Introduction
Cystic echinococcosis (CE) is caused by infection with the larval stage of the cestode Echinococcus granulosus and is one of the world's major zoonotic infections (Schantz, Reference Schantz1991). Humans acquire infection by accidental ingestion of E. granulosus eggs voided in the faeces of infected dogs. The disease is common in parts of the world where there is close contact between the intermediate and definitive hosts, usually sheep and dogs, respectively (Gottstein & Reichen, Reference Gottstein, Reichen, Cook and Zumla2003). The endemic areas are sheep-farming regions of Australia, China, Central Asia, South America, the Middle East, Mediterranean countries and North India (Fomda et al., Reference Fomda, Sofi, Thokar and Kakroo2002; Moldovan et al., Reference Moldovan, Neghina, Calma, Marincu and Neghina2012).
Even though infection may be acquired early in life, most cases become symptomatic several years after exposure because of the slow-growing nature of echinococcal cysts. Clinical manifestations are variable and determined by the site, size and condition of the cyst. A slowly growing cyst often is tolerated well until it causes dysfunction because of its size. Symptoms of disease are related to expanding mass, pressure on adjacent structures, infection and rupture of the cyst contents into the surrounding body cavity (Abu-Eshy, Reference Abu-Eshy2006).
Diagnosis of CE relies mainly on radiological and immunological techniques. Although the demonstration of scolices, hooklets or protoscolices in aspirated fluid by microscopy is very specific, aspiration of hydatid fluid is not usually recommended because of the risk of anaphylactic reaction. Casoni's intradermal skin test has low sensitivity and specificity. Imaging techniques are not cost-effective and their use is limited due to their non-availability in rural areas. Thus, routine laboratory diagnosis of CE relies heavily on the detection of a specific antibody response (Garcia, Reference Garcia and Garcia2001).
Cyst fluid (CF) of E. granulosus cysts of sheep or cattle origin is one of the most widely used antigens, and the enzyme-linked immunosorbent assay (ELISA) is one of the most commonly used techniques in serodiagnostic laboratories. In cases of CE of the liver, antibodies against CF antigens can be detected with a high diagnostic sensitivity by this method. In eight independent studies, CF-based ELISA systems detected 90% (83.2–100%) of the cases with CE (Grimm et al., Reference Grimm, Maly, Lu and Llano1998). The overall sensitivity of the tests was reported to be very high (96.0–100%; average, 99.3%) in these studies, but considerable cross-reactivity due to other parasitic infections (1.7–48.7%; average, 17.6%) was observed. Previous studies have reported that immunoglobulin (Ig) G subclasses 1–4 are differentially expressed in patients with chronic helminthic infections (Lawn et al., Reference Lawn, Bligh, Craig and Chiodini2004). The serum concentrations of IgG4 are greater in patients with symptomatic CE compared with those with asymptomatic disease (Shambesh et al., Reference Shambesh, Craig, Wen, Rogan and Padillo1997). It has therefore been suggested that IgG subclass measurements may provide a more sensitive index of disease activity than CE-specific total IgG. Serum is generally used for the detection of hydatid-specific antibodies. However, the collection of blood samples is difficult, particularly in remote rural areas, and, being an invasive procedure, may be hazardous and unsafe. Thus, it is important to assess alternative, simple, painless methods of sampling body fluids that give similar results. Saliva and urine have been suggested as possible alternatives and as non-invasive means to detect specific antibodies for the diagnosis of various diseases (Rodriguez-del Valle et al., Reference Rodriguez-del Valle, Quakyi, Amuesi, Quaye, Nkrumah and Taylor1991; Sunita et al., Reference Sunita, Dubey, Khurana and Malla2007). Studies regarding the detection of antibodies in the urine of patients infected with Echinococcus are limited (Sunita et al., Reference Sunita, Dubey, Khurana and Malla2007). To the best of our knowledge, there has been no previously published study regarding the detection of CE-specific IgG subclass (IgG1–4) antibodies in urine. Therefore, the present study was designed to assess the value of hydatid-specific antibodies of IgG, IgM, IgE and IgG subclass in urine and serum samples for the diagnosis of CE.
Materials and methods
Collection and preparation of samples
Serum and urine samples obtained from 41 patients attending outpatient departments or admitted to the Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, who were surgically confirmed for CE, were included in the present study. These patients were from different districts of the Kashmir valley. Samples from 40 patients with other diseases (ten each of amoebiasis, toxoplasmosis, ascariasis and malignancy) were taken to access cross-reactivity. Serving as controls were 16 normal healthy subjects negative for other intestinal helminthic infections by stool examination. All serum and urine samples were stored at − 70°C until further testing.
Informed consent was obtained from all participants of the present study. A detailed history was recorded, e.g. age, sex, socio-economic status, contact with sheep and dogs, any relevant past history and present complaints.
Preparation of hydatid antigens
Hydatid cysts were obtained from sheep slaughtered at a local abattoir. Hydatid fluid was aspirated aseptically from fertile hydatid cysts and centrifuged at 2000 g for 45 min. The fluid was passed through a Whatman WCN type membrane filter (cellulose nitrate, 47 mm diameter, 0.45 μm pore size) and dialysed against distilled water overnight at 4°C using dialysis tubing (Sigma Aldrich, St. Louis, Missouri, USA) with a molecular weight cut-off of 2000 Da (Wattal et al., Reference Wattal, Malla, Khan and Agarwal1986). Antigen protein concentration was estimated by the method of Lowry with bovine serum albumin as a reference standard (Lowry et al., Reference Lowry, Rosenbrough, Farr and Randall1951). The protein concentration of the antigen was 1.3 mg/ml.
Antibody detection and assays
Antibody detection in serum and urine was performed by indirect ELISA. The specific IgG, IgM and IgE in serum and IgG and IgM in urine were detected according to Wattal et al. (Reference Wattal, Malla, Khan and Agarwal1986) and Sunita et al. (Reference Sunita, Dubey, Khurana and Malla2007), respectively, with a few modifications. Briefly, microtitre plates were coated with 2 μg/100 μl of crude hydatid sheep antigen diluted in 0.1 m carbonate–bicarbonate buffer (pH 9.6). The optimum concentration of antigen had been predetermined by the checkerboard titration method. The sera of patients were diluted 1:640 for IgG, 1:320 for IgM and IgE, and 1:200, 1:100, 1:50 and 1:50 for IgG1, IgG2, IgG3 and IgG4, respectively. Urine was used undiluted for the detection of all antibodies. Diluted sera and undiluted urine were incubated for 1 h at 37°C in wells of microtitre plates and the plates were then washed three times with phosphate-buffered saline (PBS)–Tween. Assays were then continued as follows.
For the detection of antibodies in sera, goat anti-human horseradish peroxidase-conjugated IgG, IgM and IgE (Sigma Aldrich) diluted 1:4000, 1:8000 and 1:4000 in PBS–Tween, respectively, were added to each well. For the detection of antibodies in urine, goat anti-human horseradish peroxidase-conjugated IgG and IgM diluted 1:2500 and 1:2000, respectively, were added to each well. IgE was not detected in urine samples. The plates were incubated for 1 h at 37°C. The plates were then washed three times with PBS–Tween. A substrate solution of 3',3',5',5'-tetramethylbenzedine (TMB) was added and incubated at room temperature for 15–20 min. Colour development was stopped with 2 m sulphuric acid (100 μl) and absorbance values (optical density, OD) were measured at 450 nm with an ELISA plate reader.
Detection of IgG subclass was performed as described by Lawn et al. (Reference Lawn, Bligh, Craig and Chiodini2004), with some modifications. For the detection of hydatid-specific IgG subclass in serum, monoclonal mouse anti-human IgG1, IgG2, IgG3 and IgG4 (Sigma Aldrich) diluted 1:500, 1:2500, 1:1000 and 1:2500, respectively, were added to each well. For IgG subclass assays in urine, monoclonal mouse anti-human IgG1, IgG2, IgG3 and IgG4 diluted 1:250, 1:1000, 1:500 and 1:1000, respectively, were added to each well. The plates were then incubated for 1 h at 37°C and washed three times with PBS–Tween. Peroxidase-conjugated goat anti-human Fc-specific IgG was used as a secondary antibody and was diluted 1:24,000, 1:10,000, 1:4000 and 1:24,000 in PBS–Tween for the IgG1, IgG2, IgG3 and IgG4 assays in serum, respectively. For the IgG subclass assays in urine, the secondary antibody peroxidase-conjugated goat anti-human IgG diluted 1:2000 for IgG1, IgG2 and IgG3 and 1:4000 for IgG4 was used. Plates were incubated for 1 h at 37°C and then washed three times with PBS–Tween. The substrate solution was then added and the plates were read as described for the total IgG assay.
Data analysis
Results of test samples were recorded as OD; the cut-off OD was determined by the mean absorbance value derived from 100 healthy controls ± 2SD. The mean value of five negative samples was taken for each test run as a negative control. OD values more than the cut-off were considered as positives and those less than the cut-off were negative.
The criterion for true positives was based on surgical and radiological findings, as no ‘gold standard’ parameter is available for the diagnosis of human hydatidosis. The results were analysed statistically by Pearson's χ2 test and Fisher's exact test. Results were considered statistically significant when the P value was ≤ 0.05.
Results
Of the 41 CE patients included in the study, 21 were female and 20 male. Of these, 25 were from a low socio-economic class and lived in rural areas with a strong history of contact with sheep and dogs. The highest prevalence value of 26.82% occurred in the age categories of 10–19 and 40–49 years, followed by 21.95, 9.76, 7.31, 4.9 and 2.44% infections in the categories 20–29 years, 30–39 years, 50–59 years, 0–9 years and 60–69 years, respectively. Of the diagnosed patients, 21 had hepatic cysts while 20 had extrahepatic cysts (18 had cysts in the lungs, one in a kidney and one in multiple organs).
Antibody detection
Among the cases of CE studied, the numbers with positive serology were 39 (95.12%), 22 (53.65%), 35 (85.4%), 31 (75.6%), 31 (75.6%), 31 (75.6%) and 32 (78.0%) for IgG, IgM, IgE, IgG1, IgG2, IgG3 and IgG4 in sera, respectively (table 1). A statistically significant difference (P < 0.05) between total IgG and IgG subclasses (IgG1–4) was observed. However, there was no significant difference (P > 0.05) between total IgG and IgE, or IgE and IgG subclass. Cut-off values of ODs derived from the analysis of negative controls (mean ± 2SD) were low for each of the assays: total IgG, 0.181; IgM, 0.264; IgE, 0.269; IgG1, 0.118; IgG2, 0.120; IgG3, 0.462; IgG4, 0.123. The mean ± 2SD ODs of positive samples for total IgG, IgM, IgE, IgG1, IgG2, IgG3 and IgG4 were 0.633 ± 0.285, 0.580 ± 0.360, 0.450 ± 0.199, 0.620 ± 0.315, 0.281 ± 0.123, 0.997 ± 0.372 and 0.443 ± 0.188, respectively. There was a significant difference between the ODs of IgG3 and all other immunoglobulins (P < 0.05), but no significant difference was found among other immunoglobulins (P > 0.05).
Table 1 Detection of specific antibodies to cystic echinococcosis in the serum and urine samples by enzyme-linked immunosorbent assay; * statistically significant at P <0.05.
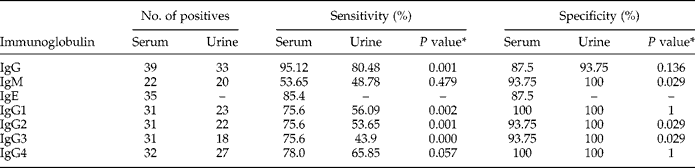
Out of 41 urine samples tested, 33 (80.5%) were IgG positive, while 20 (48.78%), 23 (56.09%), 22 (53.65%), 18 (43.9%) and 27 (65.85%) were positive for IgM, IgG1, IgG2, IgG3 and IgG4, respectively (table 1). A statistically significant difference was observed between IgM, IgG1, IgG2, IgG3 and total IgG (P < 0.05), but no significant difference was found between total IgG and IgG4 (P > 0.05). The cut-off values of ODs derived from the analysis of negative controls (mean ± 2SD) were 0.073 for IgG and 0.291, 0.075, 0.073, 0.090 and 0.087 for IgM, IgG1, IgG2, IgG3 and IgG4, respectively. The mean ± 2SD ODs for positive samples obtained in the ELISA were 0.369 ± 0.323, 0.479 ± 0.161, 0.300 ± 0.230, 0.321 ± 0.232, 0.501 ± 0.238 and 0.376 ± 0.230 for IgG, IgM and IgG1–G4, respectively. Mean ODs of serum samples were significantly higher (P < 0.05) for IgG total, IgG1 and IgG3 as compared with urine samples.
Sensitivity, specificity and cross-reactivity
In serum, the highest diagnostic sensitivity was seen with total IgG (95.12%) followed by IgE (85.4%), IgG4 (78.0%), IgG1–3 (75.6%) and IgM (53.65%). The specificity for the IgG2 and IgG3 subclasses in serum was 100%, whereas the specificity for IgG1, IgG4 and IgM was 93.75%. The lowest specificity was seen in IgE and total IgG (87.5%). In urine samples, total IgG antibody showed the highest diagnostic sensitivity of 80.5% followed by IgG4 (65.85%), IgG1 (56.09%), IgG2 (53.65%), IgG3 (43.95%) and IgM (48.78%). The specificity for IgM and all IgG subclasses (IgG1–4) in urine was 100%, whereas the specificity for IgG total was 93.75%. When the specificity and sensitivity of serum and urine were compared, it was observed that urine had a significantly higher specificity (P < 0.05) for IgM, IgG2 and IgG3 than serum, but serum showed significantly higher diagnostic sensitivity (P < 0.001) for total IgG, IgG2 and IgG3.
A total of seven cross-reactions were observed when sera from patients with different parasitic infections and malignancy were analysed for anti-Echinococcus IgG antibodies. The highest proportion was found in the sera from patients with ascariasis (3 of 10) followed by amoebiasis and malignancy (two each). An additional six cross-reactions of IgG in urine were observed (two each for ascariasis, amoebiasis and toxoplasmosis). The number of cross-reactions was reduced when specific IgG1, IgG2 and IgG4 antibodies were analysed. For IgG3 there was no cross-reaction observed in the serum and urine samples.
No non-specific reactions were observed in the urine and serum samples of healthy controls for IgE, IgG1 and IgG4. Two samples from this group had a non-specific reaction for IgG and one sample each gave a non-specific reaction for IgM, IgG2 and IgG3.
Discussion
Conventionally, serum samples are used for the demonstration of antibodies, yet few studies have also reported the utility of urine for the diagnostic testing of various diseases (Licel de Los et al., Reference Licel de Los, Rodriguez, Osmany Larralde, Raiza Martinez and Aidonis Gutierrez2003). No attempts have been made to date to detect specific IgG subclass antibodies in urine and compare them with the serum of CE patients. The present study was designed to assess the immunoglobulin levels of total IgG, IgG subclass and IgM in serum and urine and IgE in serum only of patients diagnosed with CE. In the present study the majority of patients, 31 (75.60%), were in the age group of 30–49 years. Our findings are in agreement with the previous studies of Craig (1997) and Ghorbanalinezhad et al. (Reference Ghorbanalinezhad, Assmar, Piazak and Khabiri2001). Echinococcus granulosus cysts following primary infection may inhabit any anatomical site. The two most common organs involved are the liver and the lungs. In the present study the liver was the most common organ involved (51.21%), followed by the lungs (43.90%), kidneys and multiple organs (2.43% each). Previous studies have also reported similar findings (Gottstein, Reference Gottstein1992; Khuroo, Reference Khuroo2002; Sunita et al., Reference Sunita, Dubey, Khurana and Malla2007).
In our study ELISA was used for the detection of hydatid-specific antibodies incorporating crude antigen rather than purified antigen (antigen B or antigen 5) as used in other studies (Rigano et al., Reference Rigano, Profumo, Loppolo, Notargiacomo, Ortona, Teggi and Siracusano1995; Shambesh et al., Reference Shambesh, Craig, Wen, Rogan and Padillo1997; Daeki et al., Reference Daeki, Craig and Shambash2000). Our justification was that the broad diversity of antigens in crude hydatid CF might augment the detection of subclass antibodies with different antigenic specificities. The results of our study demonstrated that total IgG, IgE and IgG4 in the sera of CE patients are the most important antibodies for the serological diagnosis of CE using crude hydatid CF antigen. There was a significant difference between the results of total IgG and IgG4 (P < 0.023), but there was no significant difference between IgG and IgE and IgG4 in the sera of CE patients. There was no significant difference among other IgG isotypes (IgG1, IgG2 and IgG3). Previous studies have also reported higher diagnostic sensitivity of total IgG, IgE and IgG4 (Khabiri et al., Reference Khabiri, Bagheri, Assmar and Siavashi2006; Sunita et al., Reference Sunita, Dubey, Khurana and Malla2007). Recent studies on IgG subclass responses in human CE have demonstrated IgG1 and IgG4 to be the most immunodominant IgG isotypes (Hira et al., Reference Hira, Bahr, Shewiki and Behbehani1990; Aceti et al., Reference Aceti, Pennica, Teggi, Fondacaro, Caferro, Leri, Tacchi, Celestino, Quaranta and De Rosa1993; Wen & Craig, Reference Wen and Craig1994; Zhang et al., Reference Zhang, Li and McManus2003). Shambesh et al. (Reference Shambesh, Craig, Wen, Rogan and Padillo1997) and Short et al. (Reference Short, Heiner, Hsiao and Andersen1990) also found predominance of IgE, IgG1 and IgG4 against CF antigen. Mean ODs of IgG, IgG1, IgG3 and IgM were significantly higher than those of IgE, IgG2 and IgG4. Lawn et al. (Reference Lawn, Bligh, Craig and Chiodini2004) also reported higher mean ODs of IgG, IgG1, IgG4 and IgG3 in their study. As compared with serum, urine samples demonstrated total IgG, IgG4 and IgG1 to be the most important antibodies for the diagnosis of CE using crude antigen. There was a significant difference among specific IgG subclasses in urine and the diagnostic specificity of IgG4 and IgG1 was higher in urine. In our study, IgM was positive only in 48.78% of cases. It has been postulated that large macromolecules such as IgM antibodies cannot pass through the glomerular filter under normal conditions. However, monomeric IgM proteins (67,000 kDa) have been detected in post-renal sources and not in the glomerular filter (Licel deLos et al., 2003). The overall diagnostic sensitivity of all immunoglobulins in urine was low as compared with sera of CE patients in the present study. Our results are in agreement with previous studies of Licel de Los et al. (Reference Licel de Los, Rodriguez, Osmany Larralde, Raiza Martinez and Aidonis Gutierrez2003) but are in contrast with the studies of Sunita et al. (Reference Sunita, Dubey, Khurana and Malla2007). The low sensitivity in urine samples could be explained by a number of factors. For example, urine may not have been collected at the optimal time (the anti-hydatid IgG, IgM, IgE and IgG1–G4 kinetics in urine are not well known), or there may have been inhibitors in the urine. Other explanations include the presence of chemicals, drugs or toxic products excreted in urine and the amount of fluid intake, which may promote fluctuation in immunoglobulin concentrations in urine. In the present study the specificity of ELISA for the detection of immunoglobulin classes and IgG subclass in urine was higher than that for serum; similar findings have been reported by Licel de Los et al. (Reference Licel de Los, Rodriguez, Osmany Larralde, Raiza Martinez and Aidonis Gutierrez2003).
In the sera of healthy controls two non-specific reactions were found in total IgG, and one each in IgM, IgG2 and IgG3. However, no non-specific reaction was found in IgE, IgG1 and IgG4. Therefore these antibodies were the most specific antibodies for this parasite and can be considered the best diagnostic markers for this disease. Similar results have been reported by Leggatt & McManus (Reference Leggatt and McManus1994) and Khabiri et al. (Reference Khabiri, Bagheri, Assmar and Siavashi2006), who also found fewer non-specific reactions in IgE and IgG4. Non-specific reactions in the urine of healthy controls were observed for total IgG, IgM, IgG2 and IgG3. Patients with other parasitic infections showed cross-reactivity against crude hydatid antigen. The highest proportion of cross-reactivity was found in the sera and urine from patients with ascariasis infection, followed by amoebiasis, malignancy and toxoplasmosis. The number of cross-reactions was reduced when specific IgG1, IgG2 and IgG4 antibodies were analysed. For IgG3 there was no cross-reaction observed in sera and urine samples.
Our results are well supported by previous studies that also reported the highest cross-reactivity in total IgG (Khabiri et al., Reference Khabiri, Bagheri, Assmar and Siavashi2006). The non-specific reactions in the sera of patients with other parasitic infections may be caused mainly by a sharing of hydatid antigen with those of other parasites, but in normal human sera the non-specific reaction may be caused by interaction with some blood-group antigens, or with non-specific host proteins (serum albumin, host IgG) in hydatid fluid (Kanwar et al., Reference Kanwar, Kaushik, Sawhney, Kamboj, Mehtas and Vinayak1992; Licel de Los et al., Reference Licel de Los, Rodriguez, Osmany Larralde, Raiza Martinez and Aidonis Gutierrez2003; Sunita et al., Reference Sunita, Dubey, Khurana and Malla2007).
From the present study it can be concluded that CE-specific total IgG, IgE and IgG4 in sera and total IgG, IgG4 and IgG1 in the urine of CE patients were the most important specific antibodies for the diagnosis of CE. However, total IgG usually persists for an extended period and has the highest cross-reactivity. Therefore detection of the IgG antibody cannot be used for diagnosis in patients previously treated surgically and in areas where other helminthic infections are endemic. However, since a significantly low level of cross-reactions was found in IgE, IgG1 and IgG4, these antibodies can be considered the best diagnostic markers for CE. The diagnostic sensitivity of hydatid-specific IgM in sera and urine samples was very low and therefore cannot be used as a diagnostic marker. In the present study there was no significant difference between IgG1 and IgG4 in serum and urine, and both showed the best correlation for the diagnosis of CE. Thus, detection of antibodies in urine could provide a new approach in the diagnosis of CE.
Financial support
The authors wish to thank the Indian Council of Medical Research (ICMR), New Delhi, India for financial support.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Indian Council of Medical Research guidelines on human experimentation which is in accordance with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the ethical clearance committee of the Sher-i-Kashmir Institute of Medical Sciences.



