sp. 1, Escherichia coli OP50 and E. coli BR at 20°C.
When grown at specific temperatures (10°C, 15°C and 20°C) and fed Pseudomonas sp. 1 to investigate the optimum conditions for growth and brood size of P. hermaphrodita DMG0001, P. hermaphrodita DMG0007 and P. californica DMG0017 it was found that 20°C was best for growth for both species (fig. 4). There was no significant difference between the numbers of offspring of both species when fed Pseudomonas sp. 1 at 20°C after three days (F (2,35) = 0.917; P = 0.41). However, after six days at 20°C P. hermaphrodita DMG0007 produced significantly more offspring than P. hermaphrodita DMG0001 and P. californica DMG0017 (F (2,31) = 5.067; P = 0.013). Production of offspring of both species was low at 10°C after six days. Eggs were laid in small numbers but they did not develop into live offspring. There was no significant difference between the numbers of viable offspring produced by both species after six days at 15°C (F (2,28) = 1.649; P = 0.212). When grown at temperatures higher than this (25°C) the hermaphrodite mothers died rapidly (Andrus & Rae, pers. obs.) so this seems to represent the upper limit for growth of these natural strains.

Fig. 4. The mean (± SE) number of offspring that were produced by P. hermaphrodita DMG0001 (black bars), P. hermaphrodita DMG0007 (white) and P. californica DMG0017 (grey) at (a) 10°C, (b) 15°C and (c) 20°C.
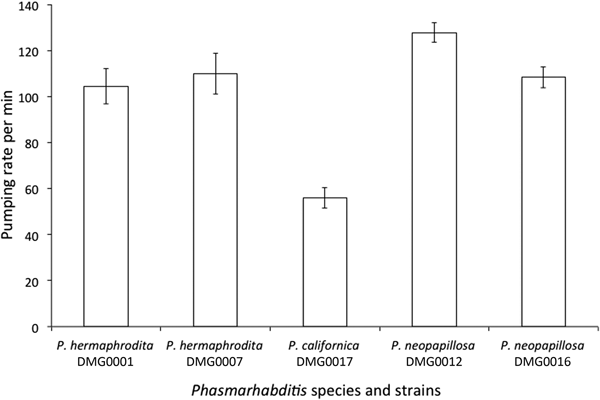
When feeding on Pseudomonas sp. 1 there was a significant difference between the pumping rates of P. hermaphrodita DMG0001 and DMG0007, P. neopapillosa DMG0012 and DMG0016 and P. californica DMG0017 (F (4, 19) = 18.577; P = 0.000) (fig. 5). Specifically, there was no difference between the pumping rates of P. hermaphrodita DMG0001 and DMG0007 and P. neopapillosa DMG0012 and DMG0016 (P > 0.05) but all differed significantly from P. californica DMG0017, which had the lowest number of pumps per minute (P < 0.05) (fig. 5).
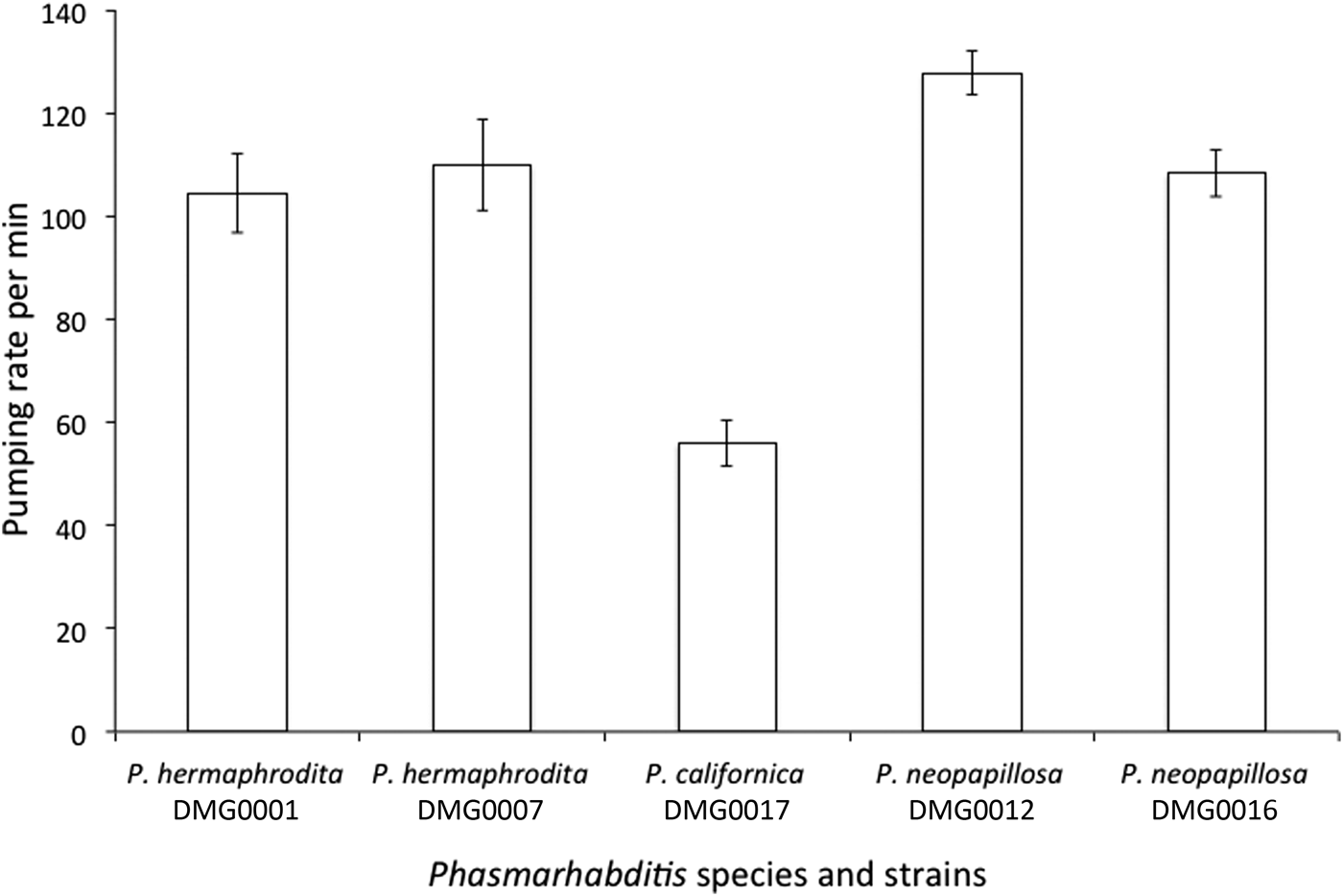
Fig. 5. The mean (± SE) pumping rate over 60 s of P. hermaphrodita DMG0001 and DMG0007, P. californica DMG0017 and P. neopapillosa DMG0012 and DMG0016 fed Pseudomonas sp. 1 at 20°C.
Natural variation in thermotolerance of Phasmarhabditis
There was a significant difference in the survival of the Phasmarhabditis isolates when exposed to 30°C (F (10, 98) = 18.389; P < 0.001) (fig. 6a). Specifically, the survival of the commercial strain of P. hermaphrodita (DMG0001) was significantly lower than that of P. hermaphrodita DMG0007 and DMG0008 (P < 0.001) but not P. hermaphrodita DMG0010 and DMG0006 (P > 0.05). When the survival of P. hermaphrodita DMG0001 was compared to other species of Phasmarhabditis there was a significant difference between it and P. neopapillosa DMG0015 and DMG0016 (P < 0.001) but not DMG0013 (P > 0.05). Also, there was a significant difference between the survival of P. hermaphrodita DMG0001 and that of P. californica DMG0019 (P < 0.05) but not DMG0017 (P > 0.05).
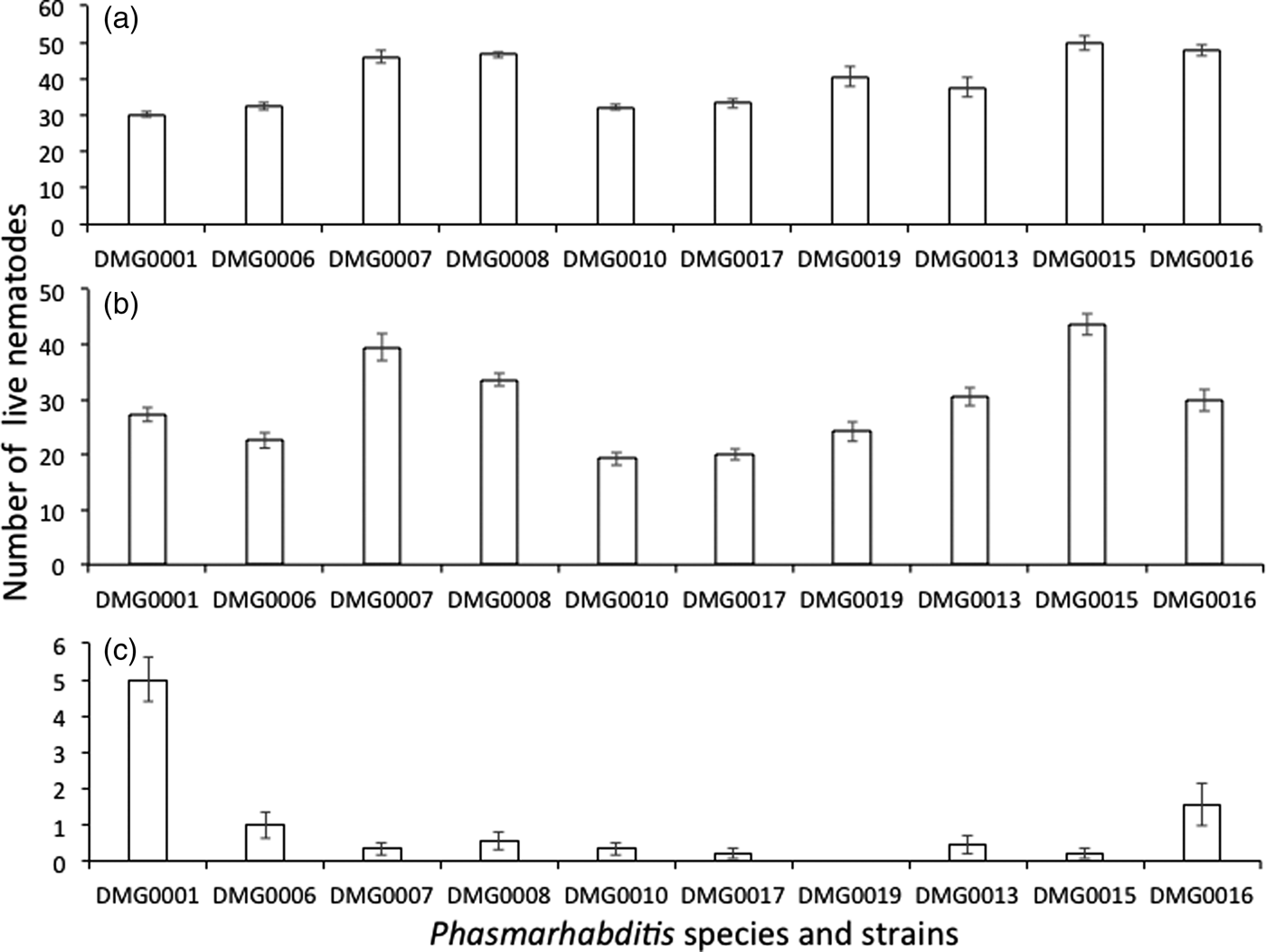
Fig. 6. The mean (± SE) number of live P. hermaphrodita DMG0001, DMG0007, DMG0010, DMG0006 and DMG0008, P. californica DMG0018 and DMG0019 and P. neopapillosa DMG0013, DMG0015 and DMG0016 exposed to (a) 31°C, (b) 37°C and (c) 41°C.
When the Phasmarhabditis isolates were exposed to 37°C there was a significant difference in their survival (F (10, 98) = 24.017; P = 0.000) (fig. 6b). The survival of P. hermaphrodita DMG0001 was significantly lower than that of P. hermaphrodita DMG0007 but significantly higher than that of DMG0010 (P < 0.05) but not of any other P. hermaphrodita strain. The survival of P. hermaphrodita DMG0001 differed from that of P. neopapillosa DMG0015 (P < 0.05) but no other species or isolate.
When the Phasmarhabditis isolates were exposed to 41°C there was a significant difference in their survival (F (10, 98) = 19.546; P = 0.000) (fig. 6c). The survival of P. hermaphrodita DMG0001 was significantly greater than that of all other species and isolates (P < 0.05).
Natural variation in pH resistance in Phasmarhabditis
When the Phasmarhabditis species and strains were exposed to pH 4 there was a significant difference in survival (F (9, 29) = 6.060; P = 0.000) (table 2), the numbers of surviving P. hermaphrodita DMG0001 being significantly greater than those of P. neopapillosa DMG0015 and DMG0016 (P < 0.05).
Table 2. The mean (± SE) number of live individuals of Phasmarhabditis hermaphrodita, P. californica and P. neopapillosa after four days of exposure to pH 4–10.
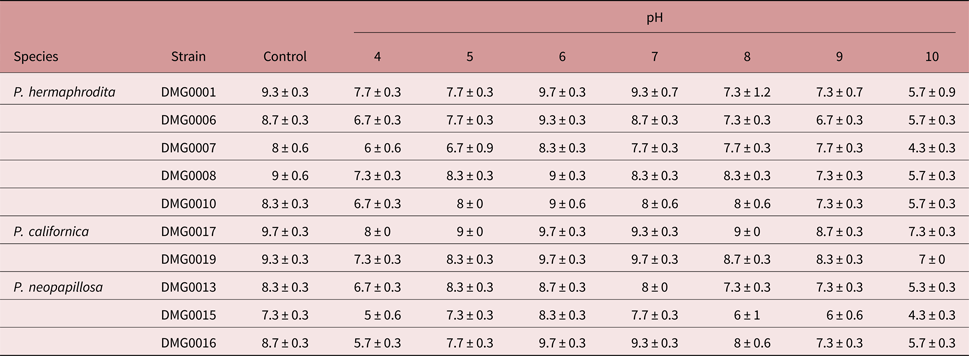
When exposed to pH 5 the survival of Phasmarhabditis species and strains was significantly different (F (9, 29) = 2.706; P = 0.031) (table 2), the survival of P. hermaphrodita DMG0007 being significantly lower than that of P. californica DMG0017 (P < 0.05). There was no significant difference between the survival of Phasmarhabditis species and strains exposed to pH 6 (F (9, 29) = 1.937; P = 0.105) or pH 8 (F (9, 29) = 1.956; P = 0.101).
The survival of Phasmarhabditis also differed at pH 7 (F (9, 29) = 3.778; P = 0.006), with the numbers of surviving P. hermaphrodita DMG0007 and P. neopapillosa DMG0015 being significantly lower than those of P. californica DMG0019 (P < 0.05).
At pH 9 (F (9, 29) = 3.378; P = 0.011) and pH 10 (F (9, 29) = 5.481; P = 0.001) survival was significantly different. In both cases the survival of P. neopapillosa DMG0015 was significantly lower than that of P. californica DMG0017 and DMG0019 (P < 0.05). Also, the survival of P. hermaphrodita DMG0007 was significantly lower than that of P. californica DMG0017 and DMG0019 when exposed to pH 10 (P < 0.05).
Rate of spontaneous male production, heat shocking and genetic crosses using Phasmarhabditis species
We observed the numbers of Phasmarhabditis dauer juveniles that developed into males when grown on NGM agar and fed Pseudomonas sp. 1 for four days. From six strains of P. hermaphrodita (DMG0001, DMG0002, DMG0010, DMG0009, DMG0003 and DMG0007) and two strains of P. californica (DMG0017 and DMG0019) no males were observed and only hermaphrodites were produced. All gonochoristic species produced males in varying numbers: P. neopapillosa DMG0012 (50% males to 50% females), DMG0015 (25% males to 75% females) and DMG0016 (46% males to 54% females).
We investigated if the number of males could be increased by heat shocking P. hermaphrodita hermaphrodites (DMG0001, DMG0007 and DMG0009) for 1, 3, 4.5, 5 and 6 h at 30°C. No offspring were produced by hermaphrodites that had been exposed to 30°C for 5 and 6 h and the number of offspring produced was low for heat treatment for 4.5 h (11–30 individuals) and 3 h (11–33 individuals) but increased when exposed for 1 h (16–115 individuals). However, no males were observed in any of the offspring. Therefore, it is problematic to find P. hermaphrodita males when grown under these conditions.
To understand whether Phasmarhabditis species could be mated under lab conditions we concentrated on using the gonochoristic species P. neopapillosa DMG0012 and DMG0016. Both strains were crossed using standard procedures based on C. elegans and were fed Pseudomonas sp. 1. Under these conditions we could show that two males to one female placed together resulted in 47.3 ± 6.7 P. neopapillosa DMG0016 and 83.9 ± 5.6 P. neopapillosa DMG0012 offspring six days later. Therefore, successful crossing of these two strains could be carried out to demonstrate inheritance of recessive and dominant mutations and to aid mapping of mutations.
Formaldehyde mutagenesis of Phasmarhabditis species
From 300 F1 P. hermaphrodita DMG0001 hermaphrodite mothers (fig. 7a) several mutants were isolated. Specifically, two small (sma) mutants (fig. 7b, c) and three uncoordinated (unc) mutants (fig. 7d, e) were found. sma mutants were significantly smaller than P. hermaphrodita WT (P < 0.05), and unc mutants strongly resembled C. elegans unc phenotype as they were lethargic, stationary and the underlying body wall muscle produced a constant twitch (Waterston et al., Reference Waterston, Thomson and Brenner1980). Therefore, P. hermaphrodita can be mutagenized using formaldehyde, allowing forward genetic screens to be carried out.
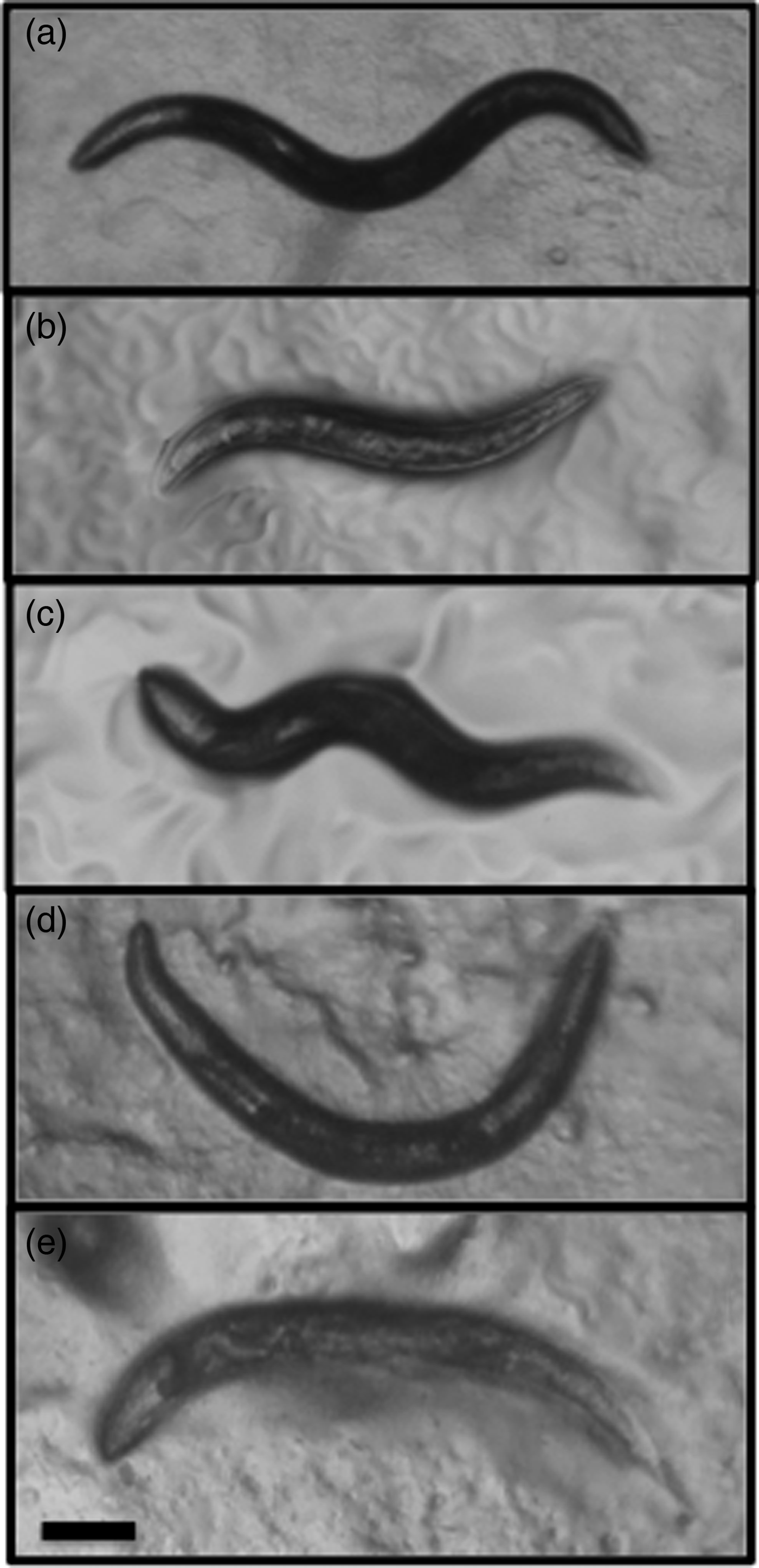
Fig. 7. (a) P. hermaphrodita DMG0001 were mutagenized with 0.1% formaldehyde, and (b, c) sma (small) mutants and (d, e) unc (uncoordinated) mutants were found in the F2 generation. Scale bar: 100 μm.
Discussion
The genus Phasmarhabditis contains 11 species: P. hermaphrodita, P. apuliae, P. papillosa, P. neopapillosa, P. valida, P. nidrosiensis, P. californica, P. tawfiki, P. bonaquaense, P. bohemica and P. huizhouensis (Andrássy, Reference Andrássy1983; Hooper et al., Reference Hooper, Wilson, Rowe and Glen1999; Azzam, Reference Azzam2003; Tandingan De Ley et al., Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014, Reference Tandingan De Ley, Holovachov, McDonnell, Bert, Paine and De Ley2016; Huang et al., Reference Huang, Ye, Ren and Zhao2015; Nermut et al., Reference Nermut, Půža and Mráček2016a, Reference Nermut, Půža, Mekete and Mráčekb, Reference Nermut, Půža, Mekete and Mráček2017). Phasmarhabditis hermaphrodita and other Phasmarhabditis species have been isolated from slugs from around the world, including the UK (Wilson et al., Reference Wilson, Glen and George1993; this study), Germany (Schneider, Reference Schneider1859; Mengert, Reference Mengert1953), France (Maupas, Reference Maupas1900; Coupland, Reference Coupland1995), Iran (Karimi et al., Reference Karimi, Kharazi-Pakadel and Robert2003), Egypt (Azzam, Reference Azzam2003; Genena et al., Reference Genena, Mostafa, Fouly and Yousef2011), Norway (Ross et al., Reference Ross, Ivanova, Hatteland, Brurberg and Haukeland2016), Chile (France & Gerding, Reference France and Gerding2000), New Zealand (Wilson et al., Reference Wilson, Burch, Tourna, Aalders and Barker2012), South Africa (Ross et al., Reference Ross, Ivanova, Sirgel, Malan and Wilson2012; Pieterse et al., Reference Pieterse, Tiedt, Malan and Ross2017a, Reference Pieterse, Malan, Kruitbos, Sirgel and Rossb), China (Huang et al., Reference Huang, Ye, Ren and Zhao2015), Japan (Waki, Reference Waki2017), Italy (Nermut et al., Reference Nermut, Půža and Mráček2016a) and the Czech Republic (Nermut et al., Reference Nermut, Půža and Mráček2010, Reference Nermut, Půža, Mekete and Mráček2016b, Reference Nermut, Půža, Mekete and Mráček2017), and P. californica has been found in the USA (Tandingan De Ley et al., Reference Tandingan De Ley, Holovachov, McDonnell, Bert, Paine and De Ley2016), Ireland (Carnaghi et al., Reference Carnaghi, Rae and Bistline-East2017) and Wales (this study). From these studies it is apparent that members of the Phasmarhabditis genus have a cosmopolitan distribution and are very easy to isolate from gastropod hosts. We found 20 separate Phasmarhabditis isolates from 426 slugs and snails from three locations around the UK. From our survey the success rate of collecting Phasmarhabditis (4.7%) seems small, yet we have found a wealth of different species, including P. californica (which had never been identified in the UK previously) and several strains of P. neopapillosa. Therefore, it seems that the UK has an underappreciated diversity of Phasmarhabditis species.
Although P. hermaphrodita is a parasite, we have shown that it can be maintained and cultured under laboratory conditions using a combination of NGM agar and naturally isolated bacteria (Pseudomonas sp. 1). For laboratory genetic model nematodes to be used successfully their bacterial food must be nutritious enough to facilitate the growth of hundreds of offspring, transparent (so nematodes are visible) and easy to grow (but does not grow too much when added to the NGM plate). This was why E. coli OP50 was selected to grow and maintain C. elegans (Brenner, Reference Brenner1974). However, regular passage of hundreds of thousands of generations of nematodes using the same culture conditions (constant temperature, lots of food and same species of food) with no interaction or variation in the environment can severely affect genetic make up (Huey & Rosenzweig, Reference Huey, Rosenzweig, Garland and Rose2009). For example, C. elegans N2 (wild type) has lost the ability to perform thermoregulatory behaviour when exposed to a temperature gradient as a result of being reared at the same temperature for over 40 years (Anderson et al., Reference Anderson, Albergotti, Proulx, Peden, Huey and Phillips2007). We propose the use of Pseudomonas sp. 1, a bacterium found in the intestine of P. entomophagus from Tübingen, Germany (Rae et al., Reference Rae, Riebesell, Dinkelacker, Wang, Herrmann, Weller, Dieterich and Sommer2008), and not an unusual food source such as E. coli OP50. Yet continual culturing on NGM plates is an unnatural culture method for these nematodes, as Phasmarhabditis are necromenic and parasitic nematodes used to reproducing on rotting cadavers of molluscs (Wilson et al., Reference Wilson, Glen and George1993; Rae et al., Reference Rae, Robertson and Wilson2009). To this end we propose growing Phasmarhabditis on decaying slugs in ‘semi-natural’ conditions using White traps, which is a more realistic environment. This method means that they can be stored at 10–15°C for months as dauers (Grewal & Grewal, Reference Grewal and Grewal2003) and cultured infrequently (every four to five months), and therefore the effect of accumulating any deleterious mutations will be reduced. Furthermore, future research will focus on the development of cryopreservation techniques for Phasmarhabditis, which will allow access to a library of ‘unevolved’ strains and species as well as mutants.
Once P. hermaphrodita has killed a slug it feeds on the bacteria growing on the rotting cadaver, and when this is depleted it turns to the dauer stage and searches for more slugs in the soil. These dauers associate with a rich diversity of bacteria that are carried in their intestines (Rae et al., Reference Rae, Tourna and Wilson2010). Previous studies have shown that the bacteria isolated from the intestine of P. hermaphrodita, from xenic cultures of P. hermaphrodita and from swabs of slugs that died from infection of P. hermaphrodita can affect the number of dauers produced as well as their virulence towards slugs (Wilson et al., Reference Wilson, Glen, George and Pearce1995a, Reference Wilson, Glen, Pearce and Rodgersb). These studies showed that P. hermaphrodita can grow on an array of bacterial species, such as Pseudomonas fluorescens, Sphingobacterium spiritivorum, M. osloensis, Serratia proteamaculans, Aeromonas sp. and Providencia rettgeri (Wilson et al., Reference Wilson, Glen, Pearce and Rodgers1995b), and P. hermaphrodita grown on bacteria such as P. fluorescens, M. osloensis and P. rettgeri can produce high yields of pathogenic nematodes that kill slugs (Wilson et al., Reference Wilson, Glen, George and Pearce1995a). However, the commercial isolate of P. hermaphrodita (DMG0001) is grown on M. osloensis, as it can produce consistently high yields of highly pathogenic nematodes (Tan & Grewal, Reference Tan and Grewal2001b; Wilson et al., Reference Wilson, Glen, George and Pearce1995a, Reference Wilson, Glen, Pearce and Rodgersb). It has been shown that when introduced into the shell cavity of D. reticulatum, M. osloensis produces a lipopolysaccharide (LPS) that acts as an endotoxin, causing rapid mortality (Tan & Grewal, Reference Tan and Grewal2002). By utilizing this collection of naturally isolated P. hermaphrodita and Phasmarhabditis species the co-evolution of these tritrophic interactions between bacteria (such as M. osloensis), nematodes and slug hosts could be analysed at the molecular level.
As well as established genetic model nematodes (C. elegans and P. pacificus) there are several other nematodes that have been proposed, including Poikilolaimus oxycercus (Hong et al., Reference Hong, Villwock and Sommer2005), Oscheius tipulae (Félix, Reference Félix2006) and Meloidogyne hapla (to study plant parasitism) (Opperman et al., Reference Opperman, Bird and Williamson2008). For these nematodes (as well as P. pacificus) to be used under laboratory conditions, information is needed about appropriate bacterial food as well as methods for genetic crosses, mutagenesis and long-term storage. We have shown that logistically and financially, nematodes such as Phasmarhabditis are easy to maintain. There is little difference in the equipment needed to keep C. elegans (Stiernagle, Reference Stiernagle2006), e.g. simple reagents and microbiological media, and incubators and freezers for growth and long-term storage. As well as these factors, another important point about model nematodes is that the ability to be isolated easily can allow tens if not hundreds of strains to be studied to investigate natural phenotypic variation, which can lead to an understanding of the underlying genotype using approaches such as RADseq (restriction site-associated DNA sequencing) (Davey & Blaxter, Reference Davey and Blaxter2010) and GWAS (genome-wide association studies) (Cook et al., Reference Cook, Zdraljevic and Tanny2016). In global sampling efforts, several hundred C. elegans strains and 26 Caenorhabditis species have been collected (Frézal & Félix, Reference Frézal and Félix2015), which are available from the Caenorhabditis Genetics Center at the University of Minnesota, USA. Studying natural variation has been successful in understanding genes involved with foraging behaviour, thermal tolerance and outcrossing (De Bono & Bargmann, Reference De Bono and Bargmann1998; Teotónio et al., Reference Teotónio, Manoel and Phillips2006; Harvey & Viney, Reference Harvey and Viney2007). A similar approach has been taken utilizing natural strains and investigating variation in behaviour, cold tolerance and dauer formation in P. pacificus (Hong et al., Reference Hong, Witte and Sommer2008; Mayer & Sommer, Reference Mayer and Sommer2011; McGaughran & Sommer, Reference McGaughran and Sommer2014). In total there are 28 species of Pristionchus (Ragsdale et al., Reference Ragsdale, Kanzaki, Herrmann and Sommer2015) and hundreds of strains of P. pacificus, which are available from the Sommer lab, Tübingen, Germany (Morgan et al., Reference Morgan, MacGaughran, Villate, Herrmann, Witte, Bartelmes, Rochat and Sommer2012). We have shown that, like both C. elegans and P. pacificus, P. hermaphrodita and a selection of Phasmarhabditis species can be isolated and maintained in the lab easily. We have shown that there is natural variation within P. hermaphrodita and Phasmarhabditis species in terms of surviving different temperatures and pHs. This means that with the development of appropriate sequencing and genomic techniques (e.g. RADseq) macroevolutionary and microevolutionary processes could potentially be unravelled at the genetic level.
The isolation of mutants via forward genetic screens using mutagenesis is a powerful technique that can identify genes responsible for specific phenotypes. The first step for any proposed genetic model nematode is to show it can be mutagenized. Here we showed P. hermaphrodita unc and sma mutants could be isolated by using similar protocols to those used for C. elegans (Johnsen & Baillie, Reference Johnsen and Baillie1988). If P. hermaphrodita can be mutated then there is no reason why unbiased forward genetic screens could not be carried out to investigate an array of evolutionary and ecologically important traits. These include finding mutants that are defective in killing slugs, inducing slug avoidance (Wilson et al., Reference Wilson, Hughes, Jefferies and Glen1999; Wynne et al., Reference Wynne, Morris and Rae2016) or failing to chemotax towards host cues such as slug mucus (Rae et al., Reference Rae, Robertson and Wilson2006, Reference Rae, Robertson and Wilson2009). As P. hermaphrodita is one of the candidates for the 959 Nematode Genomes project (Kumar et al., Reference Kumar, Schiffer and Blaxter2012) and several species are currently undergoing full genome sequencing, this will facilitate genomic comparison with closely related free-living nematodes, arthropod and mammalian parasites present in Clade 5.
We found that P. neopapillosa could be mated under lab conditions using similar procedures as for C. elegans. However, generating enough males for genetic crosses with P. hermaphrodita proved difficult. This is not unusual for hermaphroditic nematodes that are able to produce males spontaneously. Caenorhabditis elegans produces only 0.1–0.2% males in culture (Hodgkin & Doniach, Reference Hodgkin and Doniach1997). Maupas (Reference Maupas1900) noted that only one male was found in 14,888 P. hermaphrodita. Our strains under lab conditions seem not to produce males, even when heat shocked for 1–4 h at 30°C. When these strains were first isolated one was found to have males present (DMG007) (R. Rae, unpubl. data) but when grown on rotting slug and NGM plates the ability to produce males seemed to diminish rapidly over time. Future research will focus on methods used to generate males, including exposing hermaphrodites to ethanol (Lyons & Hecht, Reference Lyons and Hecht1997), and isolating a mutant (using forward genetics) that has a high incidence of males (him mutant) (Hodgkin et al., Reference Hodgkin, Horvitz and Brenner1979).
In conclusion, we have outlined the methods used to work with P. hermaphrodita and other Phasmarhabditis species under laboratory conditions. We believe that P. hermaphrodita (and other Phasmarhabditis species) could be used to identify genes that are essential for pathogenicity towards slugs. The most logical way to achieve this would be to take a natural variation approach to isolate as many P. hermaphrodita strains as possible and grow them on rotting slugs (as we have outlined here), which does not affect their virulence (Rae et al., Reference Rae, Tourna and Wilson2010), and assess their pathogenic potential towards slugs. The main aim would be to identify a strain that is more or less virulent than DMG0001 (an approach that is currently ongoing; R. Rae, unpubl. data). Through genome sequencing, potential parasitism genes could be identified and confirmed by reverse genetics, e.g. RNAi and/or CRISPR-Cas9. This information could provide deep insight into the evolution of parasitism in other Clade 5 animal, plant and invertebrate nematode parasites, and allow comparison with C. elegans and P. pacificus.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X18000305
Conflict of interest
None.