Introduction
Host specificity is a key concept for understanding the ecology and evolution of host–parasite associations (Brooks & McLennan, Reference Brooks and McLennan1993; Poulin, Reference Poulin2006). A simple and powerful approach to describe patterns of specificity is the encounter/compatibility paradigm (Combes, Reference Combes2001; Kuris et al., Reference Kuris, Goddard, Torchin, Murphy, Gurney and Lafferty2007). This paradigm states that specificity is determined by two sequential filters. The encounter filter prevents infections of potential hosts that cannot contact the parasite, whereas the compatibility filter can (1) exclude contacted hosts in which the parasite is unable to find the appropriate resources and/or escape or deter the host's defences, or (2) impact other fitness components of the parasite (e.g. life span) if the contacted host is suboptimal.
For most parasite species, patterns of specificity are described based on surveys in which samples of potential hosts from natural settings are examined. Typically, for a given parasite species, these surveys identify its hosts, in which the parasite occurs and is able to reproduce, and its non-hosts, in which the parasite does not occur or cannot reproduce. However, parasitological surveys of many host species, particularly large vertebrates, rely on the opportunistic sampling of few hosts, and this may result in problems for the interpretation of specificity patterns (e.g. Zdzitowiecki, Reference Zdzitowiecki1986; Mateu et al., Reference Mateu, Raga and Aznar2011). A helminth species could be absent from a sample of hosts because the helminth did not contact these hosts or because it was unable to establish in them. Identifying the actual cause is crucial for correct interpretation of specificity patterns and to gather information about the host's ecology (Kuris et al., Reference Kuris, Goddard, Torchin, Murphy, Gurney and Lafferty2007). One could argue that failed infections of trophically transmitted helminths can still be detected throughout the gut. However, failed infections can easily be missed if the transit time of digesta is rapid and host sample size is small (Mateu et al., Reference Mateu, Raga and Aznar2011). Small host sample sizes also increase the probability of missing rare parasites or confounding recent recruitment with an inability of the parasite to reproduce. In other words, there is a risk of mistaking true hosts as non-hosts because of sampling error (e.g. references in Zdzitowiecki, Reference Zdzitowiecki1986).
Consider, for instance, the patterns of specificity of two acanthocephalan species that infect marine mammals in the Southern Hemisphere, i.e. Corynosoma australe and C. cetaceum (Sardella et al., Reference Sardella, Mattiucci, Timi, Bastida, Rodriguez and Nascetti2005). Records of occurrence strongly suggest that C. australe is specific to pinnipeds whereas C. cetaceum is specific to cetaceans (table 1). However, the extent to which this host segregation results from a low probability of contact and/or a failure to establish in the putative non-hosts is unclear. Data on sexual maturity of female worms would suggest that C. australe and C. cetaceum cannot reproduce in non-pinniped and non-cetacean hosts, respectively. However, this conclusion is supported, in all but one case, by data obtained from 1–2 host individuals (table 1).
Table 1 Records of the acanthocephalans Corynosoma australe and C. cetaceum in mammals and birds worldwide. N, the number of hosts analysed; NI, number of infected hosts; MI, mean number of worms (or range) per infected host; G, presence of gravid females (Y, yes; N, no). Hyphens indicate that information is not available.

a 1, Shaughnessy & Ross (Reference Shaughnessy and Ross1980); 2, George-Nascimento & Marín (Reference George-Nascimento and Marín1992); 3, Aznar et al. (Reference Aznar, Cappozzo, Taddeo, Montero and Raga2004); 4, Sardella et al. (Reference Sardella, Mattiucci, Timi, Bastida, Rodriguez and Nascetti2005); 5, Delyamure & Parukhin (Reference Delyamure and Parukhin1968); 6, Stewardson & Fourie (Reference Stewardson and Fourie1998); 7, Smales (Reference Smales1986); 8, Obendorf & Presidente (Reference Obendorf and Presidente1978); 9, Morini & Boero (Reference Morini and Boero1960); 10, Morgades et al. (Reference Morgades, Katz, Castro, Capellino, Casas, Benitez, Venzal, Moraña, Rodríguez-Gallego, Scarabino and Conde2006); 11, Johnston (Reference Johnston1937); 12, Johnston & Edmonds (Reference Johnston and Edmonds1953); 13, Zdzitowiecki (Reference Zdzitowiecki1986); 14, Johnston & Best (Reference Johnston and Best1942); 15, Berón-Vera et al. (Reference Berón-Vera, Crespo, Raga and Fernández2007); 16, Figueroa & Puga (Reference Figueroa and Puga1990); 17, Torres et al. (Reference Torres, Oporto, Brieva and Escare1992); 18, Dans et al. (Reference Dans, Reyes, Pedraza, Raga and Crespo1999); 19, Corcuera et al. (Reference Corcuera, Monzón, Aguilar, Borrell and Raga1995); 20, Berón-Vera et al. (Reference Berón-Vera, Crespo and Raga2008); 21, Andrade et al. (Reference Andrade, Pinedo and Pereira1997); 22, Brownell (Reference Brownell1975); 23, Kagei et al. (Reference Kagei, Tobayama and Nagasaki1976); 24, Aznar et al. (Reference Aznar, Balbuena and Raga1994); 25, Hoberg & Ryan (Reference Hoberg and Ryan1989).
b Identified as Corynosoma sp., but considered to be C. australe by Sardella et al. (Reference Sardella, Mattiucci, Timi, Bastida, Rodriguez and Nascetti2005).
c Pansegrouw (1990), cited by Stewardson & Fourie (Reference Stewardson and Fourie1998).
Interestingly, a coprological analysis could shed light on the actual cause(s) of the observed patterns. Suppose that a reasonably large sample of scats is collected in a colony of an austral pinniped species and is examined for acanthocephalans. One could readily investigate the causes that determine the specificity, for example, of C. cetaceum, by examining whether this species is present, and whether mature females are found, in the scats. Obviously, inferences made from scat analysis are not free from sampling biases; the point is that, being non-invasive, this technique is less prone to sampling limitations than the parasitological examination of hosts themselves. Moreover, coprological analysis would be particularly useful to identify and characterize non-hosts.
In this paper we illustrate the use of coprological analysis as a tool to shed light on the causes of host–parasite specificity using C. australe and C. cetaceum as a model. In particular we test predictions derived from table 1 using faeces from South American sea lions, Otaria flavescens, and faecal contents from the rectum of the franciscana, Pontoporia blainvillei. Samples came from the Buenos Aires Province, Argentina, where both C. australe and C. cetaceum have been reported in a number of fish (paratenic) hosts and marine mammal (definitive) hosts (Sardella et al., Reference Sardella, Mattiucci, Timi, Bastida, Rodriguez and Nascetti2005). We are aware that faeces from franciscanas will hardly be obtained in the wild except from carcasses. Note, however, that the primary goal of our study was to explore the suitability of coprological analysis as a method to uncover patterns of parasite specificity whenever host carcasses are difficult to obtain.
Materials and methods
Collection and examination of samples
Sampling was carried out in three localities of Buenos Aires Province, Argentina, encompassing about 130 km of coastline. Scats from South American sea lions were collected in a permanent non-breeding rookery of males at Puerto Quequén (38°37′S, 58°50′W). This rookery is composed mostly of adult and subadult individuals, their total number ranging from 86 to 180 individuals depending on the season (Suárez et al., Reference Suárez, Sanfelice, Cassini and Cappozzo2005). Fresh scats were collected at sunrise during a one-week period in February (n = 48), May (n = 75), July (n = 75) and November (n = 54) of 2001. Scats were preserved in situ in vials with 70% ethanol. In the laboratory, scats were washed with rising water under a 0.3 mm sieve. Parasites were cleaned in saline and preserved in 70% ethanol.
A total of 43 franciscanas were found drowned in shark fishery gillnets in Necochea (38°27′S, 58°50′W) and Claromecó (38°52′S, 60°05′W), between October and December in 1988 (n = 6), 1989 (n = 16) and 1990 (n = 21). Information about the parasites from the stomach and the intestine of this host sample has already been published (see Aznar et al., Reference Aznar, Balbuena and Raga1994, Reference Aznar, Bush, Balbuena and Raga2001, and references therein). In the present study we re-analysed solid material from faeces, which had been stored in vials with 70% ethanol. Faeces usually appeared in the last sixth of the intestine and were recognized by their dark-brownish colour and loosely granular texture. Re-examination of stored material resulted in the finding of specimens of C. australe and Andracantha sp., which had been overlooked in the previous analysis.
For identification, acanthocephalans were cleared with lactophenol and observed under a stereomicroscope ( × 100). In specimens with an everted proboscis, hook patterns were drawn with the aid of a drawing tube. Some specimens were also dehydrated through an ethanol series, critical point dried and coated with a gold–paladium alloy to a thickness of 250 nm. These specimens were examined with a Hitachi 4100FE scanning electron microscope. Identification was based on Aznar et al. (Reference Aznar, Bush and Raga1999) and Sardella et al. (Reference Sardella, Mattiucci, Timi, Bastida, Rodriguez and Nascetti2005). All specimens were sexed, and female worms were classified into two categories (Aznar et al., Reference Aznar, Cappozzo, Taddeo, Montero and Raga2004): gravid (with at least some fully formed eggs) and non-gravid (with ovarian balls only).
Data analysis
Infection parameters were estimated following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997) and Rósza et al. (Reference Rósza, Reiczigel and Majoros2000). Sterne's exact 95% confidence interval (CI) was calculated for prevalence (Reiczigel, Reference Reiczigel2003) and bootstrap 95% confidence intervals (CIs) using 20,000 replications were estimated for mean intensity and mean abundance (Rósza et al., Reference Rósza, Reiczigel and Majoros2000). Sex ratio was expressed as number of males in the total sample of worms, and percent gravid females as number of gravid females in the total sample of females, in a given sample of scats (in the case of sea lions) or hosts (in the case of franciscanas). The 95% CI for these parameters was set using bootstrap re-sampling of acanthocephalan numbers per individual host or scat in the sample (Aznar et al., Reference Aznar, Bush, Balbuena and Raga2001). For instance, individuals of C. australe were found in 29 out of 252 scats analysed. The total number of worms and males in the sample were 206 and 61, respectively; therefore, the sex ratio was 29.6% (see table 2). We created a distribution of 20,000 replicates of sex ratio by sampling with replacement of the 29 scats that were positive for C. australe. Confidence intervals were corrected by the bias-corrected and accelerated percentile method (Efron & Tibshirani, Reference Efron and Tibshirani1993).
Table 2 Species of Corynosoma found in 252 scats from the South American sea lion, Otaria flavescens, collected seasonally in the Buenos Aires Province, Argentina, during 2001. Numbers in parentheses are the lower and upper limits of the 95% confidence interval of each parameter in the overall sample.

For each acanthocephalan species, prevalence among seasons (in the case of sea lions) or years (in the case of franciscanas) were compared with Fisher's tests and abundance with Krukal–Wallis tests. Differences in sex ratio and percent gravid females were tested with the Kruskal–Wallis test using the data obtained per individual scat or host. To carry out these tests, observations were assumed to be independent. This is true in the case of the sample of franciscanas but, in the case of sea lions, scats were sampled over time in the same rookery and, therefore, we cannot rule out that some of the scats belong to the same animals. Thus, tests are conservative in this case because we implicitly assume that random variability is higher than it actually is.
In franciscanas, data were available about infrapopulation size, and percent males, gravid females and non-gravid females of C. cetaceum in their typical habitat, i.e. the stomach and upper duodenum (for brevity, we will refer to this habitat as ‘stomach’) (Aznar et al., Reference Aznar, Bush, Balbuena and Raga2001). These data were used to assess the sensitivity of coprological analysis to detect infections of C. cetaceum. Sensitivity was calculated as number of true positive hosts × 100/(number of true positive hosts+false negative hosts). In ‘true positive’ hosts, specimens of C. cetaceum were found both in the stomach and faeces, while in ‘false negative’ hosts, C. cetaceum was found in the stomach but not in faeces. The 95% CI for sensitivity was set using Sterne's exact method (Reiczigel, Reference Reiczigel2003). A one-tailed logistic regression was also used to investigate whether the presence of C. cetaceum in faeces was dependent on the number of worms in the stomach. Finally, in those hosts whose faeces were positive for C. cetaceum, we used Wilcoxon tests to compare sex ratios and percent gravid females between the stomach and faeces.
A morphometric analysis was conducted to investigate the extent to which parasite growth had occurred in putative non-hosts. The size of female specimens of C. cetaceum from scats of sea lions (putative non-host) was compared with that of: (1) gravid females collected from faecal contents of franciscanas (right definitive host); and (2) female cystacanths obtained from the mesenteries of three teleost species from Península Valdés (42°52′S, 62°06′W), i.e. Xystreurys rasile, Paralichthys isosceles and Prionotus nudigula (Hernández et al., unpublished data). Likewise, the size of female specimens of C. australe from faecal contents of franciscanas (putative non-host) was compared with that of both gravid females collected from sea lion scats (right definitive host) and female cystacanths obtained from the same sample of fish mentioned above. Disk diameter was considered a more reliable surrogate of size than trunk length because the trunk was introverted to a variable degree depending on the specimen (see fig. 1). For each Corynosoma species, a one-way ANOVA was performed to test for overall significant differences in disk diameter among groups. Pairwise differences were compared with a post hoc Tukey test.

Fig. 1 Specimens of Corynosoma collected from faeces of the South American sea lion, Otaria flavescens. (A) Immature female of C. cetaceum. (B) Adult female of C. australe. The lines indicate disk diameter (see Materials and methods for details). Scale bar: 500 μm.
Infection parameters and their CIs were obtained with the free software Quantitative Parasitology v.3 (Reiczigel & Rózsa, Reference Reiczigel and Rózsa2005). Bootstrap estimates of 95% CIs for sex ratios, percent gravid females and sensitivity were calculated using the statistical package Systat v. 12 (http://www.systat.com/). The remaining analyses were carried out with SPSS v. 17 (SPSS Inc., Chicago, Illinois, USA).
Results
South American sea lion
Corynosoma australe and C. cetaceum were the only acanthocephalans collected from the scats of the South American sea lion. The majority of worms were found intact (fig. 1). The body of some specimens was distorted or broken, but they could still be identified and sexed based on size and the distribution of trunk spines. Infection parameters are shown in table 2. The prevalence and the abundance of both species did not differ significantly among seasons (prevalence: Fisher's exact test, C. australe: P = 0.73; C. cetaceum: P = 0.28; abundance: Kruskal–Wallis test, C. australe: χ2 = 1.66, 3 df, P = 0.65; C. cetaceum: χ2 = 4.49, P = 0.21). In the overall sample, the prevalence of both species was similar (11.5 versus 15.9), but the mean abundance of C. australe was half of that of C. cetaceum (table 2); the difference was statistically significant (Wilcoxon test, Z = 2.01, n = 51, P = 0.045). The maximum numbers of worms collected from a single scat were 38 (C. australe) and 82 (C. cetaceum).
The sex ratio per scat did not differ significantly among seasons, either in C. australe (Kruskal–Wallis test, χ2 = 5.605, 3 df, P = 0.132) or C. cetaceum (χ2 = 3.10, 3 df, P = 0.38). In the overall sample, the sex ratio of C. australe was strongly female-biased, whereas the sex ratio of C. cetaceum was far closer to 1:1, being slightly male-biased (table 2). Gravid females of C. australe were found in all seasons, but the percentage varied substantially, ranging between 14.3 and 85.1% (table 2). In the overall sample, the percentage of gravid females was 37.9% (95% CI: 19.7–59.4%). However, none of the 168 females of C. cetaceum that were found in 29 scats was gravid (table 2). The genital primordium was not transformed into ovarian balls in any specimen.
Franciscana
Three acanthocephalan species were found in the faeces of franciscanas, i.e. C. australe, C. cetaceum and Andracantha sp. The latter species could not be identified because single immature females with inverted proboscis were found in two hosts. All but five individuals of C. australe were in good condition and could be sexed. Infection parameters are shown in table 3. Infection levels did not differ significantly among years in either species (prevalence: Fisher's exact test, C. australe: P = 1.0; C. cetaceum: P = 0.44; abundance: Kruskal–Wallis test, C. australe: χ2 = 0.42, 3 df, P = 0.98; C. cetaceum: χ2 = 2.58, P = 0.28). In the overall sample, both the prevalence (55.8 versus 25.6) and mean abundance (3.2 versus 0.6) were clearly higher in C. australe than in C. cetaceum (table 3); differences in abundance were highly significant (Wilcoxon test, Z = 3.36, n = 30, P = 0.001). The maximum number of worms collected in the faeces from a single host were 19 (C. australe) and 9 (C. cetaceum).
Table 3 Species of Corynosoma found in the rectal faeces of 43 franciscanas, Pontoporia blainvillei. The animals were collected in the Buenos Aires Province, Argentina, during the spring of three consecutive years. Numbers in parentheses are the lower and upper limits of the 95% confidence interval of each parameter in the overall sample.

Sex ratio per host did not differ among years, either in C. cetaceum (χ2 = 0.04, 2 df, P = 0.84) or C. australe (Kruskal–Wallis test, χ2 = 5.62, 3 df, P = 0.09). In the overall sample, the sex ratio of C. cetaceum was strongly female-biased, and the upper limit of the 95% CI did not include the value 0.50 (table 3). However, the sex ratio of C. australe was far closer to 1:1 and the 95% CI did include this value (table 3). In C. cetaceum, 14 out of 17 females found in the overall sample were gravid (table 3). In contrast, none of the 139 females of C. australe collected from faeces from 19 franciscanas was gravid (table 3). The genital primordium was not transformed into ovarian balls in any specimen.
Corynosoma cetaceum was found in the faecal contents of 11 hosts, but it occurred in the stomach of all 43 franciscanas examined (mean intensity (95% CI): 703.7 (546.9–907.0)); sensitivity therefore was 25.6% (95% CI: 14.6– 40.6%). The presence of worms in faeces was not dependent on the number of worms in the stomach (logistic regression, Wald statistic = 0.245, 1 df, one-tailed P = 0.321). In the hosts that were positive for C. cetaceum in faeces, the sex ratio in the stomach was also female-biased (41.9%, 95% CI: 39.5–46.2%) and did not differ significantly from that observed in faeces (table 3, Wilcoxon test, Z = 0.71, n = 11, P = 0.477). Also, the percent of gravid females in the stomach (81.2%, 95% CI: 75.3–89.1%) was very similar to that in faeces (table 3); the difference was not significant (Wilcoxon test, Z = 0.0, n = 11, P = 1.0).
Morphological analysis
In both C. cetaceum (one-way ANOVA: F (2,103) = 16.32, P < 0.001) and C. australe (F (2,56) = 82.29, P < 0.001), disk diameter differed significantly among female specimens collected from paratenic hosts, putative non-hosts and right definitive hosts. However, Tukey tests revealed that differences (P < 0.05) occurred only between gravid females from definitive hosts and those from each of the remaining host types (fig. 2). Disk diameter in specimens from putative non-hosts was just slightly greater than that from cystacanths in both C. australe and C. cetaceum (fig. 2).
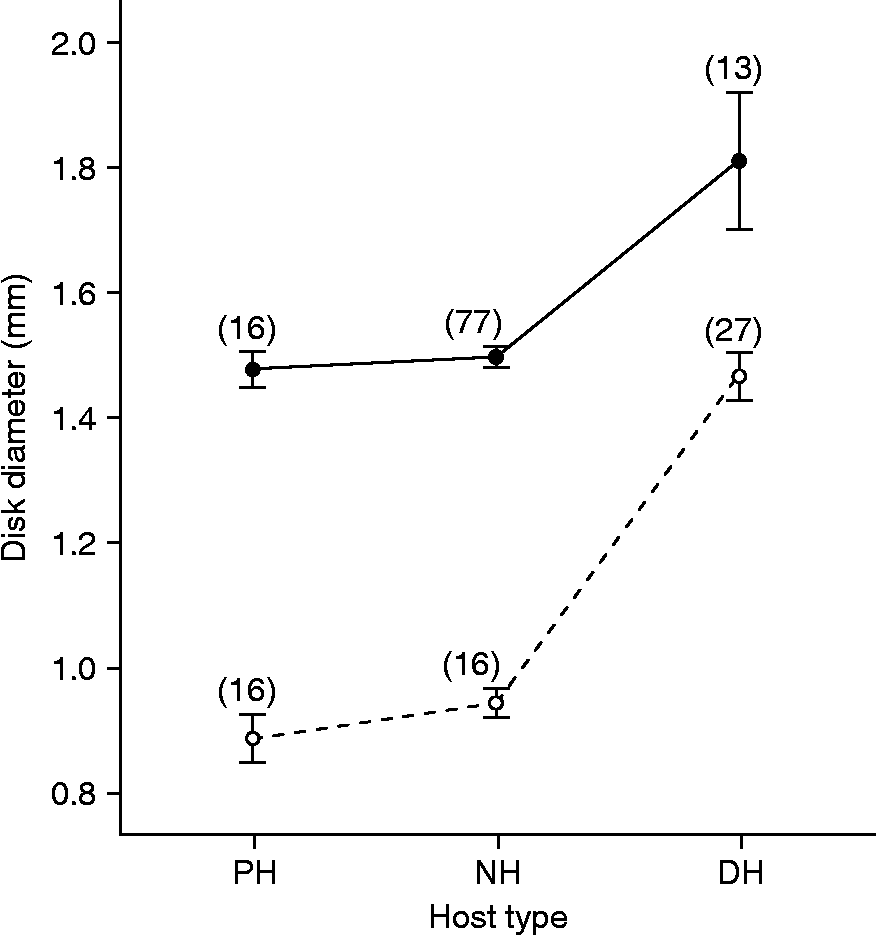
Fig. 2 Mean disk diameter (bar: standard error) in samples of female C. cetaceum (black dots) and C. australe (white dots) from several host types. Numbers on the dots are parasite sample sizes. Juvenile specimens of both C. cetaceum and C. australe were collected from the mesenteries of the following fish paratenic hosts (PH): Xystreurys rasile, Paralichthys isosceles and Prionotus nudigula. Immature specimens from putative non-hosts (NH) were obtained from faecal samples of South American sea lion, Otaria flavescens (in the case of C. cetaceum) and franciscana, Pontoporia blainvillei (in the case of C. australe). Gravid specimens from definitive hosts (DH) were obtained from faecal samples of the South American sea lion (in the case of C. australe) and franciscana (in the case of C. cetaceum).
Discussion
Coprological analysis suggests that, in the study area, both C. australe and C. cetaceum contact South American sea lions and franciscanas regularly through the local food web. In fact, Suárez et al. (Reference Suárez, Sanfelice, Cassini and Cappozzo2005) carried out a dietary analysis of sea lions based on the same scat sample used in this study, and at least 8 out of the 11 numerically most important fish prey these authors found are known to be paratenic hosts for C. australe, or C. australe plus C. cetaceum, in the Buenos Aires Province (Tanzola et al., Reference Tanzola, Guagliardo, Brizzola and Arias1997; Lanfranchi et al., Reference Lanfranchi, Rossin and Timi2009; Timi et al., Reference Timi, Lanfranchi and Etchegoin2009, and references therein). No dietary data are available for the franciscanas analysed in the present study. However, in another sample of 27 franciscanas collected in the study area, Rodríguez et al. (Reference Rodríguez, Rivero and Bastida2002) identified 2131 prey, of which 56.7% were striped weakfish, Cynoscion guatucupa, a typical paratenic host for C. australe and C. cetaceum (Timi et al., Reference Timi, Luque and Sardella2005). Cystacanths of C. australe and C. cetaceum have been recorded in many additional fish species in the study area (Lanfranchi et al., Reference Lanfranchi, Rossin and Timi2009; Timi et al., Reference Timi, Lanfranchi and Etchegoin2009, and references therein). We would therefore expect that these acanthocephalans contact piscivorous predators regularly, even if predators switch between specific preys according to temporal availability. This may explain why infection levels in sea lions and franciscanas were remarkably stable among seasons or years, respectively.
In sea lion scats, a substantial proportion of females of C. australe, but none of C. cetaceum, was gravid. Conversely, in faecal contents of franciscana, most females of C. cetaceum, but none of C. australe, were gravid. These patterns conform to the predictions that C. australe is specific to pinnipeds and C. cetaceum to cetaceans (table 1). This interpretation is hardly influenced by sampling bias. First, all the specimens we collected were intact or little distorted, suggesting that faeces likely provide a rather reliable record of failed infections. Second, sampling error could not explain the striking differences in the number of gravid females between putative hosts and non-hosts because, in the latter, the number of both C. australe and C. cetaceum was significantly higher. Third, the evidence obtained from C. cetaceum in franciscanas strongly suggests that the proportion of gravid females in faeces reflect their proportion in their reproductive population. Thus, it seems clear that C. australe and C. cetaceum hardly reproduce, if at all, in franciscanas and sea lions, respectively.
Other lines of evidence also support this conclusion. In the faeces of putative non-hosts, females of C. cetaceum and C. australe were all observed to be in an initial stage of sexual development. Also, the size of these females was very similar to juvenile females from paratenic hosts, implying that worms had passed through the gut of non-hosts without substantial growth. On the other hand, the sex ratio of C. cetaceum and C. australe in putative non-hosts was remarkably close to 1:1. In acanthocephalans, sex ratios generally are 1:1 in intermediate/paratenic hosts but become female-biased in definitive hosts because adult females live longer than males (Crompton, Reference Crompton, Crompton and Nickol1985). We therefore interpret that C. cetaceum and C. australe conserved the juvenile sex ratio in sea lions and franciscanas, respectively, because they did not reproduce in these hosts. There was, however, a slight but significant bias in favour of males in the sample of C. cetaceum from sea lion scats (lower 95% CI: 53.0%). Excluding the possibility of sampling bias, this departure from 1:1 could indicate that males of C. cetaceum remain somewhat longer than females in the gut of sea lions and/or that the sex ratio is already male-biased in the paratenic hosts.
A significantly female-biased sex ratio has been reported in the reproductive populations of C. cetaceum and C. australe (Aznar et al., Reference Aznar, Bush, Balbuena and Raga2001, Reference Aznar, Cappozzo, Taddeo, Montero and Raga2004). In the present study, this bias was observed also in faecal samples from suitable hosts. However, considering that faeces contain senescent worms and that adult males have a shorter life span than females, why was the sex ratio not male-biased in faecal samples? A likely explanation is that the probability of finding senescent worms of a given sex depends, not only on life span, but also on the relative proportion of each sex in the population. Insofar as the relative proportion of females increases in mature infections (Valtonen et al., Reference Valtonen, Helle and Poulin2004) so will the likelihood of finding them in faeces. In fact, our results indicate that the overall sex ratio of C. cetaceum was consistently female-biased in both the stomach reproductive population and the corresponding faecal sample from franciscanas.
In summary, our study suggests that, in the study area, specificity of both C. cetaceum and C. australe is determined by a compatibility filter, which apparently precludes establishment of C. cetaceum in sea lions and C. australe in franciscanas. Moreover, non-hosts likely act as a significant population ‘sink’ for both parasites, a conclusion that could not easily be drawn based on previous data (table 1). Also, our study provides a more solid foundation to the inference that pinnipeds are unsuitable hosts for C. cetaceum (Aznar et al., Reference Aznar, Bush and Raga1999; table 1). The fact that a member of Corynosoma is unable to mature in pinnipeds is rather exceptional (Aznar et al., Reference Aznar, Pérez Ponce de Leon and Raga2006), and adds to the long-standing controversy as to the generic status of C. cetaceum. Morphological data strongly suggest that C. cetaceum is a member of Corynosoma (Aznar et al., Reference Aznar, Bush and Raga1999, Reference Aznar, Berón-Vera, Crespo and Raga2002, Reference Aznar, Pérez Ponce de Leon and Raga2006), whereas molecular data raise the possibility that it may belong to another genus (García-Varela et al., Reference García-Varela, Aznar, Pérez-Ponce de León, Piñero and Laclette2005; Sardella et al., Reference Sardella, Mattiucci, Timi, Bastida, Rodriguez and Nascetti2005).
Results from the present study stress the potential of coprological analysis to obtain information about the specificity of helminths, particularly from hosts that are endangered/protected, or are otherwise difficult to obtain. Obviously, the method also suffers from several shortcomings. First, it is not amenable for parasites of several vertebrate groups. For instance, obtaining useful faecal samples from cetaceans is particularly challenging (but not impossible, see for example Smith & Whitehead, Reference Smith and Whitehead2000; Dunshea, Reference Dunshea2009). Second, the method assumes that senescent worms are not massively destroyed while they are passing through the gut. Third, helminth specimens must be identified at the species level, and this is not always possible, particularly if only immature worms are collected. However, the increasing use of molecular techniques will circumvent this problem. Finally, coprological analysis has a low sensitivity to detect infections (Torres et al., Reference Torres, Pérez, Segovia and Miquel2001). For instance, we collected only 24 individuals of C. cetaceum in rectal faeces of franciscanas, but as many as 30,259 in the stomach. Likewise, only 3 out of 3240 specimens of Corynosoma strumosum collected from 100 individuals of harbour seal, Phoca vitulina, were found in rectal faeces (Aznar et al., unpublished data). This suggests that a large number of scats will usually be necessary to minimize the risk of declaring false negatives and to obtain reliable samples of helminth specimens for host-compatibility assessment. In summary, coprological analysis should be viewed as a non-invasive tool that complements, rather than supplants, traditional parasitological surveys.
Acknowledgements
We thank Fabián Tricárico from the Scanning Electronic Microscopy Service (MACN - CONICET) for assistance with scanning electron microscopy procedures, and Eleonora Bocci, Javier Corcuera, Ana de Juán, Natalia Fraija, Florencia Monzón, Leandro Tamini, Victoria Panebianco and Daniela Sanfelice for field and laboratory assistance. This study was supported by projects PIP-02193 (CONICET, Argentina) and CGL2007-63221 from the Ministry of Science and Innovation of Spain.







