Introduction
The newly established order Rhinebothriidea Healy, Caira, Jensen, Webster and Littlewood, Reference Healy, Caira, Jensen, Webster and Littlewood2009 is not a well-studied group of elasmobranch tapeworms. The genus Rhinebothrium Linton, 1890 comprises 41 species (Reyda & Marques, Reference Reyda and Marques2011), but revision of most of them is still pending, with the exception of the unpublished thesis of Healy (Reference Healy2006a). Among the different genera belonging to this order, this genus is much more interesting, because not only have several marine species been described so far, but there are also some species adapted to the freshwater rays in inland waters of South America and Asia (table 1). Some of these freshwater species have been revised recently and several new species have been described (Menoret & Ivanov, Reference Menoret and Ivanov2009, Reference Menoret and Ivanov2011; Reyda & Marques, Reference Reyda and Marques2011). However, the most recently described marine species was redescribed by Healy (Reference Healy2006b) and molecular data of many undescribed species have been published (Healy et al., Reference Healy, Caira, Jensen, Webster and Littlewood2009; Caira et al., Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014). Also, many rays have not been examined yet, or have not been examined throughout the distribution range of the host species, and studying parasitic communities of these hosts is very important. The Gulf of Oman is among several regions in the world in which the presence of rhinebothriidean cestodes has not been evaluated systematically. In a 3-year survey, the occurrence of rhinebothriidean cestodes was examined in some batoids of the Gulf of Oman. As a result of the present survey, several rhinebothriidean cestodes were identified and, among them, two new species of Rhinebothrium inhabiting the granulated guitarfish Glaucostegus granulatus are described. The granulated guitarfish has been categorized as a Vulnerable (VU) species in the IUCN Red List (Marshall & Last, Reference Marshall and Last2006). Healy (Reference Healy2006b) stated that most species of Rhinebothrium are known to be strictly host specific, therefore parasites of this vulnerable ray might be also at risk, and require appropriate attention.
Table 1 Morphological characteristics of valid species of Rhinebothrium. N, Number of specimens examined; ranges in size or number of organs are included in parentheses.
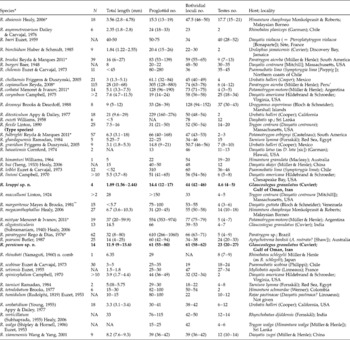
* Freshwater species. Six marine species – R. brooksi, R. copianullum, R. fulbrighti, R. mistyae, R. paratrygoni and R. urobatidium – were reported from additional hosts and additional localities (see Reyda & Marques (Reference Reyda and Marques2011) for respective details). ** The only marine Rhinebothrium species with an additional host is R. margaritense, which has also been reported from Dasyatis americana Hildebrand & Schroeder, but from the same locality of type host.
During a recent ecological survey conducted by the authors, bioconcentration capacities of two undescribed species of Rhinebothrium (Rhinebothrium sp. 1 and Rhinebothrium sp. 2) from G. granulatus and Himantura cf. gerrardi (Gray), along with some non-parasitic species, were examined (Golestaninasab et al., Reference Golestaninasab, Malek, Roohi, Karbassi, Amoozadeh, Rashidinejad, Ghayoumi and Sures2014). However, the present study is the first systematic survey of the rhinebothriidean parasites of rays in the Gulf of Oman. Before the present study, only one valid species of Rhinebothrium, R. oligotesticularis (Subramaniam Reference Subramaniam1940) Healy, 2006, had been described from G. granulatus in nearby Indian Ocean waters.
To have a more reliable comparison, important morphological characteristics and the biogeographical distribution of all valid species are provided in table 1. Previously, total size and total number of the proglottids were among the most common discriminating characteristics for identifying and evaluating new species of Rhinebothrium (Healy, Reference Healy2006b). However, a recent study of the species of Rhinebothrium in freshwater stingrays conducted by Reyda & Marques (Reference Reyda and Marques2011) reported that there is a great amount of intraspecific variation in terms of total size and, in turn, proglottid numbers of worms whenever a great number of specimens are examined. Therefore, it is highly recommended that these two characteristics should be used with much more caution in cases where specimens available for describing new cestode species are limited in number. A similar comprehensive study on marine species of Rhinebothrium is required to verify whether or not marine species of Rhinebothrium follow the same patterns.
Materials and methods
A total of 40 G. granulatus were collected from northern waters of the Gulf of Oman in five collecting trips during 2010–2012. Specimens were collected with the help of local fishermen from Jod village (25°27'1.6”N, 59°30'30.3”E) and of fishing vessels from Ferdous and Kavian (25°15'–25°17'N, 59°02'–59°24'E). After recording biometric characteristics for subsequent identification, fish were dissected and spiral intestines were opened through a longitudinal incision. Opened intestines were fixed in 4% neutral buffered formalin and shaken rigorously to ensure complete separation of scolices from intestine and penetration of the fixative into the tissues. Intestines were transferred to the Laboratory of Zoology, Department of Animal Biology, University of Tehran for detailed examination. Host identification followed Randall (Reference Randall1995). Data for infected hosts are available under the collection code MM at the website http://tapewormdb.uconn.edu/index.php/hosts/specimen_search/elasmobranch.
Worms that were still attached to intestine, if any, were separated from tissue under a stereoscope and stored in 70% ethanol (EtOH) for later examination. Specimens for study by light microscopy were prepared according to Healy (Reference Healy2006b): hydrated in 35% EtOH, dH2O; stained in Delafield's haematoxylin; differentiated in tap water; dehydrated in 35% EtOH and then 70% EtOH; destained in acidic 70% EtOH; neutralized in basic 70% EtOH; dehydrated in 100% EtOH; cleared in methyl salicylate and mounted in Canada balsam on glass slides. Specimens used in scanning electron microscopy (SEM) were also prepared according to Healy (Reference Healy2006b): hydrated in 35% EtOH to dH2O alcoholic series, incubated overnight in 1% osmium tetroxide (Merck, Darmstadt, Germany), dehydrated in a graded ethanol series, dried using hexamethyldisilizane (Merck), mounted on aluminium stubs covered with double-sided carbon tape, and sputter coated with 8–10 nm of gold. Scanning electron micrographs were taken with a field emission scanning election microscope (Hitachi, HIT4160102, Tokyo, Japan) at the School of Electrical and Computer Engineering (ECE), University of Tehran. Prepared slides of appropriate quality were measured and studied using Leica Application Suite V.3 software mounted on a Leica DM500 microscope (Buffalo Grove, Illinois, USA) equipped with Leica ICC50 HD built-in camera. Species drawings were prepared using a drawing tube.
All measurements are in micrometres unless otherwise indicated. Measurements of all genitalia were made on the terminal proglottid, except in those specimens possessing a terminal proglottid with atrophied testes and expanded vas deferens, in which cases testes were measured on the mature subterminal proglottid. For each measurement, the range is provided followed by mean and standard error; number of specimens measured (N) and total number of measurements (n) are presented in parentheses. Chervy (Reference Chervy2009) was followed for microthrix terminology. Most original descriptions of the species of Rhinebothrium were taken from the Global Cestodes Database at www.tapewormdb.uconn.edu (Caira et al., 2012). Museum abbreviation: ZUTC, Collection of the Zoological Museum, University of Tehran, Tehran, Iran.
Rhinebothrium kruppi sp. n.
Taxonomic summary
-
Order: Rhinebothriidea Healy, Caira, Jensen, Webster and Littlewood, Reference Healy, Caira, Jensen, Webster and Littlewood2009
-
Family: Rhinebothriidae Euzet, Reference Euzet1953
-
Genus: Rhinebothrium Linton, 1890
-
Type host. Glaucostegus granulatus (Cuvier), granulated guitarfish (Rajiformes: Rhinobatidae) (host code: MM1046).
-
Type locality. Off Jod, Iran, Gulf of Oman (25°45.8'90”N, 59°51.5'57”E).
-
Prevalence. 2.5% (1 of 40 individuals examined).
-
Intensity. Five specimens in a single infected host.
-
Type-material. Holotype (ZUTC Platy. 1471), three paratypes (ZUTC Platy. 1472–ZUTC Platy. 1474), one SEM voucher (ZUTC Platy. 1475).
-
Etymology. This species is named after Dr Friedhelm Krupp for his longstanding contribution to the biodiversity in the Middle East.
Description
Based on whole mounts of four mature worms and one scolex prepared for SEM and its voucher, partially measured.
Worms (fig. 1A) euapolytic, slightly craspedote proglottids; length 1.56–2.44 mm (1.89 ± 0.15 mm; N= 5), maximum width 561–1032 (813 ± 92; N= 5) at level of scolex, with 12–17 (14.4 ± 1.12; N= 5) proglottids. Scolex (figs 1B, 2A) consisting of scolex proper bearing four stalked bothridia. Bothridia hinged, slightly constricted at centre, with muscular rims (fig. 1B), 453–591 (526 ± 10.2; N= 5; n= 15) long, 104–191 (138 ± 6.21; N= 5; n= 15) wide, divided by 20–22 (21 ± 0.3; N= 5; n= 12) transverse septa and one medial longitudinal septum into 42–46 (44 ± 0.6; N= 5; n= 12) transversely orientated loculi; anterior and posterior halves of each bothridium approximately equal in width. Medial longitudinal septum conspicuous, extending from posterior margin of anteriormost loculus to anterior margin of the posteriormost loculus of bothridium. Anteriormost loculus single, 27.9–38.3 (32.9 ± 1.69; N= 5) long, 29.4–44.6 (35.8 ± 2.94; N= 5) wide; widest loculus 21.3–33.2 (27.1 ± 1.35; N= 5; n= 10) long, 58.7–93.6 (72.2 ± 3.77; N= 5; n= 10) wide, located in the middle of each half of bothridium; posteriormost loculus single, 27.5–37.4 (32.3 ± 1.8; N= 5) long, 22.9–36 (30.2 ± 2.32; N= 5) wide. Stalk 179–357 (240 ± 14.3; N= 5; n= 11) long, 24.3–47.8 (38.5 ± 2.05; N= 5; n= 11) wide, attached to middle of bothridium. Cephalic peduncle 65.2–103 (88.9 ± 8.2; N= 4) long, 51.2–73.4 (62.6 ± 3.62; N= 4) wide (fig. 1A).

Fig. 1 Line drawings of Rhinebothrium kruppi sp. n. (A) Whole worm, voucher; (B) single bothridium of the holotype; (C) subterminal mature proglottid of the holotype; (D) terminal mature proglottid of holotype with expanded vas deferens and atrophied testes. Abbreviations: c, cirrus; ds, distal surface of bothridium; gp, genital pore; ls, longitudinal septum; mg, Mehlis' gland; o, ovary; ps, proximal surface of bothridium; sr, seminal receptacle; t, testes; u, uterus; v, vitellaria; va, vagina; vd, vas deferens. Scale bars: A = 200 μm; B–D = 50 μm.
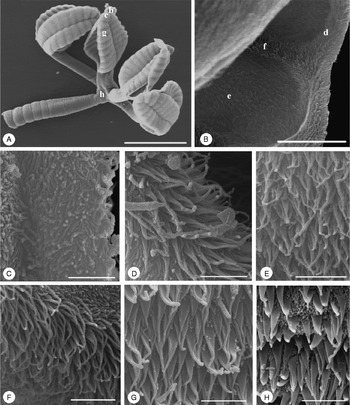
Fig. 2 Scanning electron micrographs of Rhinebothrium kruppi sp. n. (A) Scolex (letters indicate the locations of details shown in B, C, G, H); (B) distal surface of bothridium (letters indicate the location of details shown in D–F); (C) proximal bothridial surface near bothridial rim; (D) distal bothridial surface between middle of loculi and rim; (E) distal bothridial surface in the middle of loculi; (F) transverse septum on distal bothridial surface; (G) proximal bothridial surface away from bothridial rim; (H) cephalic peduncle surface. Scale bars: A = 200 μm; B = 12 μm; C–H = 2 μm.
Entire proximal surface of bothridia and stalks covered with densely arranged aristate gladiate spinitriches and few capilliform filitriches (fig. 2G), bothridial rim with densely arranged gladiate spinitriches and few capilliform filitriches (fig. 2C) and a margin of capilliform filitriches (fig. 2B–C); space between bothridial rim and surface of the middle portions of loculi with densely arranged aristate gladiate spinitriches and scattered capilliform filitriches (fig. 2D). Distal surfaces of bothridia on transverse septa and on the middle portions of loculi with only densely arranged aristate gladiate spinitriches; spaces between septa and middle portions of loculi with only a band of densely arranged short capilliform filitriches (fig. 2B, E–F); cephalic peduncle with aristate gladiate spinitriches and densely arranged capilliform filitriches (fig. 2H); strobila with densely arranged capilliform filitriches.
Strobila: greatest proglottid width 97.2–114 (105 ± 3.49; N= 5) at subterminal mature proglottid (figs 1A, C). Majority of proglottids wider than long; terminal proglottids 4–8 (5.4 ± 0.7; N= 5) in number, longer than wide (fig. 1A); mature proglottids 2–3 (2.2 ± 0.2; N= 5) in number, including one posteriormost proglottid with atrophied testes and vas deferens filled with sperm. No gravid proglottids observed (fig. 1C).
Terminal proglottid (fig. 1D): 339–438 (395 ± 21.2; N= 5) long, 79.3–98.8 (91.2 ± 3.53; N= 5) wide, length to width ratio 3.46–4.92 (4.35 ± 0.25; N= 5). Genital pores muscular, lateral, irregularly alternating, 61.1–76.9% (69.4 ± 2.74; N= 5) of proglottid length from posterior end. Testes in mature proglottids irregularly oval in dorsal view (fig. 1C), 17.1–30.2 (25.1 ± 1; N= 5; n= 18) long, 31.3–51.4 (37.5 ± 1.45; N= 5; n= 18) wide, all in primary field, 4–5 (4.6 ± 0.22; N= 5) in total number, in two irregular columns, extending from near the anterior margin of proglottid to level of genital pore. Vas deferens in terminal proglottids highly expanded, coiled, spanning from level of anteriormost testes and vitelline follicles to near ovarian isthmus, entering cirrus-sac at anterior margin. Cirrus-sac elongated, oval to pyriform, bent posteriorly (fig. 1C, D), extending medially well past midline of proglottid, posteriorly to anterior ovarian margins, containing coiled cirrus. Cirrus-sac in terminal proglottids 30.7–219 (85.5 ± 44.8; N= 4) wide, 59–289 (119 ± 56.7; N= 4) long. Cirrus 10.7–57.4 (23.3 ± 11.4; N= 4) wide, with enlarged proximal base bearing conspicuous spinitriches. Vagina thin-walled in mature and thick-walled in terminal proglottids, sinuous, varying in width along its length, with darkly staining cells, extending laterally from common genital atrium, then posteriorly along medial line of proglottid to ootype, highly coiled posterior to cirrus-sac, not crossing but slightly overlapping cirrus-sac (fig. 1C, D). Proximal portion of vagina slightly expanded. Vaginal sphincter absent. Ovary throughout posterior half of proglottid, lobulated, H-shaped in dorsoventral view (fig. 1C, D), symmetrical, 155–230 (193 ± 12; N= 5) long, maximum width 52.9–73.6 (60.1 ± 4.61; N= 5), occupying 44.7–54.5% (49 ± 2.09; n= 5) of proglottid length; ovarian isthmus located near or slightly anterior to mid-point of ovary. Anterior margin of ovary at 36.6–49.8 (44.5 ± 2.87; N= 4) from genital pore. Mehlis' gland at the level of ovarian isthmus; seminal receptacle 29.3–34.8 (32.2 ± 1.81; N= 4) long, 13–21.8 (16.7 ± 1.91; N= 4) wide. Vitellarium follicular, follicles with irregular shapes 5.1–15 (9.4 ± 0.791; N= 5; n= 18) long, 7.31–17.8 (11 ± 0.63; N= 5; n= 18) wide, in one dorsal and one ventral column on each side of proglottid, extending from nearly anterior to posterior margin of proglottid, posterior to ovary and uterus, uninterrupted by cirrus-sac, vagina or ovary, and even at the level of genital atrium. Uterus medial, extending from posteriormost row of testes to posterior level of ovarian lobes (fig. 1C, D). Free gravid proglottids and eggs not observed.
Remarks
In comparison to 34 valid marine species of Rhinebothrium (seven are freshwater species), Rhinebothrium kruppi sp. n. is categorized among species with few testes (4–5), and is easily distinguishable from 19 other species of Rhinebothrium with 10 or more testes included in table 1. However, 15 other species of Rhinebothrium bear fewer than 10 testes. Among them, Rhinebothrium kruppi sp. n. possesses more testes than R. asymmetrovarium Dailey & Carvajal, 1976, R. biorchidum Huber & Schmidt, 1985, R. ditesticulum Appy & Dailey, 1977, R. spinicephalum Campbell, 1970, and R. tetralobatum Brooks, 1977 (4–5 vs. only 2 in all the above five species).
Ten other species of Rhinebothrium with 10 or fewer testes include: R. chollaensis Friggens & Duszynski, 2005 (4), R. gravidum Friggens & Duszynski, 2005 (8–10), R. lintoni Campbell, 1970 (5–8), R. maccallumi Linton, 1924 (4–5), R. margaritense Mayes & Brooks, 1981 (3–6), R. oligotesticularis (4–7), R. rhinobati (Yamaguti, Reference Yamaguti1960) Healy, Reference Healy2006 (7–9), R. taeniuri Ramadan, 1984 (4–8), R. urobatidium (Young, 1955) Appy & Dailey, 1977 (6–12) and R. walga (Shipley & Hornell, Reference Shipley and Hornell1906) Euzet, Reference Euzet1953 (4–6). The possession of fewer bothridial loculi (42–46) distinguishes R. kruppi sp. n. from R. lintoni (54–56) and R. margaritense (53–55), whereas this new species possesses more bothridial loculi than R. maccallumi (33) and R. taeniuri (18–22). Furthermore, R. kruppi sp. n. seems to be euapolytic, whereas R. chollaensis and R. gravidum are apolytic. Rhinebothrium kruppi sp. n. can be distinguished from R. urobatidium in bearing genital pores located at the anterior rather than posterior half of the mature proglottid. Apparently shorter size and fewer proglottid and testes numbers distinguish R. kruppi sp. n. from R. rhinobati (1.56–2.44 mm vs. 6.35 mm, 12–17 vs. 29 and 4–5 vs. 7–9, respectively).
Rhinebothrium kruppi sp. n. closely resembles R. oligotesticularis and R. walga. However, R. kruppi sp. n. is conspicuously smaller and possesses fewer proglottid numbers than R. oligotesticularis (1.56–2.44 mm vs. 14.5 mm and 12–17 vs. 66, respectively). It is noteworthy that, according to Subramaniam (Reference Subramaniam1940), most R. oligotesticularis worms were immature, while, on the other hand, specimens of R. kruppi sp. n. with the above-mentioned details were completely mature, and hence the size difference might be more significant when mature worms are compared. In addition, it seems that in mature proglottids of R. oligotesticularis, vitelline follicles are interrupted by terminal genitalia, whereas, in R. kruppi sp. n., vitelline follicles are extended throughout the proglottid without any interruption, even at the level of terminal genitalia.
The specimens of R. walga redescribed by Subhapradha (Reference Subhapradha1955) as Echeneibothrium walga Shipley and Hornell, Reference Shipley and Hornell1906 are different in comparison to those used in the original description of this species by Euzet (Reference Euzet1953), and will be discussed later. Nonetheless, Euzet (Reference Euzet1953) transferred this species to Rhinebothrium and redescribed it based on specimens collected from Dasyatis pastinaca (Linnaeus) in Concarneau, France; Alexandrette, off Syria; and Agigea, Romania. This redescription is very similar to R. kruppi sp. n., in possession of 40 loculi and 4–6 testes. However, these two species are still distinguishable based on ovarian morphology. The specimens of R. walga possess an ovary with an apparently posterior ovarian isthmus, resulting in conspicuously longer anterior lobes than posterior lobes (Euzet, Reference Euzet1953) whereas R. kruppi sp. n. possesses an ovary with a median isthmus and hence four approximately equal lobes (H-shaped) (fig. 1C, D). Furthermore, R. walga, as redescribed by Euzet (Reference Euzet1953) from the Mediterranean, possesses a relatively longer cephalic peduncle (200 vs. 65–103 μm in R. kruppi sp. n.). Also, according to the illustrations of Euzet (Reference Euzet1953), in R. walga the vas deferens expands from testes field to the cirrus-sac, not extending posterior to it, whereas in R. kruppi sp. n. the vas deferens extends apparently posterior to the cirrus-sac, near to the seminal receptacle (fig. 1C, D).
Rhinebothrium persicum sp. n.
Taxonomic summary
-
Order: Rhinebothriidea Healy, Caira, Jensen, Webster and Littlewood, Reference Healy, Caira, Jensen, Webster and Littlewood2009
-
Family: Rhinebothriidae Euzet, Reference Euzet1953
-
Genus: Rhinebothrium Linton, 1890
-
Type host. Glaucostegus granulatus (Cuvier), granulated guitarfish (Rajiformes: Rhinobatidae) (host code: MM696, MM700, MM761 (Type)).
-
Type locality. Off Bandar Jask, Iran, Gulf of Oman (25°17'N, 59°24'E).
-
Prevalence. 7.5% (3 of 40 individuals examined).
-
Intensity. 1–9 (4.67 ± 2.33) worms per host.
-
Type material. Holotype (ZUTC Platy. 1476), 11 paratypes (ZUTC Platy. 1477–ZUTC Platy. 1487), two SEM vouchers (ZUTC Platy. 1488–ZUTC Platy. 1489).
-
Etymology. This species is named after its type locality, Persia, the former name of Iran.
Description
Based on whole mounts of 12 mature worms and two scoleces prepared for SEM and their vouchers, partially measured.
Worms (fig. 3A) euapolytic, slightly craspedote proglottids, 9.04–15.55 mm (11.86 ± 0.65 mm; N= 12) long, with maximum width 505–1.714 (927 ± 81.1; N= 14) at level of scolex; 55–80 (61.4 ± 2.02; N= 13) proglottids per worm. Scolex (figs 3A, 4A) consists of scolex proper bearing four stalked bothridia. Bothridia fusiform with a distinct constriction at centre or slightly posterior, with muscular rims (figs 3C, 4E), 562–1.237 (900 ± 33.1; N= 13; n= 28) long, 125–342 (249 ± 10.7; N= 12; n= 22) wide, divided by 28–30 (29.1 ± 0.4; N= 6; n= 7) transverse septa and 1 medial longitudinal septum (fig. 3C) into 58–62 (60.7 ± 0.84; N= 6; n= 7) transversely orientated loculi; bothridial rims apparently twisted toward distal surface of bothridia cover longitudinal septum in most cases, anterior and posterior halves of each bothridium approximately equal in width. Medial longitudinal septum extending from posterior margin of anteriormost loculus to anterior margin of the posteriormost loculus of bothridium, difficult to see in most as a result of inward curling of bothridial rim (fig. 3C). Anteriormost loculus single, covered by bothridial rims, difficult to measure; widest loculus 24.5–74.1 (46 ± 3.29; N= 11; n= 17) long, 74.6–136 (111 ± 4.58; N= 11; n= 17) wide, located in the middle of bothridium; posteriormost loculus single, 25.3–30.2 (27.6 ± 1.25; N= 3; n= 4) long, 34.7–45.8 (40.3 ± 3.11; N= 3; n= 4) wide. Stalk 79.9–233 (153 ± 10.8; N= 10; n= 20) long, 99–194 (140 ± 5.27; N= 10; n= 20) wide, attached to bothridium at middle or slightly posterior to middle of bothridium. Cephalic peduncle 80–291 (171 ± 22.3; N= 11) long.
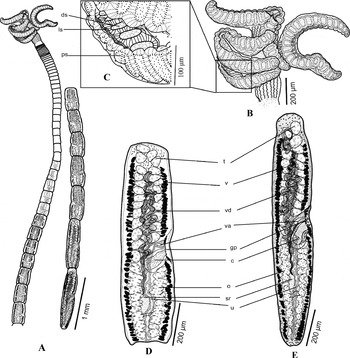
Fig. 3 Line drawings of Rhinebothrium persicum sp. n. (A) Whole worm, voucher; (B) scolex of the holotype; (C) distal surface of bothridium with distinct longitudinal septum; (D) subterminal mature proglottid of the holotype with testes; (E) terminal mature proglottid of the holotype with expanded vas deferens (testes are either present or atrophied). Abbreviations: c, cirrus; ds, distal surface of bothridium; gp, genital pore; ls, longitudinal septum; o, ovary; ps, proximal surface of bothridium; sr, seminal receptacle; t, testes; u, uterus; v, vitellaria; va, vagina; vd, vas deferens. Scale bars: A = 1 mm; B, D, E = 200 μm; C = 100 μm.
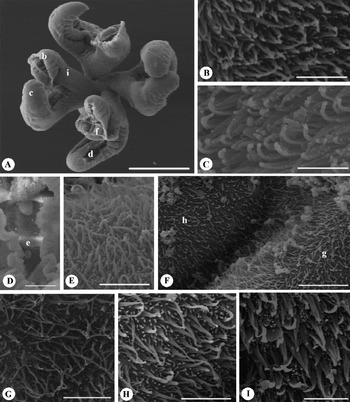
Fig. 4 Scanning electron micrographs of Rhinebothrium persicum sp. n. (A) Scolex (letters indicate the locations of details shown in B, C, D, F, I); (B) proximal bothridial surface near bothridial rim; (C) proximal bothridial surface away from bothridial rim; (D) distal surface of bothridial surface (letter indicates the location of details shown in E); (E) transverse septum on distal bothridial surface; (F) distal bothridial surface at the location of longitudinal septum (letters indicate location of details shown in G and H); (G) distal bothridial surface in the middle of loculi; (H) longitudinal septum on distal bothridial surface; (I) stalk surface. Scale bars: A = 200 μm; B, C, E, G–I = 2 μm; D = 20 μm; F = 6 μm.
Entire proximal surface of bothridia away from bothridial rim with only densely arranged gladiate spinitriches (fig. 4C), and with both densely arranged gladiate spinitriches and acicular filitriches near bothridial rim (fig. 4B); stalks covered with densely arranged gladiate spinitriches and capilliform filitriches (fig. 4I). Distal surfaces of bothridia on transverse (fig. 4D, E) and longitudinal septa (fig. 4F, H) with apparently gladiate spinitriches and scattered acicular filitriches; distal surfaces of bothridia on middle portions of loculi with apparently aristate gladiate spinitriches and scattered acicular filitriches (figs 4F, G).
Strobila: greatest proglottid width 187–307 (247 ± 14.2; N= 8) at subterminal mature proglottid. Majority of proglottids wider than long; terminal proglottids 15–41 (25 ± 2.24; N= 11) in number, longer than wide (fig. 3A); mature proglottids (fig. 3D) 8–16 (11.2 ± 0.76; N= 12) in number, including 0–2 (0.71 ± 0.19; N= 14) terminal proglottids with atrophied testes and vas deferens filled with sperm. No gravid proglottids observed.
Terminal proglottid (fig. 3E): 687–1,201 (769 ± 38.5; N= 13) long, 207–287 (245 ± 7.99; N= 13) wide, length to width ratio 2.4–5.36 (3.31 ± 0.22; N= 13). Genital pores marginal, irregularly alternating, small tubercles on tegument surrounding genital pore, 47.2–63.3% (55.9 ± 1.28; N= 13) of proglottid length from posterior end. Testes in mature terminal or subterminal proglottids irregularly oval in dorsal view (fig. 3D), 19.1–52.2 (29.4 ± 0.93; N= 14; n= 66) long, 23.9–67.7 (43 ± 1.29; N= 14; n= 66) wide, all in primary field, 20–27 (23.4 ± 0.59; N= 14) in total number, in two irregular columns in terminal, and 3–4 irregular columns in anterior, mature proglottids, extending from near anterior margin of proglottid to level of genital pore. Vas deferens in terminal proglottids coiled, spanning from level of anteriormost testes to level of ovarian anterior margins, entering cirrus-sac at anterior margin. Cirrus-sac short, oval or cylindrical, bent posteriorly, slender in subterminal mature proglottids (fig. 3D, E), not extending beyond midline of proglottid, extending posteriorly to anterior ovarian margin with some degree of overlapping, containing coiled cirrus. Cirrus-sac in terminal proglottids 47.3–98.9 (77.2 ± 3.78; N= 13) wide, 110–155 (127 ± 4.02; N= 13) long. Cirrus 32–51.1 (42.2 ± 1.54; N= 13) wide, proximal portion of cirrus enlarged approximately as wide as cirrus-sac basal width, bearing conspicuous spinitriches. Vagina thick-walled, approximately straight, varying in width along its length, with darkly staining cells, extending laterally from common genital atrium, then posteriorly along medial line of proglottid to ootype, not crossing or overlapping with cirrus-sac (fig. 3D, E). Proximal portion of vagina expanded. Vaginal sphincter absent. Ovary, throughout posterior half of proglottid, follicular with conspicuously large follicles on each side, H-shaped in dorsoventral view, symmetrical, 275–506 (345 ± 17.6; N= 13) long, with maximum width 129–217 (165 ± 7.43; N= 13), occupying 39.8–48.4% (43.3 ± 0.78; n= 13) of proglottid length; ovarian isthmus located in posterior half of ovary. Anterior margin of ovary at 43.9–96.6 (64.1 ± 3.79; N= 12) from genital pore. Mehlis' gland anterior to ovarian isthmus at level of seminal receptacle; seminal receptacle 70.4–135 (100 ± 5.87; N= 12) long, 34.5–62.9 (45.2 ± 2.17; N= 12) wide. Vitellarium follicular, follicles 6.86–34.6 (15.1 ± 0.69; N= 14; n= 61) long, 12.7–53.8 (27.2 ± 1.08; N= 14; n= 61) wide, in one dorsal and one ventral column on each side of proglottid, extending anteriorly with a distance from proglottid margin at level of anteriormost second or third row of testes, posteriorly past ovary and uterine margins, uninterrupted by cirrus-sac, vagina or ovary, interrupted at the level of genital atrium. Uterus medial, sacciform, extending from posterior margin of proglottid to near anterior margin of proglottid approximately alongside extension of vas deferens (fig. 3D, E). Free gravid proglottids and eggs not observed.
Remarks
The two new species described in the present study exhibit a number of significant morphological differences. Apart from differences in terms of the bothridial shape, specimens of R. kruppi sp. n. and R. persicum sp. n. are completely distinguishable based on their total length (1.56–2.44 vs. 9.04–15.55 mm), the number of proglottids (12–17 vs. 55–80), bothridial loculi (42–46 vs. 58–62) and testes (4–5 vs. 20–27). The extension of the cirrus-sac past the proglottid midline in R. kruppi sp. n., the lobulated (in R. kruppi sp. n.) rather than follicular ovary (in R. persicum sp. n.) and the uninterrupted extension of the vitelline follicles in R. kruppi sp. n. are among other significant features to differentiate these two new species.
Another main difference between these two species concerns scolex microthrix patterns. Not only do the two species bear different types of spinitriches and filitriches in the corresponding areas of scolices, but patterns are also different. In general, both R. kruppi sp. n. and R. persicum sp. n. possess gladiate spinitriches and acicular and capilliform filitriches. Regarding distribution patterns, in R. kruppi sp. n. distal surfaces are mainly covered by discontinuous bands of densely arranged spinitriches and filitriches, i.e. the septa and middle portions of loculi are covered merely with gladiate spinitriches, whereas a band of only densely arranged short capilliform filitriches separates septa from middle portions of loculi (fig. 2D, E, G). However, in R. persicum sp. n. the gladiate spinitriches are not as densely arranged as in R. kruppi sp. n., and in most cases, both scattered spinitriches and filitriches with approximately even densities are visible in the same parts (fig. 4D–I) and no discontinuous band of spinitriches and filitriches is distinguishable on the distal surface of R. persicum sp. n.
Rhinebothrium persicum n. sp. is similar to, and even overlaps with, the type species R. flexile in most importance characteristics, including total length (9.04–15.55 mm vs. 7.5–16 mm), proglottid number (55–80 vs. 21–50), bothridial loculi number (58–62 vs. 50–54) and testes number (20–27 vs. 14–20) (Healy, Reference Healy2006a). Hinged rather than fusiform bothridia, extension of the cirrus-sac past the proglottid midline rather than being limited to the aporal side, bearing rather than lacking an external seminal vesicle, and vas deferens extension from level of anterior lobes of ovary to posteriormost rather than anteriormost row of testes are among the features that help in distinguishing R. flexile from this new species. In addition, apart from the bothridial and proglottid differences discussed above, these two species are different with regards to microthrix patterns. It seems that both species are similar in terms of possession of gladiate spinitriches and acicular filitriches on the proximal surfaces; however, microthrix patterns on the distal surface are different. In R. persicum n. sp. an approximately invariable pattern of relatively scattered gladiate spinitriches and acicular filitriches appears throughout the bothridial distal surface. However, in R. flexile, surfaces of inner loculi bear only acicular filitriches, while the septa are merely covered with gladiate spinitriches (as presented by Healy, Reference Healy2006a).
In addition to R. persicum n. sp., seven other Rhinebothrium species bear 20–30 testes: R. burgeri Baer, 1948 (30–35), R. corymbum Campbell, 1975 (18–34), R. devaneyi Brooks & Deardoff, 1988 (30–43), R. himanturi Williams, 1964 (19–20), R. pearsoni Butler, 1987 (20–33), R. scobinae Euzet & Carvajal, 1973 (18–24) and R. setiensis Euzet, Reference Euzet1955 (27–34). Rhinebothrium persicum n. sp. possesses more bothridial loculi than R. burgeri, R. pearsoni, R. scobinae and R. setiensis (58–62 vs. 48–50, 20–33, 19 and 47, respectively), while it bears fewer bothridial loculi than R. devaneyi (58–62 vs. 94–152). Rhinebothrium persicum n. sp. and R. corymbum are very similar in terms of all main features, including total size, proglottid, bothridial loculi and testes numbers; however, R. persicum n. sp. is euapolytic rather than apolytic, lacks a seminal vesicle, and its vas deferens extend to the anteriormost rather than posteriormost row of testes. Another similar species is R. himanturi, with an incomplete original description; however, possession of the typical hinged bothridia of Rhinebothrium (vs. entwined fusiform in R. persicum sp. n.), along with a conspicuously posterior rather than median to anterior genital pore, help in distinguishing it from R. persicum n. sp.
Before the present study, apart from misidentified specimens of R. flexile Linton, 1890, another species R. oligotesticularis (both described as Echeneibothrium) has been reported from G. granulatus in Indian waters by Subramaniam (Reference Subramaniam1940) and Subhapradha (Reference Subhapradha1955), respectively. Rhinebothrium flexile is the type species, and has been reported from Dasyatis centroura (Linton) from off Wood's Hole, Massachusetts, USA. However, there are some previous reports of this species from other localities, by Yamaguti (Reference Yamaguti1960) (as E. flexile from off Japan), Subhapradha (Reference Subhapradha1955) (as E. flexile from the Indian Ocean) and Williams (Reference Williams1958) (from off Sri Lanka). But all these reports are misidentifications and belong to different species (see Healy, Reference Healy2006a).
Bearing in mind the relatively unfamiliar shape of bothridia (entwined fusiform) in R. persicum n. sp., this species resembles another species previously misidentified from G. granulatus in Indian waters. Subhapradha (Reference Subhapradha1955) described Echeneibothrium flexile (mentioned as a synonym of R. longicolle) from both rays Rhynchobatus djiddensis (Forsskål, 1775) and G. granulatus. Description of this species in most respects corresponds with R. persicum n. sp., including: significant width of stalk at the place of attachment to bothridia, inward curling of bothridial rim, testes number (20–27 vs. 20–32), cirrus-sac not extending past proglottid midline, and follicular ovary. However, Subhapradha (Reference Subhapradha1955) mentioned and emphasized that careful examination of bothridia revealed that it lacks a longitudinal septum, the main characteristic of Rhinebothrium, so she included it in Echeneibothrium as E. flexile. The differentiation of R. persicum n. sp. and dissimilarity of the species described by Subhapradha (Reference Subhapradha1955) is reconfirmed here, as a longitudinal septum is partly visible in most specimens of R. persicum n. sp. (figs 3B, 4A) and, also, a few other specimens lacking the longitudinal septum and corresponding to E. flexile were collected from G. granulatus, and will be published in future as a species of another rhinebothriidean genus, Scalithrium Ball, Neifar & Euzet (Reference Ball, Neifar and Euzet2003).
The other species of Rhinebothrium reported from G. granulatus so far, R. oligotesticularis, was discussed earlier as being different from R. kruppi sp. n. However, regarding R. persicum sp. n., it may be distinguished easily from R. oligotesticularis based on the greater number of testes (20–27 vs. 4–7). In addition, the specimens of E. flexile described by Subhapradha (Reference Subhapradha1955) lacked a longitudinal septum, and therefore do not belong to Rhinebothrium and are different from not only R. kruppi sp. n. and R. persicum sp. n., but also from any other species of Rhinebothrium. Subhapradha (Reference Subhapradha1955) has also reported R. walga (as Echeneibothrium walga) from Trygon walga ( = Himantura walga [Müller & Henle]) in Indian waters, but her description differs from the original description of R. walga provided by Shipley & Hornell (Reference Shipley and Hornell1906) and reviewed by Euzet (Reference Euzet1953). These specimens were reported as being up to 7 mm long, with 38–42 proglottids, 34–42 bothridial loculi and 11–16 testes, and therefore can be easily distinguished from both R. kruppi sp. n. and R. persicum sp. n. reported from G. granulatus in the Gulf of Oman.
Discussion
Euzet (Reference Euzet1953) placed Rhinebothrium within the subfamily Rhinebothriinae Euzet, Reference Euzet1953. According to Euzet (Reference Euzet, Khalil, Jones and Bray1994) the members of this subfamily possess a four-pedunculated bothridia, and the surface of the bothridium is divided by one longitudinal and several transverse muscular septa into two columns of loculi. After a detailed phylogenetic study, Healy et al. (Reference Healy, Caira, Jensen, Webster and Littlewood2009) established the new Order Rhinebothriidea to include members previously described within the subfamily Rhinebothriinae and some other taxa with similar characteristics. This designation has been confirmed recently by Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014). However, there are significant inconsistencies at the generic and specific levels. In the most recent study conducted on the members of the order Rhinebothriidea, Ruhnke et al. (Reference Ruhnke, Caira and Cox2015) have confirmed four families for this order, including Rhinebothriidae Euzet, Reference Euzet1953, Echeneibothriidae de Beauchamp, 1905, Anthocephaliidae Ruhnke, Caira and Cox, Reference Ruhnke, Caira and Cox2015, and Escherbothriidae Ruhnke, Caira and Cox, Reference Ruhnke, Caira and Cox2015. The family Rhinebothriidae is designated to include members of the genus Rhinebothrium, along with the genera Rhabdotobothrium, Rhinebothroides, Rhodobothrium, Scalithrium and Spongiobothrium.
Healy (Reference Healy2006a) believed that among the different genera belonging to this order, Rhinebothrium is the most speciose genus, with more than 36 valid species, and several other species to be identified and described throughout the world in future studies. Subsequently, five other new valid species, i.e. R. copianullum Reyda, 2008, R. corbatai Menoret and Ivanov, Reference Menoret and Ivanov2011, R. mistyae Menoret and Ivanov, Reference Menoret and Ivanov2011, R. brooksi Reyda and Marques, Reference Reyda and Marques2011 and R. fulbrighti Reyda and Marques, Reference Reyda and Marques2011 have been included in this genus. Reyda & Marques (Reference Reyda and Marques2011) have amended the diagnosis of the genus to include a new feature, defined as marginal longitudinal septa as observed in the freshwater species R. copianullum and R. brooksi. The present study increases the number of valid species of Rhinebothrium to 43.
The two new species of Rhinebothrium described above are reported from G. granulatus. Several other batoid species examined during this project were not infected with these cestodes; these included Aetobatus flagellum (Bloch and Schneider) (N= 13), Aetobatus narinari (Euphrasen) (N= 2), Aetomylaeus nichofii (Bloch and Schneider) (N= 12), Aetomylaeus maculatus (Gray) (N= 3), Aetomylaeus sp. (N= 4), Glaucostegus halavi (Forsskål) (N= 4), Glaucostegus typus (Bennett) (N= 2), Gymnura cf. poecilura (Shaw) (N= 110), Himantura cf. gerrardi (N= 150), Himantura imbricata (Bloch and Schneider) (N= 60), Himantura uarnak (Gmelin) (N= 3), Pastinachus cf. sephen (Forsskål) (N= 56), Rhina ancylostoma Bloch and Schneider (N= 1), Rhinoptera javanica Müller and Henle (N= 34), Rhynchobatus djiddensis (Forsskål) (N= 8) and Torpedo spp. (N= 53). Most rhinebothriideans seem to have strict (oioxenous) host specificity. Of the 36 described valid species plus the 11 new unpublished species in the phylogenetic study of Healy (Reference Healy2006a) and the five recently described species reported from freshwater stingrays, only one marine species, R. margaritense Mayes & Brooks, 1981 was reported from two hosts, and the other marine species are 100% host specific (table 1); however, six freshwater species inhabited more than one host. These two new species are found to be 100% host specific.
The order Rhinebothriidea is a recently erected taxon and many regions and hosts have not been examined yet for the presence of these cestodes. Studies conducted by Healy (Reference Healy2006a) and Healy et al. (Reference Healy, Caira, Jensen, Webster and Littlewood2009) were mainly focused on the Gulf of Mexico, Gulf of California, Indo-Pacific waters of Malaysian Borneo and northern Australia, with some few cases from Senegal, France, India and Venezuela (table 1). However, in total, 13 cestode species have been reported from G. granulatus so far, including Acanthobothrium satyanarayanaraoi Sarada, Lakshmi and Rao, 1993 (Fayler and Caira, 2006); Anthobothrium sasoonense Srivastav and Srivastava, 1988; Echinobothrium rhynchobati (Khalil and Abdul-Salam, 1989) Tyler, 2006; Marsupiobothrium rhinobati Shinde and Deshmukh, 1980 (considered by Ruhnke (Reference Ruhnke2010) as species incertae sedis); Orectolobicestus chiloscyllii Subhapradha, Reference Subhapradha1955 (invalid according to Ruhnke (Reference Ruhnke2010)); Polypocephalus affinis Subhapradha, 1951; P. radiatus Braun, 1878; P. rhinobatidis Subhapradha, 1951; Scalithrium filamentosum (Subhapradha, Reference Subhapradha1955) Healy, Reference Healy2006; Tylocephalum rhinobatii (Deshmukh, 1980) Jensen, 2005; and three species of Rhinebothrium, including R. oligotesticularis, R. flexile and R. walga. At the moment, only R. oligotesticularis and the two current new species are considered to be valid species of Rhinebothrium from G. granulatus. Specimens reported as E. flexile probably belong to Scalithrium, and specimens described as R. walga have been proved to be inconsistent with the original description of the taxon, therefore need to be redescribed as a new valid species (Healy, Reference Healy2006a). In total, there are no species described by Subhapradha (Reference Subhapradha1955) or Subramaniam (Reference Subramaniam1940) in adjacent waters (Indian Ocean) which completely correspond to either R. kruppi sp. n. or R. persicum sp. n. This diversity among rhinebothriidean cestodes of G. granulatus reveals that a given ray species may harbour different cestode species at different localities. This has also been reported by Healy (Reference Healy2006a), where she mentioned that a given rhinebothriidean species reported from one locality was not detected in the same host from localities elsewhere.
The Persian Gulf and the Gulf of Oman have been the subject of intensive studies on marine cestodes in recent years by Hassan et al. (Reference Hassan, Palm, Mahmoud and Al Jamie2002), Haseli et al. (Reference Haseli, Malek and Palm2010, Reference Haseli, Malek, Valinasab and Palm2011, Reference Haseli, Malek, Palm and Ivanov2012), Malek et al. (Reference Malek, Caira and Haseli2010), Caira et al. (Reference Caira, Malek and Ruhnke2011), Haseli (Reference Haseli2013), Maleki et al. (Reference Maleki, Malek and Palm2013) and Meraji & Haseli (Reference Meraji and Haseli2014). This has resulted in significant findings and, apart from many as yet undescribed new species, ten new species and a new genus have been described (Caira et al., Reference Caira, Malek and Ruhnke2011). A more comprehensive examination of cestode diversity in these regions would shed more light on our current knowledge, and provide a more realistic picture of the diversity of this taxon throughout the world.
Acknowledgements
We would like to thank Dr Claire Healy for providing valuable references and helpful communications. We also thank Dr Tooraj Valinasab from the Fisheries Research Organization of Iran for supporting the host collection, the Iranian Ministry of Science, Research and Technology, the University of Tehran, the Department of Environment of Iran for their financial support and the Field Emission Electron Microscopy centre at the University of Tehran, School of Electrical and Computer Engineering (ECE). We appreciate invaluable efforts of our colleagues Loghman Maleki and Atabak Roohi Aminjan in collecting host samples. We would like to express our appreciation to local fishermen and people of Jod village, especially Mr Naser Salari, for their help in collecting host samples and for their hospitality.
Financial support
This study has been carried out within the framework of the NSF planetary Biodiversity and Inventory Project (PB and I) awards (Award Nos. 0818696 and 0818823).
Conflict of interest
None.







