Introduction
Onchocerciasis is a filarial disease which affects several millions of people, mainly in Africa, America and Asia. About 90% of the affected countries are in Africa (World Health Organization, 1995). Approximately 37 million persons are infected, 270,000 are blind and 500,000 are visually impaired (Osei-Tweneboana et al., Reference Osei-Tweneboana, Eng, Boakye, Gyapong and Prichard2007). The burden of the disease causes disability, social stigmatization and forces the affected population to abandon the infested areas, which usually have high agricultural potential. Thus a high burden of onchocerciasis in a country leads primarily to low productivity and consequently to an economic loss and the slowdown of development. During the past three decades, much progress has been made in the control of onchocerciasis but the disease is still a major public health concern in those countries in which it is highly endemic. From 1987 to date, the control of the disease has been based on two approaches: vector control using insecticides and mass drug administration using mainly ivermectin. These approaches have failed for several reasons. Early in the past decade resistance to both insecticides and ivermectin was observed (Winnen et al., Reference Winnen, Plaisier, Alley, Nagelkerke, van Oortmarssen, Boatin and Habbema2002). Also, the use of insecticides showed toxicity against non-targeted insects involved in beneficial activities for the equilibrium of the ecosystem. A re-infestation phenomenon was also observed in insecticide-treated areas. Following the observed limits of these approaches, the control of blackflies using insecticides was stopped and several combinations of drug therapy were used against onchocerciasis, but all of them were limited. Some drugs have strong adverse effects, and interference in the case of co-endemicity with loasis and parasite resistance has been reported (Moussala et al., Reference Moussala, Fobi, Zogo, Hiag, Bengono and McMoli2004). Macrofilaricides against the adult worm are rare, making the need for such drugs an important concern for endemic areas. The ideal drug would be one that overcomes all the limits observed with the previous approaches and has high efficiency within a short time of treatment. Faced with these problems, several plants have been assessed for their nematocidal activity against the bovine parasite Onchocerca ochengi, mostly used as a laboratory model of onchocerciais (Chagas et al., Reference Chagas, Viera, Freitas, Araujo-Filho, Aranguao and Navarro2008, Ndjonka et al., Reference Ndjonka, Ajonina-Ekoti, Djafsia, Lüersen, Abladam and Liebau2012a). Onchocerca ochengi is the closest species to Onchocerca volvulus, the human parasite, with which it shares the same vector and presents an identical pathological manifestation, such as nodule formation (Achukwi et al., Reference Achukwi, Harnett and Renz2000). Previously, several plants used traditionally against worms were screened using some gastrointestinal parasite of veterinary importance, such as Haemonchus contortus or Trychostrongylus columbriformis (Monglo et al., Reference Monglo, Njongmeta, Musongong, Ngassoum and Nukenine2006; Cho-Ngwa et al., Reference Cho-Ngwa, Abongwa, Ngemenya and Nyongbela2010; Ndjonka et al., Reference Ndjonka, Agyare, Lüersen, Djafsia, Achukwi, Nukenine, Hensel and Liebau2011). Most of them were shown to be efficacious against those worms. The close biological relationship of O. ochengi and O. volvulus suggests that the recorded efficacy of a given plant in the bovine model might be reproduced similarly on the human parasite, and therefore be a potential source of an antifilarial drug. Ethnoveterinary medicine cannot therefore be neglected as a source for anthelmintic or antifilarial drugs.
Some selected plants have shown toxicity against the free-living and bacteria-feeding nematode Caenorhabditis elegans (Chagas et al., Reference Chagas, Viera, Freitas, Araujo-Filho, Aranguao and Navarro2008), which is easily maintained in the laboratory. This species has been used in the field of genetics and pharmacology as a suitable model to assess the toxicity of plant extracts. The most promising plant appeared to be the axlewood tree Anogeiossus leiocarpus (DC.) Guill & Perr (Combretaceae) with a high toxicity against O. ochengi and C. elegans (Ndjonka et al., Reference Ndjonka, Ajonina-Ekoti, Djafsia, Lüersen, Abladam and Liebau2012a). A phytochemical analysis of an extract of A. leiocarpus showed a high amount of tannins, which have been reported in several studies to have a certain anthelmintic activity (Ademola et al., Reference Ademola, Fagbemi and Idowu2004; Chagas et al., Reference Chagas, Viera, Freitas, Araujo-Filho, Aranguao and Navarro2008; Hoste et al., Reference Hoste, Brunet, Paolini, Bahuaud, Chauveau, Fouraste and Lefrileux2009). Tannins comprise several phenolic groups and phenols such as ellagic, gallic and gentisic acids. Gallic and gentisic acids have been reported to be toxic for C. elegans (Smith et al., Reference Smith, Pontiggia, Waterman, Lichtenwalner and Wasserman2009) and could be toxic for the bovine parasite. Interest in the free-living nematode C. elegans is associated with a mutation which can be readily induced in this species. In fact, the genetic resistance of several filariae could be reproduced using C. elegans, thereby demonstrating resistance to a selected drug. Such a mutant would provide a suitable model for screening new compounds. Using both C. elegans and O. ochengi, the efficacy of compounds from plant extracts and any responses to resistance can then be observed. Therefore the aim of the present work was to demonstrate the activity of ellagic, gentisic and gallic acids on O. ochengi and on albendazole-, ivermectin- and levamisole-resistant strains of the free-living nematode C. elegans.
Materials and methods
Chemicals and sample preparation for anthelmintic assays
All chemicals were purchased from Sigma-Aldrich (Deisenhofen, Germany). Ivermectin, levamisole, albendazole, ellagic, gallic and gentisic acids (purity >95%, high-pressure liquid chromatography (HPLC)) were prepared as described by Ndjonka et al. (Reference Ndjonka, Ajonina-Ekoti, Djafsia, Lüersen, Abladam and Liebau2012a, Reference Ndjonka, Bergmann, Agyare, Lüersen, Hensel, Wrenger and Liebaub). Briefly, ivermectin and levamisole were dissolved in 10% dimethyl sulphoxide (DMSO) while albendazole was dissolved in 50% DMSO. The three drugs were diluted with M9 to obtain a final concentration of 2.5 mm. The maximal final concentration of DMSO in test preparations was 1%.
Gallic and gentisic acids were dissolved in 25% ethanol (EtOH) and diluted in 0.5% DMSO to a final concentration of 200 mm. Ellagic acid was dissolved in 0.3 m KOH and equilibrated with 1 × PBS (phosphate-buffered saline) to a final concentration of 50 mm. The final concentration of KOH was 10 mm and the highest concentration in test preparations was 2 mm. Samples were centrifuged and aliquoted to determine their activity on O. ochengi and C. elegans.
Monoxenic and axenic cultures of C. elegans
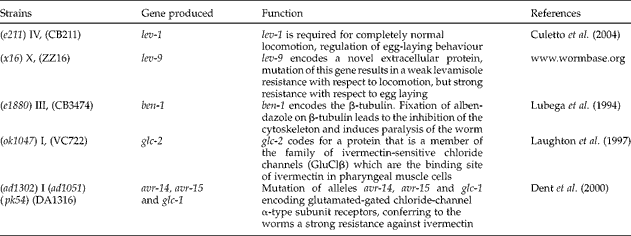
The wild-type C. elegans (N2 Bristol) and mutants CB211 lev-1(e211) IV, CB3474 ben-1(e1880) III, VC722 glc-2(ok1047) I, ZZ16 lev-9(x16) X and DA1316 avr-14(ad1302) I avr-15(ad1051) glc-1(pk54) (table 1) were purchased from Caenorhabditis Genetics Center (Minneapolis, Minnesota, USA). A monoxenic culture was performed in Petri dishes at 20°C on NGM-agar (Nematode Growth Medium: 2.5 g peptone from casein, 3 g NaCl, 17 g agar, 0.5% cholesterin, 1 mm CaCl2, 1 mm MgSO4, 25 mm KH2PO4/K2HPO4 in 1 litre of water) and seeded with Escherichia coli OP50. The culture was synchronized to initiate axenic worm cultures using the alkaline bleaching method (Ndjonka et al., Reference Ndjonka, Ajonina-Ekoti, Djafsia, Lüersen, Abladam and Liebau2012a).
Table 1 A summary of the alleles and functions of the mutated gene of different strains of Caenorhabditis elegans.

In vitro screening assay of O. ochengi and C. elegans
Onchocerca ochengi worms were extracted from nodules following their collection in the communal slaughter house of Ngaoundere in Adamaoua Region of Cameroon. Nodules removed from the skin were treated following the method described by Ndjonka et al. (Reference Ndjonka, Ajonina-Ekoti, Djafsia, Lüersen, Abladam and Liebau2012a). The isolated worms and microfilariae were incubated at 37°C in RPMI 1640 supplemented with 100 μg/ml streptomycin and 100 U/ml penicillin in 24-well plates. The number of worms (six individuals per 1-ml well) was defined according to the protocol of Borsboom et al. (Reference Borsboom, Boatin, Nagelkerke, Agoua, Akpoboua, Alley, Bissan, Renz, Yameogo, Remme and Habbema2003).
Synchronized C. elegans (approximately 30 L4/young adult worms) were transferred from liquid axenic medium into 24-well sterile plates, each well containing 500 μl M9 medium (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 0.25 g MgSO4.7H2O, in 1 litre of water) supplemented with 2% glucose and 0.5% cholesterin. Assays were incubated at 20°C. In both cultures (C. elegans and O. ochengi), increasing concentrations (0–40 mm) of gallic, gentisic or ellagic acids were added and the mortality rate was determined after 48 h.
Worm mortality and LC50 determination
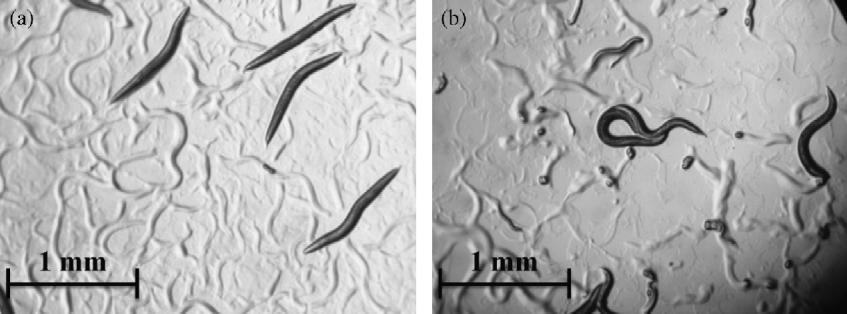
Worm viability was checked by observation under the binocular microscope. After shaking, immotile and fully elongated worms were considered to be dead (fig. 1). The viability rate was calculated as follows:
where N L is the number of living worms in each well at various concentrations and N T is the total number of worms in each well at various concentrations. Gallic, gentisic and ellagic acids, together with the respective control groups, were tested in three duplicate independent determinations. LC50 values were determined, with LC50 being the concentration of the extract required to induce 50% worm mortality. Results are presented as mean values ± standard error. Ivermectin, levamisole and albendazole were used for the preparation of positive control groups. KOH-PBS, EtOH-DMSO, DMSO or M9-DMSO was used as the negative control.

Fig. 1 Worms of Caenorhabditis elegans on NGM-agar to show (a) immotile and elongated dead specimens and (b) actively moving live specimens; adult worms were transferred on to NGM-agar plates (10 worms per plate) supplemented with 10 mm ellagic acid.
Experimentation with rats
Eight to 10-week-old albino rats, with an average weight of 100 g were bred and maintained at the Veterinary Research Laboratory of the Institute of Agricultural Research for Development, Wakwa Regional Centre, Ngaoundere, Cameroon. Rats were allowed to fast for 24 h before the administrations. Gallic, gentisic and ellagic acids, at doses of 1000 mg/kg body weight (175 mm), 3000 mg/kg body weight (530 mm) and 5000 mg/kg body weight (750 mm), respectively, were administered orally as suspensions in DMSO to six male and six female rats. After dosing, each rat was carefully observed at 2-, 4-, 24- and 48-h intervals for clinical signs of morbidity and mortality; and thereafter twice daily for a continuous period of 14 days. This study was performed in compliance with the Organization for Economic Cooperation and Development (OECD) guidelines for testing of chemicals.
Results and discussion
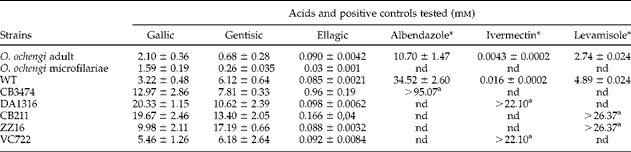
The present results focus on describing the anthelmintic activity of ellagic, gallic and gentisic acids on the bovine nematode parasite, O. ochengi, and on the resistant strains of C. elegans. Five resistant strains of the free-living nematode C. elegans were used, namely CB211 and ZZ16 resistant to levamisole, CB3474 resistant to albendazole, DA1316 and VC722 resistant to ivermectin.
The effect of ellagic, gallic and gentisic acids on O. ochengi
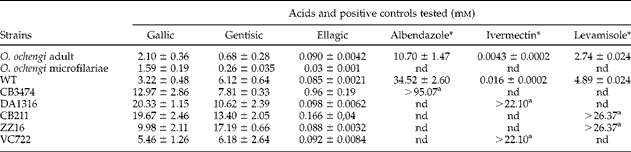
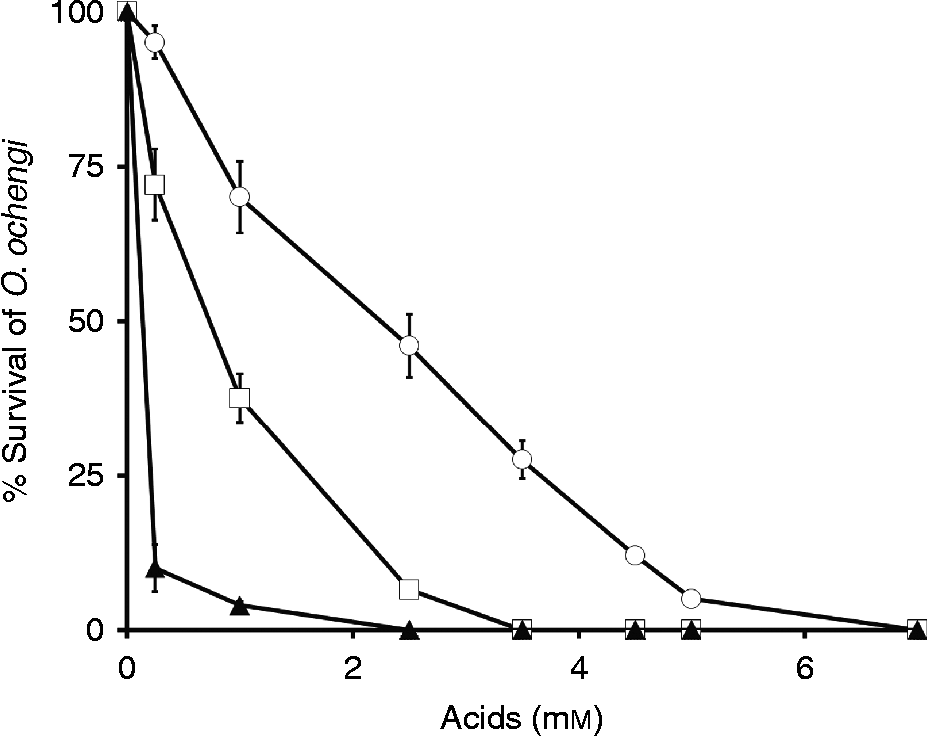
Different tannins from the plant A. leiocarpus (ellagic, gentisic and gallic acids) were used to screen for any in vitro activity against adults and microfilariae of the bovine filarial nematode O. ochengi, an established model for human onchocerciasis (table 2). The anthelmintic effect of these tannins was found to be time and concentration dependent (fig. 2) and in adult worms after 48 h produced LC50 values of 0.090 mm, 0.68 mm and 2.10 mm, respectively, for ellagic, gentisic and gallic acids. For microfilariae, ellagic acid showed the highest LC50 of 0.03 mm, followed by values of 0.26 mm and 1.59 mm in the case of gentisic and gallic acids, respectively (table 2). Ellagic acid showed the highest activity for both adult worms and microfilariae, resulting in a 100% worm mortality at 2.5 mm (fig. 2). As positive controls, LC50 values of 0.0043 μ m for ivermectin, 2.74 μ m for levamisole and 10.70 μ m for albendazole were observed (table 2). As negative controls, EtOH-DMSO, DMSO, M9-DMSO and PBS-KOH showed no effect whatsoever. To our knowledge, the tannins ellagic, gallic and gentisic acids have never been tested against Onchocerca spp. However, previous studies on the parasitic protozoans Plasmodium falciparum, Plasmodium bergheii, Trypanosoma spp., the nematodes H. contortus, O. ochengi and the fungi Aspergillus spp. and Penicillium spp. reported no effects with A. leiocarpus from which the phenols ellagic, gentisic and gallic acids were derived (Mann et al., Reference Mann, Banso and Clifford2008; Shuaibu et al., Reference Shuaibu, Wuyep, Yanagi, Hirayama, Ichinose, Tanaka and Kouno2008a; Ademola & Eloff, Reference Ademola and Eloff2011; Akanbi et al., Reference Akanbi, Omonkhua, Cyril-Olutayo and Fasimoye2012). Our study confirms that ellagic or gentisic acids may be the active compounds in A. leiocarpus. In addition, it has been shown that ellagic, gentisic and gallic acids have antiplasmodial potential against P. falciparum (Asres et al., Reference Asres, Bucar, Knauder, Yardley, Kendrick and Croft2001; Shuaibu et al., Reference Shuaibu, Pandey, Wuyep, Yanagi, Hirayama, Ichinose, Tanaka and Kouno2008b; Gansane et al., Reference Gansane, Sanon, Ouattara, Traoré, Hutter, Ollivier, Azas, Traore, Guissou, Sirima and Nebié2010). Other tannins, such as proanthocyanidins, castalagin and flavogallonic acid, affect the survival of different gastrointestinal worms and protozoans, such as H. contortus, P. falciparum and Leishmania (Fakae et al., Reference Fakae, Campbell, Barrett, Scott, Teesdale-Spittle, Liebau and Brophy2000; Shuaibu et al., Reference Shuaibu, Pandey, Wuyep, Yanagi, Hirayama, Ichinose, Tanaka and Kouno2008b; Gansane et al., Reference Gansane, Sanon, Ouattara, Traoré, Hutter, Ollivier, Azas, Traore, Guissou, Sirima and Nebié2010). The effect of polyphenol KSI-4088 on wild-type C. elegans has also been reported, with LC50 value of 28.7 μ m (Kaewintajuk et al., Reference Kaewintajuk, Cho, Kim, Lee, Lee, Choi and Park2010).
Table 2 LC50 values for ellagic, gallic, gentisic acids and positive controls tested against adults and microfilariae of Onchocerca ochengi and wild-type (WT) and mutant strains (CB211, CB3474, DA1316, ZZ16 and VC722) of Caenorhabditis elegans after 48 h exposure.
(*)μ m; a lowest survival resistance dose; nd, not determined.

Fig. 2 Mortality rates of cultures of Onchocerca ochengi following exposure for 48 h to 0–7 mm concentration of gallic (○), gentisic (□) and ellagic acids (▲).
Inhibition of gentisic, gallic and ellagic acids on C. elegans
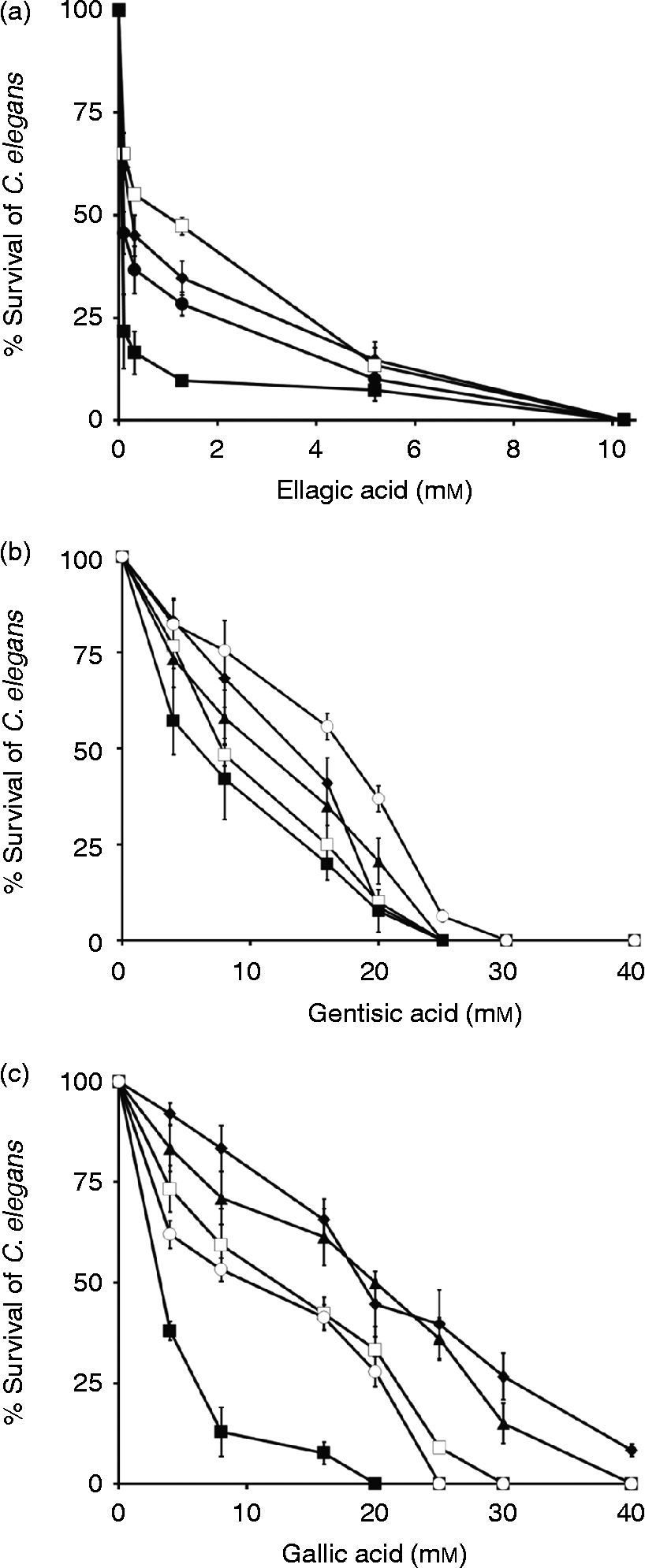
Gentisic acid exhibited moderate activity towards wild-type C. elegans, while gallic and ellagic acids exhibited high activity towards the wild-type C. elegans and the C. elegans mutants. Worm motility decreased with increasing acid concentrations (fig. 3). The lowest concentrations required to inhibit worms by 50% were 6.12 mm, 3.22 mm and 0.085 mm for gentisic, gallic and ellagic acids, respectively (table 2). Similar to O. ochengi, ellagic acid was strongly active against C. elegans wild type and almost all drug-resistant mutant strains, with LC50 values ranging between 0.085 and 0.166 mm. A tenfold increase in the LC50 value in the C. elegans albendazole-resistant mutant CB3474 was observed for ellagic acid (0.96 mm compared with 0.085 mm in the wild type). This result is difficult to explain, but it was noticed that high concentrations of albendazole were needed to kill wild-type C. elegans, the albendazole-resistant mutant and O. ochengi (table 2). To our knowledge, ellagic acid has never been tested against C. elegans. Since LC50 values of all mutants are almost similar to the LC50 value of the wild type, this result suggests that ellagic acid may have a different molecular target or mode of action from that of ivermectin, levamisole and albendazole on C. elegans. All wild-type and all mutant worms were killed by ellagic acid at 10 mm after 48 h (fig. 3a). LC50 values similar to those obtained with N2 wild-type worms (6.12 mm) were determined for the albendazole-resistant strain CB3474 (7.81 mm) of C. elegans with gentisic acid. This result can suggest that the binding site of gentisic acid on C. elegans may be different from that of albendazole. The ivermectin- and levamisole-resistant strains DA1316 and CB211, respectively, were slightly sensitive towards gentisic acid, exhibiting LC50 values of 10.62 mm and 13.40 mm after 48 h (table 2). With the exception of the levamisole-resistant strain ZZ16, 100% of worms were killed by gentisic acid at 25 mm after 48 h (fig. 3b). The ivermectin- and levamisole-resistant strains DA1316 and CB211 were not sensitive towards gallic acid and exhibited LC50 values of 20.33 and 19.67 mm after 48 h, respectively. An almost sevenfold increase in both LC50 values was observed for gallic acid, compared with 3.22 mm in the wild type. This result shows that gallic acid may act on these two mutants (DA1316 and CB211) like ivermectin and levamisole. The LC50 value with gallic acid (5.42 mm) for the ivermectin-resistant strain VC722 is higher than the LC50 for the wild type (3.22 mm), 1.5-fold lower than the LC50 for the levamisol-resistant strain ZZ16 (9.98 mm) and two times lower than the LC50 for the albendazole-resistant strain CB3474 (12.97 mm). All wild-type worms were killed by gallic acid at 20 mm after 48 h (fig. 3c). Negative controls indicated that EtOH-DMSO, M9-DMSO and KOH-PBS used in these tests showed no effect whatsoever.

Fig. 3 Mortality rates of axenically cultured Caenorhabditis elegans following 48-h exposure to 0–40 mM concentrations of (a) ellagic acid: C. elegans (□) CB3474, (♦) CB211, (●) VC722 and (■) wild type, (b) gentisic acid: C. elegans (○) ZZ16, (♦) CB211, (▲) DA1316, (□) CB3474 and (■) wild type and (c) gallic acid: C. elegans (♦) CB211, (▲) DA1316, (□) CB3474, (○) ZZ16 and (■) wild type.
A levamisole-resistant strain developed from a wild-type strain was used by Smith et al. (Reference Smith, Pontiggia, Waterman, Lichtenwalner and Wasserman2009). They compared at two concentrations (6.5 and 12 mm), the activity of gentisic and gallic acids during 24 h, using two different solvents, HPLC water versus M9 medium. Comparing the present results with those of Smith et al. (Reference Smith, Pontiggia, Waterman, Lichtenwalner and Wasserman2009), it can be concluded that activity varies with each strain, as seen with the activity of the two levamisole- and ivermectin-resistant strains used in the present study. Also, viability varies with the solvent and the length of time. In the current study 0.5% DMSO was used and the incubation lasted 48 h.
Apart from examining the anthelmintic activities of ellagic, gentisic and gallic acids, in vivo toxicity assays were performed using rats. The toxicity of ellagic, gentisic and gallic acids was determined in vivo on rats at doses of 1000 mg/kg body weight (175 mm), 3000 mg/kg body weight (530 mm) and 5000 mg/kg body weight (750 mm). These values were used to determine the selectivity index, being 252.4, 257.4 and 8333.3 for gallic, gentisic and ellagic acids, respectively (table 3). The in vivo testing demonstrated that there was no alteration in the mean body weight of animals before and after the test period in both the control and acid-treated rats (data not shown). Administration of gallic, gentisic and ellagic acids at the dose of 1000 mg/kg body weight did not cause mortality after 2–14 days. The same result was obtained with ellagic acid at the dose of 5000 mg/kg body weight, while 40% of rats were killed with gentisic and gallic acids at the doses of 3000 mg/kg and 5000 mg/kg, respectively, and 100% with gentisic acid at doses of 5000 mg/kg after 2 days (table 3). In contrast, Rajalakshmi et al. (Reference Rajalakshmi, Devaraj and Devaraj2001) reported no effect on mice with gallic acid at 5000 mg/kg. Also gentisic and ellagic acids are non-toxic for cells (Curto et al., Reference Curto, Kwong, Hermersdorfer, Glatt, Santis, Virador, Hearing and Dooley1999; Soh et al., Reference Soh, Witkowski, Olagnier, Nicolau, Garcia-Alvarez, Berry and Vical2009). The absence of mortality and any adverse effects upon the administration of a dose of 5000 mg/kg body weight to rats clearly indicates the non-toxic nature of ellagic acid. Toxicologists agree that any test substance that is not lethal when administered at a concentration of 5000 mg/kg body weight is essentially non-toxic according to the internationally acceptable guidelines published by the OECD (2001).
Table 3 Percentage (%) mortality of male and female rats 48 h after administration of ellagic, gentisic or gallic acid.

SI, selectivity index; LC50, lethal concentration required to kill 50% of worms.
Binding and active sites of ellagic, gentisic and gallic acids in the worms
Data on the effects of the three acids on drug-resistant mutants offer some new insights into the binding on molecular targets. Ivermectin, by acting on C. elegans via glutamate-gated chloride channels, causes hyperpolarization of cell membranes (Yates et al., Reference Yates, Portillo and Wolstenholme2003) and induces the excitation of muscles, which leads to worm paralysis and mortality (Laughton et al., Reference Laughton, Lunt and Wolstenholme1997; Dent et al., Reference Dent, Smith, Vassilatis and Avery2000). The mutation of alleles avr-14, avr-15 and glc-1 coding for the nicotinic receptors has been reported as the origin of resistance of nicotinic receptors to the ligands of ivermectin-resistant strain DA1316, and confers a strong resistance to ivermectin (Laughton et al., Reference Laughton, Lunt and Wolstenholme1997; Dent et al., Reference Dent, Smith, Vassilatis and Avery2000). Ivermectin-resistant strain DA1316 was very sensitive to ellagic acid, moderately sensitive to gentisic acid and slightly sensitive to gallic acid. Thus, according to the effect on the DA1316 strain, ivermectin and gallic acid could have the same molecular target and therefore the same mechanism of action. The allele glc-1 of DA1316 is the binding site of ivermectin in the nervous system and this result is confirmed by the action of gallic acid on the mutant VC722, where the binding site of ivermectin in pharyngeal muscle cells is mutated. This implies that the binding site of gallic acid in the worm may be in the nervous system and not in the pharyngeal muscle cells. VC722 is also very sensitive to ellagic, gentisic and gallic acids. Since gentisic and ellagic acids affect both mutants VC722 and DA1316 ivermectin-resistant strains to a similar degree, it can be concluded that ellagic and gentisic acids may act both on nervous system and on pharyngeal muscle cells, while gallic acid may act only on nervous system.
Albendazole is an anthelmintic drug used for several decades against gastrointestinal worms. Its fixation on β-tubulin leads to the inhibition of the formation of microtubules of the cytoskeleton (Roos et al., Reference Roos, Boersema, Borgsteede, Cornelissen, Taylor and Ruitenberg1990; Lubega et al., Reference Lubega, Klein, Geary and Prichard1994). These microtubules, which are involved in mitosis, nutrient absorption, secretion, intracellular transport and cell mobility, induce worm paralysis and a reduction in growth. The β-tubulin is encoded by the allele ben-1 (Driscoll et al., Reference Driscoll, Dean, Reilly, Bergholz and Chalfie1989). Compared to the wild type, the albendazole-resistant strain CB3474 was highly sensitive to ellagic acid (0.96 ± 0.19 mm) and moderately sensitive to gallic and gentisic acid (12.97 ± 2.86 and 7.81 ± 0.33 mm, respectively). These observations suggest that the molecular targets involved in the action of those acids are different from that of albendazole. Additionally, it is possible that molecular targets of ellagic acid are more sensitive to its ligand or that ellagic acid must be more specific to these molecular targets than gentisic and gallic acids.
Levamisole is a nicotinic receptor agonist (Aceves et al., Reference Aceves, Erlij and Martínez-Marañón1970; Aubry et al., Reference Aubry, Cowell, Davey and Shevde1970) and causes hypercontraction of muscles and lethality due to prolonged activation of the excitatory nicotinic acetylcholine (nACh) receptors on the muscular body wall. It has been shown that three genes unc-38, unc-29 and lev-1 encode non-alpha nACh receptor subunits which confer resistance to levamisole when mutated (Culetto et al., Reference Culetto, Baylis, Richmond, Jones, Fleming, Squire, Lewis and Sattelle2004). lev-1 is required for completely normal locomotion, regulation of egg-laying behaviour and forms a cation channel when co-expressed with unc-38 or unc-63 and unc-29, and it is expressed in the muscular body wall (Culetto et al., Reference Culetto, Baylis, Richmond, Jones, Fleming, Squire, Lewis and Sattelle2004). lev-9 encodes a novel extracellular protein; mutation of this gene results in a weak levamisole resistance with respect to locomotion, but leads to a strong resistance with respect to egg laying (www.wormbase.org).
The alleles lev-1 and lev-9 are also secreted in muscle cells and localized at cholinergic neuromuscular junctions (Gendrel et al., 2009). Since the nematocidal activity of the ellagic acid against both levamisole-resistant strains ZZ16 (lev-9) and CB211 (lev-1) was almost similar to the effect on the N2 wild type (table 2), it can be concluded that the mode of action of ellagic acid is likely to be different from that of levamisole. CB211 is very sensitive to ellagic acid, moderately sensitive to gentisic acid and slightly sensitive to gallic acid. Since resistance with gallic acid was observed, it can be concluded that the mutated allele lev-1 as the binding site of levamisole on muscular body wall may also be the binding site for gallic acid. However as gallic and ellagic acid affect the mutant ZZ16 levamisole-resistant strain, it can be expected that there are at least two binding sites of levamisole on the muscular body wall of the worm and that gallic acid may bind to lev-1 while gentisic acid binds to lev-9. Gentisic acid affects mutant CB211 (lev-1) but not mutant ZZ16 (lev-9), while gallic acid has the opposite effect on these two strains. Such antagonistic effects of gentisic and gallic acid on these two mutants might be explained by the fact that the two genes lev-1 and lev-9 are involved in levamisole resistance.
In conclusion, the present study assessed the in vitro anthelmintic effect of ellagic, gallic and gentisic acids on drug-resistant mutant strains of C. elegans and on the bovine parasite O. ochengi. Among the three acids involved in this study, ellagic acid exhibits the highest activity on O. ochengi, C. elegans and all albendazole-, levamisole- and ivermectin-resistant strains. This result with ellagic acid tends to imply that the mechanism of action of ellagic acid may be different from that of albendazole, levamisole and ivermectin. Our results also suggest that gallic acid may need lev-1 and gentisic acid lev-9 for their binding. Further studies must be undertaken to identify the binding sites of ellagic acid and to confirm these results in vivo.
Acknowledgements
This research was supported in part by the Deutsche Forschungsgemeinschaft (DFG) grant LI 793/5-1 to the Cameroonian–German Cooperation Project (CGCP; http://www.cameroon.uni-muenster.de) and a fellowship of the Alexander von Humbolt Foundation (AVH) awarded to D.N.