Introduction
The distribution and dynamics of fish parasites in the aquatic environment are influenced by biotic and abiotic factors, and the parasitofauna in a host population may, therefore, reflect changes in the environmental conditions (Marcogliese, Reference Marcogliese2002; Poulin, Reference Poulin2006). Accordingly, several authors have applied parasites in fish as indicators to reflect the biology of fish stocks (Williams et al., Reference Williams, MacKenzie and McCarthy1992; MacKenzie, Reference MacKenzie2002; Marcogliese, Reference Marcogliese2002). This approach was also used for Baltic Sea fishes (Reimer, Reference Reimer1970; Køie, Reference Køie1999; Sobecka et al., Reference Sobecka, Łuczak, Więcaszek and Antoszek2011; Unger et al., Reference Unger, Klimpel, Lang and Palm2014; Mehrdana et al., Reference Mehrdana, Marana, Skov, Bahlool, Sindberg, Mundeling, Overgaard, Kania and Buchmann2015) addressing salinity variations in the Baltic (Herlemann et al., Reference Herlemann, Labrenz, Jürgens, Bertilsson, Waniek and Andersson2011). The Baltic cod is a subpopulation of the Atlantic cod (Gadus morhua) residing in the Baltic Sea since the last glacial age. It performs local migrations within this brackish water area but has limited exchange with other cod populations (Sick, Reference Sick1965). Due to the low salinity in the Baltic, the cod diet composition is less diverse, but shifts from mainly invertebrates in small cod (below 40 cm body length) to a predominantly piscine diet in larger fish (Zuo et al., Reference Zuo, Huwer, Bahlool, Al-Jubury, Christensen, Korbut, Kania and Buchmann2016). During the latest decade, cod in the eastern part of the Baltic Sea has been in a critical state and a range of biotic and abiotic causes have been suggested to explain poor growth and performance – including oxygen depletion, climate, salinity, food availability and parasite infections (Eero et al., Reference Eero, Hjelm and Behrens2015; Horbowy et al., Reference Horbowy, Podolska and Nadolna-Ałtyn2016). We performed a parasitological investigation of Baltic cod – caught in 2018 in a specific habitat east of the island of Bornholm in the Baltic sea – and compared the recorded parasite fauna with a similar study on cod from the same area conducted in 1983. By analysing differences and similarities, we discuss if any ecological change over three decades in this part of the Baltic sea may be reflected in the parasite fauna.
Materials and methods
Fish
Baltic cod (G. morhua) (body length 31–50 cm) were caught along the east coast of the island Bornholm in the Baltic sea (ICES subdivision 25) both in 1983 (40 specimens) and 2018 (33 specimens). Total fish length was recorded both in 1983 and 2018. All fish were frozen after capture and kept at −20°C until examination. We divided fish into length groups (31–40 cm and 41–50 cm) to minimize size group bias. By necropsy, organs were separated, placed in Petri dishes and inspected under the dissection microscope (magnification × 40–400) (Leica MZ125, Wetzlar, Germany). Endohelminths were isolated, recorded and conserved immediately in plastic vials containing 70% ethanol.
Morphological identification
Haematoxylin-stained parasites (ten per species if applicable) were mounted on microscope slides in Aquatex (Merck, Darmstadt, Germany) and studied in the compound light microscope (Leica DM5000B, Wetzlar, Germany).
Molecular identification
A section of the individual parasite was used for molecular analysis (lysis, DNA-purification, polymerase chain reaction (PCR) sequencing) (primers in table 1). Five out of 11 parasite species were genetically identified, whereas six were not due to insufficient parasite material. Lysis was performed with the QIAGEN® DNeasy Blood & Tissue Kit (Ballerup, Denmark), and PCR performed in a 60 µl PCR set-up (Zuo et al., Reference Zuo, Kania, Mehrdana, Marana and Buchmann2018) using pre-denaturation at 95°C for 5 min; amplification starting with denaturation at 95°C for 30 s, annealing at an assay-specific temperature for 30 s, elongation at 72°C and post-elongation at 72°C (7 min). All PCR products were examined by 1.5% ethidium bromide containing agarose gel electrophoresis, purified using the illustra™ GFX™ PCR DNA Purification Kit (GE Healthcare, Brøndby, Denmark), sequenced (Macrogen, Seoul, Korea) and analysed (CLC Main Workbench v7.9.1, Qiagen, Aarhus, Denmark) by BLAST® (Bethesda, Maryland, USA) analysis at GenBank.
Table 1. Primers applied for the molecular identification of Baltic cod parasites.
Statistics
The sample sizes (40 and 33, respectively) were relatively small but allowed us to perform significance tests. Prevalence (percentage of hosts infected), mean intensity (mean number of parasites per infected fish), variance-to-mean ratio (reflecting overdispersion if >1) and range (lowest and highest number of parasites in a host) was calculated (Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997). Intensity differences between years were evaluated by the Mann–Whitney U-test. Prevalence differences were evaluated using a contingency table (Chi-square). All tests performed (Graph Pad Prism version 7.2, www.graphpad.com) applied a 5% significance level.
Results
Parasite occurrence
We recorded 11 species of helminths comprising trematodes, cestodes and acanthocephalans (table 2). Low infections due to the Bothriocephalus sp., Hemiurus luehei and Podocotyle atomon, and Cucullanus cirratus were recorded in both 1983 and 2018, and no significant changes were evident for these species. We recorded a slight but non-significant increase of infection for the intestinal nematode Capillaria gracilis and a lowered Hysterothylacium aduncum infection. A dominant helminth in both years was the acanthocephalan Echinorhynchus gadi, which occurs in the intestinal lumen of cod, but in 2018 the infection intensity was slightly lower for small cod (31–40 cm body length). Occurrence of the nematode third-stage larva Contracaecum osculatum increased significantly during the three decades from almost absence in 1983 to 100% prevalence in 2018, with intensities increasing from one parasite (1983) to more than 200 per fish liver (2018). The larval acanthocephalan Corynosoma semerme occurrence increased significantly as well.
Table 2. Prevalence (% of fish infected), intensities (MI, mean intensity), standard deviation (SD) and variance-to-mean ratio (V/M) of endohelminth infection of Baltic cod (captured along the east coast of Bornholm island, ICES subdivision 25) in 1983 and 2018.
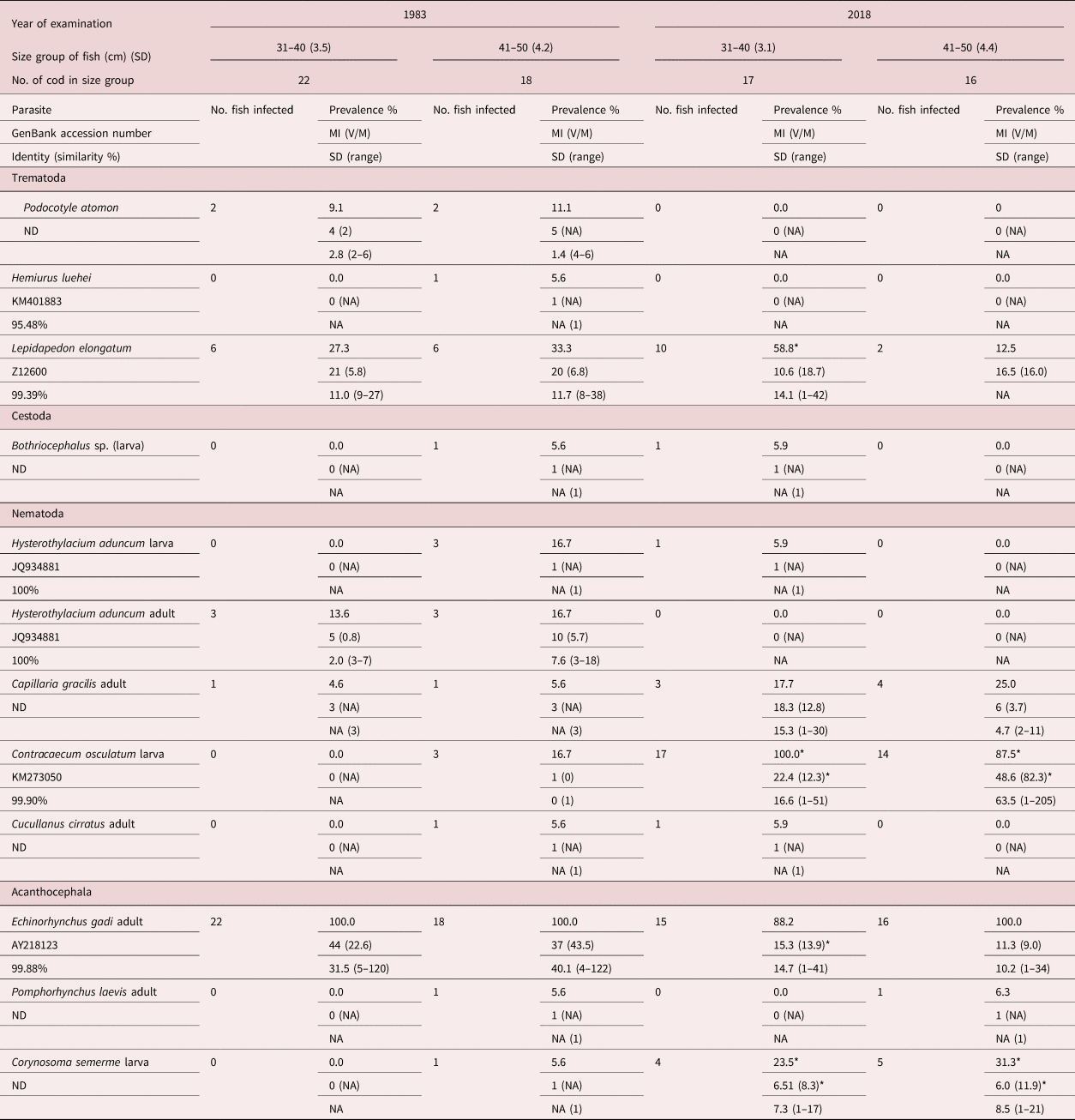
ND, molecular identification was not performed due to limited samples or negative PCR (in these cases, ID is based on morphological characters only); NA, not applicable.
* Significant differences between years, P < 0.05 (Mann–Whitney U-test for intensity and Chi-square for prevalence).
Discussion
Communities of certain fish parasites with complex life cycles are dependent on the presence of specific hosts, whereby the parasite fauna in a fish reflects availability of these organisms. The main final host of C. osculatum (Rudolphi, 1802) in the Baltic Sea is the grey seal (Køie & Fagerholm, Reference Køie and Fagerholm1995; Skrzypczak et al., Reference Skrzypczak, Rokicki, Pawliczka, Najda and Dzido2014; Lunneryd et al., Reference Lunneryd, Bostrom and Aspholm2015) and its population has exhibited a significant expansion since 1990 (Harding et al., Reference Harding, Härkönen, Helander and Karlsson2007, Haarder et al., Reference Haarder, Kania, Galatius and Buchmann2014; Zuo et al., Reference Zuo, Kania, Mehrdana, Marana and Buchmann2018). Thus, this biotic factor is likely to explain the marked increase of C. osculatum infection from a low level in the 1980s and 1990s (Myjak et al., Reference Myjak, Szostakowska, Wojciechowski, Pietkiewicz and Rokicki1994) to present levels (Haarder et al., Reference Haarder, Kania, Galatius and Buchmann2014; Nadolna & Podolska, Reference Nadolna and Podolska2014; Rodjuk, Reference Rodjuk2014; Sokolova et al., Reference Sokolova, Buchmann, Huwer, Kania, Krumme, Galatius, Hemmer-Hansen and Behrens2018; Zuo et al., Reference Zuo, Kania, Mehrdana, Marana and Buchmann2018). The associated and elevated C. semerme infection, also using seal as final host (Sinisalo & Valtonen, Reference Sinisalo and Valtonen2003), adds to seal signatures in the cod parasitofauna. Generally, the variance-to-mean ratio was higher for all species indicating a large variation of intensity between individual fish. The acanthocephalan E. gadi, commonly occurring in Baltic cod (Nordenberg, Reference Nordenberg1963; Buchmann, Reference Buchmann1986; Sobecka et al., Reference Sobecka, Łuczak, Więcaszek and Antoszek2011), showed a slightly decreased infection level in the smallest cod (31–40 cm) in 2018 when compared to 1983. The intermediate hosts are amphipods such as Gammarus spp. (Buchmann, Reference Buchmann1986) and a lowered infection may reflect decreased ingestion of this food source. Less prevalent species (and thereby useless as indicators) were the cestode Bothriocephalus sp., the nematode C. cirratus and the acanthocephalan Pomphorhynchus laevis. Minor non-significant differences were noted for the trematodes H. luehei and P. atomon using molluscs and crustaceans as intermediate hosts (Køie Reference Køie1981, Reference Køie1990), the nematode H. aduncum using the isopod Saduria entomon (Pawlak et al., Reference Pawlak, Nadolna-Ałtyn, Szostakowska, Pachur and Podolska2018) and other crustaceans (Køie, Reference Køie1993) as first intermediate host. These changes are too limited to draw any strict conclusions about availability of intermediate or transport hosts, but we suggest that future eco-parasitological studies should include these species. Larval parasites are expected to increase their numbers in cod during growth, but rarely occurring species (Bothriocephalus sp., H. aduncum) occurred sporadically more often in smaller cod, probably due to chance effects. The prevalence of the pyloric digenean Lepidapedon elongatum in smaller cod increased from 27.3% in 1983 to about 58.8% in 2018, but no significant difference of the intensity was recorded. The trematode uses the prosobranch snail (Anoba aculeus) and polychaetes as first and second intermediate hosts, respectively (Køie, Reference Køie1985). Hence, the study suggests a higher availability of these invertebrates on the feeding grounds of Baltic cod. Likewise, the increased C. gracilis infection does not indicate any decrease over the study period in availability of invertebrate intermediate hosts. Chironomids, oligochaetes and sand goby serve as intermediate hosts (Køie, Reference Køie2001), suggesting presence of these organisms on the feeding grounds. When evaluating ecological differences in the Baltic Sea between the years 1983 and 2018, as reflected by parasites in cod, the increasing population of grey seal (Halichoerus grypus) (Harding et al., Reference Harding, Härkönen, Helander and Karlsson2007; Haarder et al., Reference Haarder, Kania, Galatius and Buchmann2014; Zuo et al., Reference Zuo, Kania, Mehrdana, Marana and Buchmann2018) comes up as the main influential biotic factor because the seal is the final host of the nematode C. osculatum and the acanthocephalan C. semerme. The present investigation found no significant indications on extirpation of invertebrate intermediate hosts when compared to 1983, but we suggest to record the presence of endohelminths in cod in future ecological studies as they may serve as indicators for these populations.
Financial support
The Indonesia Endowment Fund for Education (LPDP) is acknowledged for PhD stipend support to Agung Cahyo Setyawan (contract PRJ-88/LPDP.3/2016). The study was supported by J.P.A. Espersen and Mrs Dagny Espersen Foundation and the European Fisheries Fund/Danish Fisheries Agency (33113-B-16-070).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.




