Introduction
Dipetalonema caudispina was described by Molin (Reference Molin1858), who initially named the species Filaria caudispina and subsequently revised the taxonomy and considered it to be Filaria gracilis Rudolphi, Reference Rudolphi1809 (Boulenger, Reference Boulenger1920); it was validated as D. caudispina by Freitas (Reference Freitas1943). The occurrence of D. caudispina in Brazil was reported by Molin (Reference Molin1858) from the following species: Ateles paniscus (L.) (syn. Cebus paniscus Fischer) (Atelidae); Sapajus apella (L.) (syn. Cebus apella Linnaeus) (Cebidae); Brachyteles arachnoides (G.) (syn. Cebus arachnoides Geoffroy) (Atelidae); Saimiri sciureus (L.) (syn. Callithrix sciureus Geoffroy) (Cebidae); Lagothrix lagotricha (H.) (syn. Cebus Lagothrix E. Geoffroy) (Atelidae); Alouatta seniculus (L.) (syn. Cebus ursinus Linnaeus) (Atelidae); Leontopithecus rosalia (L.) (syn. Jacchus rosalia Fischer) (Callitrichidae). It was also reported in Ateles paniscus from French Guiana by Bain et al. (Reference Bain, Petit and Rosales1986). Molin reported D. caudispina in non-human primates from Brazil but did not provide quantitative or morphometric data on the helminths found, nor the condition of the hosts and accurate geographical records. Moreover, some observations by Freitas (Reference Freitas1943) suggest mistaken records of parasitism by filariae in some hosts mentioned by Molin (Reference Molin1858).
Dipetalonema gracile (Rudolphi, 1809), on the other hand, has been reported in Bolivia, Ecuador, Mexico, Colombia, Venezuela, Panama, Paraguay, Peru and Brazil as a parasite of several non-human primates (Notarnicola et al., Reference Notarnicola, Pinto and Navone2008). However, complete morphometric data were provided only for specimens of D. gracile diagnosed from S. sciureus by Bain et al. (Reference Bain, Petit and Rosales1986) in specimens from French Guiana, and for specimens from the Ecuadorian Amazon by Notarnicola et al. (Reference Notarnicola, Pinto and Navone2008). Until now, the presence of filariae of the same genus in the same host has not been reported. This work records for the first time the co-infection with D. caudispina and D. gracile in two species of cebid primates, Sapajus macrocephalus (Spix, 1823) and Cebus albifrons (Humboldt, 1812), in free-living conditions in the Peruvian Amazon, where these primates are used for human consumption and harvested through subsistence hunting. As well as expanding the host range for D. caudispina, and the geographical range for both D. gracile and D. caudispina, we provide additional morphological data for the latter species based on scanning electron microscopy (SEM).
Materials and methods
From 2009 to 2013, 44 large-headed capuchins S. macrocephalus (30 males, 14 females) and ten white-fronted capuchins C. albifrons (four males, six females) were harvested by subsistence hunters in the north-eastern Peruvian Amazon at the Yavarí-Mirín river basin (04°19′53′′S, 71°57′33′′W) in the region of Loreto, Peru. As subsistence hunting is a common activity in the region, hunters and local residents were included in a natural resource management programme and advised on the removal and identification of abdominal and thoracic organs and their preservation in 10% formaldehyde fixative solution.
Helminths were collected, preserved in 70% ethanol and sent to the Laboratory of Cell Biology and Helminthology of the Biological Sciences Institute of the Federal University of Pará (UFPA) under an import license to Brazil (No. 02309-MINAGRI-SERFOR). Helminths were cleared in 50% Aman lactophenol (Gardner, Reference Gardner, Wilson, Cole, Nichols, Rudran and Foster1996) and examined under an Olympus BX41 microscope and an Olympus SZX12 stereo microscope (Olympus Corporation, Tokyo, Japan). SEM samples were prepared following the protocol of Furtado et al. (Reference Furtado, Melo, Giese and Santos2010) and analysed under a VEGA3 LMU microscope (TESCAN, Brno, Czech Republic) at the Laboratory of the Federal Rural University of the Amazon (UFRA). Specimens of D. caudispina (one male, MPEG 0115, and one female, MPEG 0116) and D. gracile (two males, MPEG 0117–0118 and two females, MPEG 0119–0120), were deposited in the Invertebrate Collection of Museu Paraense Emílio Goeldi (MPEG, Belém, Pará state, Brazil).
Results
A detailed analysis of the reproductive structures and other taxonomic characteristics of 345 filarial specimens from S. macrocephalus and 260 filarial specimens from C. albifrons allowed the identification of two species co-parasitizing these two hosts in the Amazon basin of the Yavarí-Mirín River in the Peruvian Amazon: D. caudispina and D. gracile. Both male and female specimens of D. caudispina examined during this study were smaller in body size compared to descriptions by Freitas (Reference Freitas1943) and Bain et al. (Reference Bain, Petit and Rosales1986) (table 1). According to studies of experimental infections on the development of the nematode under the influence of the host, these body size variations may be influenced by the acquired immunity and the age of the host (Chylinski et al., Reference Chylinski, Boag, Stear and Cattadori2009). These effects probably occur with more dynamism in free-living animals.
Table 1. Morphometric comparison of the descriptions of male and female Dipetalonema caudispina found in Sapajus macrocephalus and Cebus albifrons (present study) and in Ateles paniscus by Freitas (Reference Freitas1943) and Bain et al. (Reference Bain, Petit and Rosales1986). All measurements are in μm except body length and area rugosa length (in mm).
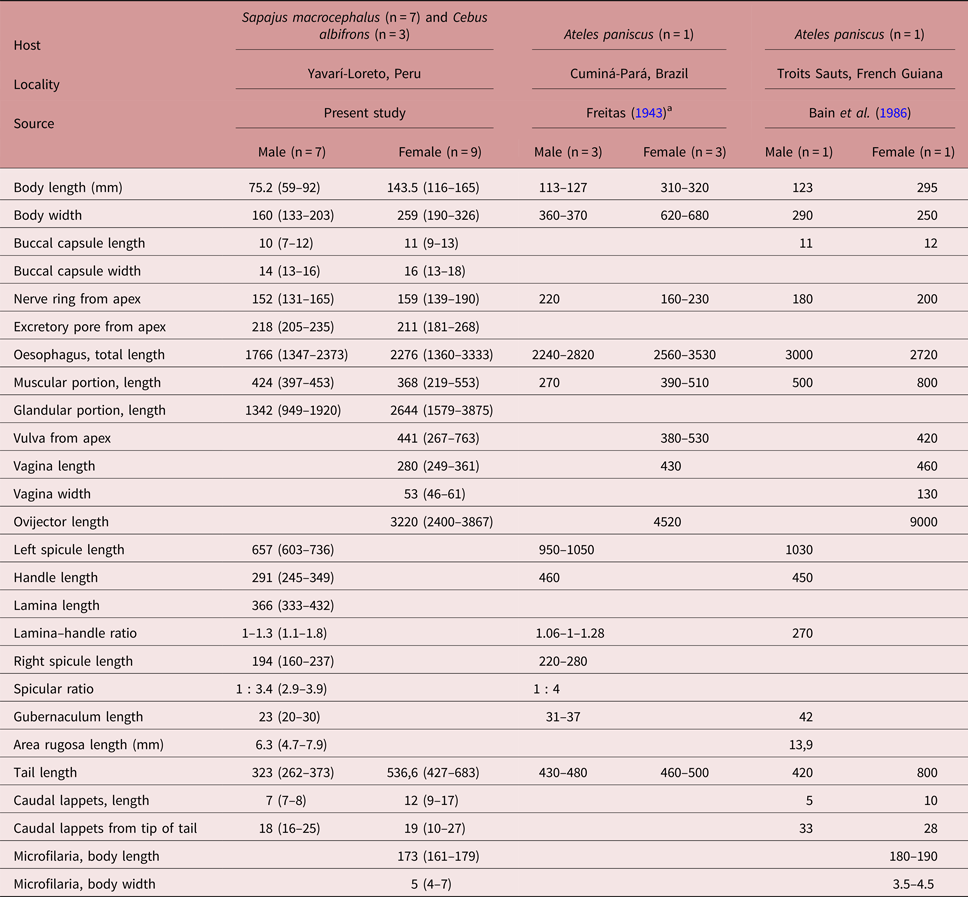
a Measurements of D. caudispina provided by Freitas (Reference Freitas1943) converted from micrometers to millimeters.
The presence of a discrete excretory pore in both sexes had been reported by Notarnicola et al. (Reference Notarnicola, Pinto and Navone2008) for D. gracile but not for D. caudispina. For this reason it was decided to examine specimens of D. caudispina from the helminthological collection of Instituto Oswaldo Cruz (CHIOC, Rio de Janeiro, Brazil); specimens deposited under number CHIOC 9027 had been studied by Freitas (Reference Freitas1943) and Bain et al. (Reference Bain, Petit and Rosales1986). The excretory pore of D. caudispina collected from S. macrocephalus and C. albifrons is immediately posterior to the nerve ring, at a mean distance of 218 μm from the anterior end in males and 211 μm in females; this is similar to the measurements obtained from specimens from A. paniscus deposited under CHIOC 9027, in which the excretory pore is immediately posterior to the nerve ring and situated at a distance of 251 μm from the anterior end in a single male and at a mean distance of 229 μm (195–259 μm) in six females.
Male specimens of D. caudispina from S. macrocephalus and C. albifrons differed in the number and arrangement of caudal papillae (fig. 1B) compared to those described by Freitas (Reference Freitas1943) and Bain et al. (Reference Bain, Petit and Rosales1986). Both earlier studies described 12 circular papillae differing in size and a median pre-cloacal papilla differing in shape, in addition to four papillae near the end of the tail. The present SEM study revealed one additional pair of papillae in the caudal region (fig. 1C, F) of three specimens studied, situated between the two pairs of caudal papillae directly following the cloaca and the posteriormost group of four caudal papillae (fig. 1A, E); these additional papillae could be an intraspecific variation observed in these three. Another important characteristic of male D. caudispina from S. macrocephalus and C. albifrons are the postcloacal bands, located on the left side only and which stop at a level that is approximately as far removed from the posteriormost group of caudal papillae as that group is removed from the tail tip, similar to the specimens of D. caudispina examined by Bain et al. (Reference Bain, Petit and Rosales1986). SEM micrographs of this structure are provided for the first time (fig. 1D, E). Specimens of D. gracile examined in the present study (supplementary table S1) match descriptions of D. gracile provided by Bain et al. (Reference Bain, Petit and Rosales1986) and Notarnicola et al. (Reference Notarnicola, Pinto and Navone2008) in having a sinuous vagina, a left spicule that is divided into three parts and a left postcloacal band that extends further posteriorly than the right one.

Fig. 1. Scanning electron micrographs of male Dipetalonema caudispina. (A) Posterior end, with detail of lateral caudal papilla (arrow); scale bar: 5 μm. (B) Posterior end, with details of group of four caudal papillae (cp); scale bar: 20 μm. (C) Posterior end, with detail of lateral caudal papilla (arrow); scale bar: 5 μm. (D) Posterior end, ventral view with detail of left postcloacal band of area rugosa (arrow); scale bar: 20 μm. (E) Posterior end, ventral view with detail of left postcloacal band, cloacal papillae (pp) and lateral caudal papilla (arrow); the posterior end of the right spicule is broken; scale bar: 50 μm. (F) Posterior region, lateral caudal papilla; scale bar: 5 μm.
Twenty filariae of D. caudispina were recovered from S. macrocephalus and five from C. albifrons, with a mean intensity of infection of 2.9 (1–6) in S. macrocephalus and 1.7 (1–2) in C. albifrons. The overall prevalence of D. caudispina was 18.5% (15.9% in S. macrocephalus, 7/44 hosts examined, four males and three females, and 30.0% in C. albifrons, 3/10 hosts examined, only in males). Of D. gracile, 325 filariae were recovered from S. macrocephalus and 255 from C. albifrons, with a mean intensity of infection of 10.5 (1–61) in S. macrocephalus and 28.3 (1–114) in C. albifrons. The overall prevalence of D. gracile was 74.1% (70.5% in S. macrocephalus, 31/44 hosts examined, 21 males and ten females, and 90.0% in C. albifrons, 9/10 hosts examined, four males and five females). In general, the combined prevalence of D. caudispina and D. gracile was 87% (47/54 hosts examined), with a prevalence of mixed infection of 18.5% (7/44 hosts examined, four males and three females, from S. macrocephalus; 3/10 hosts examined, males only, from C. albifrons).
Discussion
It is noteworthy that D. caudispina was not observed as an independent parasite in any host but was always associated with D. gracile. This is the first record of co-infection with these two filariae. Occurrences of co-infections of helminths are common in wild hosts because they are exposed to these parasites in nature, but the difficulty in collecting specimens makes it difficult to record them. These co-infections could have effects not only on the host but also on the parasites themselves, their number and pathogenicity. The distribution of Dipetalonema spp. and other filarial species is important, as it complements data on the diversity of primate hosts in the Amazon region and provides epidemiological evidence of natural infections caused by this parasite in wildlife populations.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X18000287
Acknowledgements
The authors gratefully acknowledge the people of Nueva Esperanza, who participated actively in data collection, demonstrating that communal participation is an important step in the development of wildlife management, and the institutional support provided by SERFOR – Ministerio de Agricultura del Peru, Earthwatch Institute and Fundació Autònoma Solidària (Universidad Autónoma de Barcelona). We thank the Laboratory of Animal Histology and Embryology (LHEA/UFRA). This work is part of the doctoral thesis of D. F. Conga, in the Post-Graduate Program in Biology of Infectious and Parasitic Agents of UFPA.
Financial support
This study was funded by CAPES-Brazil (grant CAPES–Parasitologia Básica/2010), Grant PAPQ 2017-PROPESP/UFPA, and CNPq-Brazil, Research Grant for the work of J. N. Santos).
Conflict of interest
None.
Ethical standards
The research and sample collection authorization protocol used in this study was approved by Servicio Forestal y de Fauna Silvestre of Peru (Ethics Committee for research on wild animals; No. 0229-2011-DGFFS-DGEFFS).




