Introduction
Members of the cosmopolitan family Clinostomidae Lühe, 1901 are parasites of the buccal cavity, oesophagus or intestine of birds, reptiles and occasionally mammals (Ukoli, Reference Ukoli1966; Kanev et al., Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002). The taxonomic status and phylogenetic relationships among the genera allocated to this family have been uncertain. Currently, the family comprises seven genera in four subfamilies according to Kanev et al. (Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002): Clinostominae Lühe, 1901 with the cosmopolitan genus Clinostomum Leidy, 1856 containing c. 14 species parasitizing fish-eating birds as definitive hosts (see Locke et al., Reference Locke2015; Pérez-Ponce de León et al., Reference Pérez-Ponce de León2016; Caffara et al., Reference Caffara2017), Clinostomatopsis Dollfus, 1932 (two species), and the monotypic Clinostomoides Dollfus, 1959; Euclinostominae Yamaguti, 1958, with Euclinostomun Travassos, 1928 containing eight species parasitizing birds (Caffara et al., Reference Caffara2016); Nephrocephalinae Travassos, 1928, with two genera parasitic in crocodilians, i.e. Odhneriotrema Travassos, 1928 and Nephrocephalus Odhner, 1902, with two and one species, respectively (Woodyard et al., Reference Woodyard, Rosser and Rush2017); and Ithyoclinostominae Yamaguti, 1958, with the monotypic Ithyoclinostomum dimorphum (Diesing, 1850) Witenberg, 1925, as a parasite of birds. Even though great progress has been made in recent years to increase the generic library for species of Clinostomum, the most species-rich genus in the family, in studies aimed at establishing robust species delimitation criteria, few attempts have been made to generate sequence data to perform a phylogenetic analysis at the family level (see Woodyard et al., Reference Woodyard, Rosser and Rush2017).
During survey work on the helminth fauna of Middle American freshwater fishes, unusually large clinostomid metacercariae were found unencysted in the body cavity of some cichlid species in several locations of Mexico and Costa Rica. Based on the size of the metacercariae and the position of the genital complex in the posterior fourth of the body, we first identified the species as Clinostomum heluans Braun, 1899. An additional piece of information was that C. heluans had been previously recorded as a parasite of the great blue heron, Ardea herodias Linnaeus, 1758 in north-eastern Mexico (Bravo-Hollis, Reference Bravo-Hollis1947). In a recent study, Briosio-Aguilar et al. (Reference Briosio-Aguilar2018) characterized molecularly the metacercariae of C. heluans; in addition, these authors established a molecular link between the metacercariae and the adults and determined that the distributional range of the species extends between northern Mexico and Brazil. However, once we obtained ribosomal and mitochondrial DNA sequences of the unusually large clinostomid metacercariae, we discovered that they are not conspecific with C. heluans. The objective of this paper is twofold: to characterize morphologically and molecularly the clinostomid metacercariae found in Middle American cichlids, and to accomplish their identification at genus level, establishing their phylogenetic position within the phylogeny of the family Clinostomidae.
Materials and methods
Specimen collection
Specimens of metacercariae were sampled between 2014 and 2016 in six species of cichlids from six localities, four in Mexico and two in Costa Rica (table 1). In total, 45 individual fish were collected using seine nets and electrofishing, kept alive and transported to the laboratory, pith sacrificed, and examined for parasites under a stereomicroscope. Some specimens were fixed by sudden immersion in hot (near boiling) 4% formalin, subsequently washed in distilled water and stored in 70% ethanol; some specimens were also preserved in vials with 100% ethanol for molecular analysis.
Table 1. Localities sampled across Middle America for Ithyoclinostomum sp., with the host species and the geographical coordinates of each locality, and the data source.

Morphological study
For morphological identification, 29 specimens (14 whole specimens and 15 hologenophores) were stained with Mayer´s paracarmine, dehydrated in a graded ethanol series, cleared with methyl salicylate, and mounted on permanent slides with Canada balsam. Voucher specimens were deposited at the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City. All the specimens were examined using a bright-field Axio Zoom V16 microscope (Zeiss, Oberkochen, Germany). Images were obtained through an Axio Cam Mrc5 attached to the microscope, and specimens were measured using the software ZEN-Zeiss Efficient Navigation; measurements are presented in millimeters, with the range followed by the mean in parentheses. Drawings of the metacercariae were made using a drawing tube attached to the microscope. For the scanning electron microscopy (SEM) study, two specimens were dehydrated through a graded series of ethyl alcohol, and then critical-point dried with carbon dioxide, mounted on metal stubs with silver paste, coated with gold, and examined in a Hitachi Stereoscan model SU1510 (Hitachi High-Technologies Mexico S.A. de C.V, Mexico) at 15 kV.
DNA extraction, amplification and sequencing
Seventeen specimens were placed individually in tubes and digested overnight at 56°C in a solution containing 10 mM Tris–HCl (pH 7.6), 20 mM NaCl, 100 mM Na2 EDTA (pH 8.0), 1% Sarkosyl, and 0.1 mg/ml proteinase K. DNA was extracted from the supernatant using DNAzol (Molecular Research Center, Cincinnati, Ohio). Two regions of nuclear ribosomal DNA (rDNA), and the mitochondrial cytochrome c oxidase subunit 1 (cox1) were amplified via polymerase chain reaction (PCR). The ITS1, 5.8S and ITS2 regions were amplified using the forward primer BD1, 5´-GTCGTAACAAGGTTTCCGTA-3´and the reverse primer BD2, 5´-ATCTAGACCGGACTAGGCTGTG-3´ (Luton et al., Reference Luton, Walker and Blair1992). The D1–D3 domains of the 28S rRNA gene were amplified using the primers 502 5'-CAAGTACCGTGAGGGAAAGTTGC-3' (forward) and 536 5'-CAGCTATCCTGAGGGAAA-3' (reverse) (García-Varela & Nadler, Reference García-Varela and Nadler2005). The mitochondrial cox1 was amplified using the forward primer MplatCOX1dF 5´-TGTAAAACGACGGCCAGTTTWCITTRGATCATAAG-3´ and the reverse primer MplatCOX1dR 5´-CAGGAAACAGCTATGACTGAAAYAAYAIIGGATCICCACC-3´ (Moszczynska et al., Reference Moszczynska2009). PCR reactions (25 μl) consisted of 10 μM of each primer, 2.5 μl of 10 X buffer, 1.5 μl of 2 mM MgCl2, 0.5 μl of dNTPs (10 mM), 1 U of Taq DNA polymerase (Platinum Taq, Invitrogen Corporation, São Paulo, Brazil), 2 μl of the genomic DNA plus 16.7 μl of distilled water. PCR cycling parameters for rDNA amplifications included denaturation at 94°C for 5 minutes, followed by 35 cycles of 94°C for 1 minute, annealing at 50°C for 1 minute for the three molecular markers, and extension at 72°C for 1 minute, followed by a post-amplification incubation at 72°C for 10 minutes. PCR products for ITS1, 5.8S, ITS2 and 28S rDNA were sequenced with the PCR primers plus the internal primers BD3 5′-GAACATCGACATCTTGAACG-3′ and BD4 5′-ATAAGCCGACCCTCGGC-3′ (Hernández-Mena et al., Reference Hernández-Mena, García-Prieto and García-Varela2013) and 503 5′-CCTTGGTCCGTGTTCAAGACG-3′ (forward) (Stock et al., Reference Stock, Campbell and Nadler2001) and 504 5′-CGTCTTGAAACACGGACTAAGG-3′ (reverse) (García-Varela & Nadler, Reference García-Varela and Nadler2005), respectively. Sequencing reactions were performed using ABI Big Dye (Applied Biosystems, Boston, Massachusetts, USA) terminator sequencing chemistry, and reaction products were separated and detected using an ABI 3730 capillary DNA automated sequencer. Contigs were assembled and base-calling differences resolved using Codoncode Aligner version 6.0.2 (Codoncode Corporation, Dedham, Massachusetts, USA). Sequences obtained in the current research for ITS, 28S and cox1 were aligned with sequences of other genera of clinostomids downloaded from GenBank. Three alignments were built. For ITS and cox1 alignments, newly generated sequences were aligned along with one to three representative sequences of 23 species/lineages of Clinostomum, plus Odhneriotrema incommodum (Leidy, 1856) and Euclinostomum heterostomum (Rudolphi, 1908). For 28S, an alignment was built considering two newly generated sequences in addition to six validated species of Clinostomum, plus newly generated sequences of five genetic lineages of the genus, and one sequence of Clinostomoides, two of Euclinostomum and one of Odhneriotrema. In addition, sequences of the diplostomids Diplostomum baeri Dubois, 1937 and Alaria marcianae (La Rue, 1917) Walton, 1949 were used as outgroups for rooting the trees. A fourth alignment was built to perform a concatenated analysis of 28S-ITS-cox1. Due to the number of sequences available for the 28S rRNA gene, the concatenated analysis was performed considering sequences of six validated species of Clinostomum, plus those of five genetic lineages, two of Euclinostomum, one of Odhneriotrema, with Alaraia marcianae as outgroup.
Phylogenetic analyses
Sequences were aligned with the software Clustal Omega (Sievers et al., Reference Sievers2011), implemented at https://www.ebi.ac.uk/Tools/msa/clustalo/. Phylogenetic analysis for each dataset was run under Bayesian inference (BI) and maximum likelihood (ML), employing the nucleotide substitution model GTR + GAMMA, which was calculated in the program jModelTest v2.1.10 (Darriba et al., Reference Darriba2012). The concatenated analysis was also run under ML and BI. Bayesian inference was performed in MrBayes v. 3.2.6 (Ronquist et al., Reference Ronquist2012), running two independent MCMC of four chains each run (heating parameter = 0.5) for 10 million generations and sampling trees every 1000 generations (printfreq = 1000, samplefreq = 1000, diagnfreq = 10,000), and burn-in periods were set to the first 2500 generations. A 50% majority-rule consensus tree and nodal support (posterior probability values) were calculated from the remaining trees. ML inference (100 replicates), model parameters and bootstrap support (1000 replicates) were estimated with RAxML v. 8.2.X (Stamatakis, Reference Stamatakis2006). Phylogenetic trees obtained from the analysis were visualized in FigTree v.1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/). Molecular divergence for all markers was estimated using uncorrected p distances (p-distances) with the software PAUP* 4.0a (Swofford, Reference Swofford2002).
Results
Morphology
The clinostomid metacercariae were identified as members of the monotypic genus Ithyoclinostomum Witenberg, 1925, based on body size, the presence of an oral collar, simple caeca lacking diverticula, gonads being located in the posterior fourth of the body, and having a pre-testicular genital pore. Even though body size was variable among collected individuals (11.6–29.5 mm), the position, size and shape of internal organs in proportion to the body length were very similar, particularly for characters related to the reproductive organs (fig. 1).

Fig. 1. Metacercariae of Ithyoclinostomum sp. obtained from different host species. Processed specimens show the same shape and position of gonads, irrespective of body size.
Ithyoclinostomum sp. (Metacercariae)
Taxonomic summary Hosts. Mayaheros urophthalmus (Günther, 1862), Vieja melanura (Hubbs, 1935), Herichthys deppii (Heckel, 1840), Cribroheros longimanus (Günther, 1867), Cribroheros alfari (Meek, 1907), Parachromis managuensis (Günther, 1867).
Localities. Mexico – Gregorio Méndez, Tabasco (Grijalva river basin); Naha, Chiapas (Usumacinta river basin); Nautla (Nautla river basin); Filipinas Creek (Nautla river basin). Costa Rica – Irigaray River at Liberia, Guanacaste; Orosi River, Guanacaste; Pithaya Creek (Orosi river basin), Guanacaste.
Specimens deposited. Colección Nacional de Helmintos (CNHE), vouchers 10716–10721.
Representative DNA sequences. MH159738-MH159752 (cox1), MH159753-MH159770 (ITS), MH159736-MH159737 (28S).
Description. (Based on measurements of 14 specimens; measurements in table 2): Body large, elongate and widest in equatorial region of body. Tegument surface lacking spines. Anterior end forming an oral collar; oral collar with a ventral constriction. Oral sucker rounded, terminal, smaller than ventral sucker, embedded in oral collar. Prepharynx absent. Pharynx well developed, leading immediately to intestinal bifurcation. Intestinal caeca run laterally to posterior end of body. Ventral sucker rounded, in anterior fourth of body; triangular aperture. Testes in tandem, in posterior fourth of body, irregularly shaped, with smooth margins, entirely intracaecal. Anterior testis H-shaped, with anterior lobes longer than posterior ones. Posterior testis X-shaped, forming four lobes of same size; inter-testicular space wide. Cirrus sac ovoid, overlapping anterior testis in dextral position. Genital pore pre-testicular, in mid-level of body. Ovary small, rounded, smooth, located in inter-testicular space on right side of body. Uteroduct emerging from ootype, running around left margin of anterior testis to form tubular uterine sac, well developed, extending anteriorly to short distance from posterior border of ventral sucker, descending straight into genital pore; metraterm not observed. Vitellaria undeveloped.
Taxonomic remarks. The metacercariae sampled from Middle American cichlids correspond in general with the diagnosis of the genus Ithyoclinostomum following Kanev et al. (Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002). Our specimens possess a large and elongate body with a small oral sucker surrounded by an oral collar; a considerable space free of internal organs between ventral sucker and anterior testis; simple, long caeca without lateral diverticula; gonads in the posterior fourth of body; and inter-testicular ovary. Overall comparison of morphometric characters showed that our specimens are very similar to those described for I. dimorphum (table 2); this species was found in some locations across South America (see Benigno et al., Reference Benigno2014; Costa et al., Reference Costa, Monteiro and Brasil-Sato2015). In addition, the ultrastructure of the body surface of I. dimorphum was previously studied by Dias et al. (Reference Dias2003) in adults obtained from Ardea cocoi (L.), and by Benigno et al. (Reference Benigno2014) in metacercariae from Hoplerytrinus unitaeniatus, both in Brazil. Our SEM specimens (fig. 3) are similar, except that that they possess a triangular shaped ventral sucker aperture and a well-defined constriction of the oral collar (see figs 3a, c). Moreover, two main facts prevented us from identifying our specimens as the monotypic I. dimorphum. First, we relied solely on the morphology of the metacercarial stage, as we did not collect the adults from their definitive hosts. Second, our specimens were morphologically different from I. dimorphum regarding some traits. For instance, some differences were found in the overall body shape, size and shape of testes, and the position of the genital pore between I. dimorphum and our specimens (table 2). In I. dimorphum, the body is long and slender, and testes are small and irregularly shaped, whereas in our specimens, the body is elongate and robust, testes are well developed and their form is well defined, the anterior testis is H-shaped and the posterior testis is X-shaped. The cirrus sac in our specimens is located in the mid-level of the anterior testis, and the genital pore is medial; in I. dimorphum the cirrus sac is dextral to the right margin of the anterior testis, and the genital pore is dextrally located. A striking difference was found in the body length/body width ratio. On average, in our specimens the ratio is 3.8, whereas in I. dimorphum the ratio is around 18 (table 2).
Molecular data. Phylogenetic analyses inferred with three independent datasets (cox1, ITS, 28S) through BI and ML unequivocally recovered all the sequenced metacercariae as a monophyletic assemblage, with high bootstrap and posterior probability values (figs 4–6). However, the three datasets yielded different topologies regarding the position of Ithyoclinostomum sp. with respect to other clinostomids for which DNA sequences are available.
Cox1. The alignment was 474 bp long and consisted of 15 newly sequenced metacercariae, two sequences of Euclinostomum heterostomum, three of Odhneriotrema incommodum, and one or two replicates of each of the 11 valid species of Clinostomum, plus one or two replicates of each of the 12 genetic lineages of Clinostomum not yet described. The phylogenetic analysis suggested that the metacercariae of Ithyoclinostomum sp. occupy a basal position with respect to the other genera of clinostomids, as follows: [Ithyoclinostomum sp. (Euclinostomum (Odhneriotrema (Clinostomum)))] (fig. 4).
ITS. The alignment was 1142 bp long and consisted of 17 newly sequenced metacercariae, two sequences of Euclinostomum heterostomum, two of Odhneriotrema incommodum, and one or two replicates of each of the 11 valid species of Clinostomum, plus two replicates of each of the 12 genetic lineages of Clinostomum not yet described. The phylogenetic analysis showed that the metacercariae of Ithyoclinostomum sp. are recovered as the sister group of the genus Odhneriotrema, as follows: [Euclinostomum (Odhneriotrema + Ithyoclinostomum sp. (Clinostomum))] (fig. 5).
28S. The alignment was 1414 bp long, and included only two newly generated sequences of Ithyoclinostomum sp., two sequences of Euclinostomum heterostomum, one of Odhneriotrema incommodum, one or two replicates of six valid species of Clinostomum for which this molecular marker has been sequenced and an unidentified species from Australia, two replicates of five newly sequenced genetic lineages of Clinostomum, and one sequence of Clinostomoides brieni Dollfus, 1950. The phylogenetic analysis revealed that the metacercariae of Ithyoclinostomum sp. were recovered as the basal group of the Clinostomidae as follows: [Ithyoclinostomum (Odhneriotrema (Euclinostomum (Clinostomum)))] (fig. 6). In this analysis, C. brieni nests within the group of Clinostomum species that occur in the old world.
Concatenated analysis (cox1 + ITS + 28S). As the mitochondrial gene and the two ribosomal genes yielded different topologies regarding the position of the newly generated sequences in the phylogeny of Clinostomidae, a fourth alignment was built for a concatenated analysis of the three molecular markers through BI and ML. The final alignment was 3094 bp long and included fewer representative sequences of clinostomids; only species or lineages with sequences of the three markers were included in the analysis. Alignment consisted of two sequences of Ithyoclinostomum sp., two of Euclinostomum, one of Odhneriotrema, one or two replicates of six valid species of Clinostomum, and two replicates of five genetic lineages of Clinostomum. The concatenated tree recovered Odhneriotrema incommodum as the basal member of the group, as the sister taxon of the metacercariae of Ithyoclinostomum sp. plus Euclinostomum and Clinostomum, as follows: [Odhneriotrema (Ithyoclinostomum sp. (Euclinostomum (Clinostomum)))] (fig. 7).
Genetic divergence. The genetic distance estimated through uncorrected p distances showed a high level of divergence between the sequences of Ithyoclinostomum sp. and the other clinostomids for the three molecular markers. On average, for the 28S rRNA gene, our metacercariae from Euclinostomum, Odhnerietrema and Clinostomum varied 3.69–9.97%, 7.78% and 6.02–7.92%, respectively; for ITS they varied 19.72%, 15.82% and 17.15–20.14%, and for cox1 divergence values varied 18.14%, 19.62% and 16.66–23.62% with respect to the species included in the aforementioned genera. Intraspecific divergence among isolates of our metacercariae was very low or null (0.00–0.85% for cox1, 0.00–0.09% for ITS, and 0.0% for 28S), indicating that irrespective of body size (see fig. 1) and geographical location (Mexico or Costa Rica), all metacercariae are conspecific.
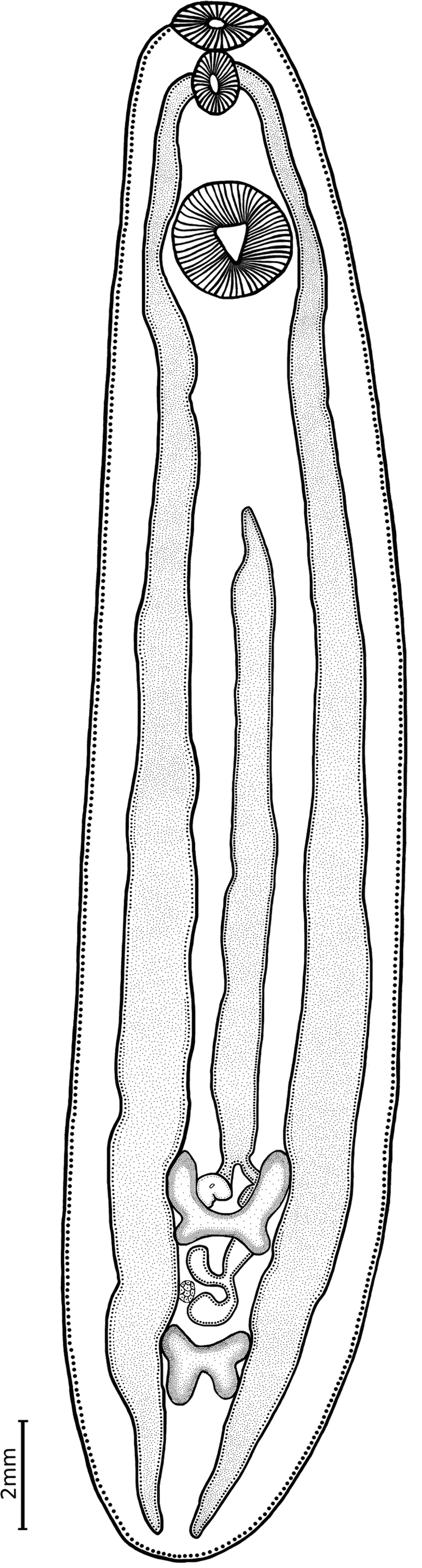
Fig. 2. Line drawing of a metacercaria of Ithyoclinostomum sp. from Herichthys deppi, Nautla River, Veracruz State, Mexico. Ventral view.

Fig. 3. Scanning electron microscopy images of Ithyoclinostomum sp. from Cribroheros alfari, Orosi River, Costa Rica. (a) Entire worm; scale bar = 2 mm. (b) Oral collar, showing ventral constriction; ventral view; scale bar = 0.5 mm. (c) Oral collar, showing ventral constriction; ventro-lateral view; scale bar = 0.5 mm. (d) Oral collar, showing ventral constriction and oral aperture; en face view; scale bar = 0.5 mm.

Fig. 4. Bayesian inference tree for Ithyoclinostomum sp. based on the cytochrome c oxidase subunit 1 gene (cox1) dataset. Dots above branches represent posterior probability values and bootstrap values higher than 0.8/80%. Newly generated sequences of Ithyoclinostomum sp. in this study are in bold.

Fig. 5. Bayesian inference tree for Ithyoclinostomum sp. based on the internal transcribed spacers (ITS1–5.8S–ITS2) dataset. Dots above branches represent posterior probability values and bootstrap values higher than 0.8/80%. Newly generated sequences of Ithyoclinostomum sp. in this study are in bold.

Fig. 6. Bayesian inference tree for Ithyoclinostomum sp. based on the 28S rRNA gene dataset. Dots above branches represent posterior probability values and bootstrap values higher than 0.8/80%. Newly generated sequences in this study for Ithyoclinostomum sp., as those for Lineages 1–5 (sensu Pérez-Ponce de León et al., Reference Pérez-Ponce de León2016) are in bold.

Fig. 7. Bayesian inference tree for Ithyoclinostomum sp. based on the concatenated dataset (28S + ITS1–5.8S–ITS2 + cox1). Numbers above branches represent posterior probability values and bootstrap values. The definitive host, either a fish-eating bird or a crocodilian, for each species/genetic lineage of clinostomid is included in the figure. * cox1 sequences; ° ITS sequences; ^ 28S sequences.
Table 2. Morphometric data for the metacercariae of Ithyoclinostomum sp. from Middle American cichlids sampled in this study, and comparison with published descriptions of metacercariae and adults of I. dimorphum. Measurements are expressed as a range, in mm; mean values are in parentheses. * represents ratios not provided in the original description/redescription; these were calculated as reference values for comparative purpose only.

Abbreviations: BL, body length; BW, body width; OCL, oral collar length; OCW, oral collar width; OSL, oral sucker length; OSW, oral sucker width; PhL, pharynx length; PhW, pharynx width; VSL, ventral sucker length; VSW, ventral sucker width; DBS, distance between suckers; DOC&VS, distance between oral collar and ventral sucker; ATL, anterior testis length; ATW, anterior testis width; PTL, posterior testis length; PTW, posterior testis width; DBVS&AT, distance between ventral sucker and anterior testis; DBT, distance between testis; OL, ovary length; OW, ovary width; CSL, cirrus sac length; CSW, cirrus sac width.
Discussion
The morphology of the specimens reported in our study resembled that of members of the genus Ithyoclinostomum. Molecular results confirm that our specimens form a monophyletic assemblage and that they represent an independent genetic lineage, not closely related with species of Clinostomum, the most species-rich genus within the family Clinostomidae; moreover, the phylogenetic relationships of the metacercariae with other clinostomids remain uncertain, as the three molecular markers used in this study resolved contradictory sister-group relationships (figs 4–6). Even though we hypothesized that the metacercariae recovered from Middle American cichlids belong in the genus Ithyoclinostomum, morphologically, they cannot be considered conspecific with I. dimorphum. Several differences show that our specimens may actually represent an undescribed species. In addition to morphology, three pieces of information were useful to complement the species differentiation: habitat, host specificity, and geographical distribution. In terms of habitat, records of I. dimorphum as a metacercaria in most locations of South America indicate the body cavity (mesentery) of their hosts is the preferential habitat; more rarely, metacercariae were found in the musculature, opercula, stomach and gills. It is unclear, however, if the metacercariae were always encysted in the different habitats where they occurred. For instance, Szidat (Reference Szidat1969) found metacercariae encysted in the gill arches of their hosts in Tucumán, Argentina; Gallio et al. (Reference Gallio2007) recovered them encysted in the musculature of their hosts in Rio Grande do Sul, Brazil; and Belei et al. (Reference Belei2013) found them encysted in the visceral cavity of their hosts in Minas Gerais, Brazil. In contrast, some authors have reported metacercariae of I. dimorphum from the mesentery/musculature, coelomic cavity/stomach, and body cavity of their fish hosts, although none of them mentioned specifically whether or not the metacarcariae were encysted (Benigno et al., Reference Benigno2014; Costa et al., Reference Costa, Monteiro and Brasil-Sato2015; Delgado et al., Reference Delgado2017). In our study, the metacercariae of Ithyoclinostomum sp. were found exclusively in the body cavity of their cichlid hosts, and in all cases they were unencysted, and actively moving when collected.
Furthermore, geographical distribution and host specificity patterns were also used to differentiate I. dimorphum from the specimens sampled in this study. The metacercariae of I. dimorphum show a strong host specificity, having been reported only from three species of Characiform fishes (Family Erythrinidae Valenciennes, 1847): Hoplias malabaricus Bloch, 1794, Hoplias intermedius (Günther, 1864) and Hoplerytrinus unitaeniatus (Spix & Agassiz, 1829) in Brazil, Peru and Argentina (Szidat, Reference Szidat1969; Travassos et al., Reference Travassos, Freitas and Kohn1969; Pavanelli et al., Reference Pavanelli, Schaeffer and Santos1990; Weiblen & Brandão, Reference Weiblen and Brandão1992; Paraguassú & Luque, Reference Paraguassú and Luque2007; Gallio et al., Reference Gallio2007; Belei et al., Reference Belei2013; Benigno et al., Reference Benigno2014; Costa et al., Reference Costa, Monteiro and Brasil-Sato2015; Delgado et al., Reference Delgado2017). The species has also been found as a parasite of Schizodon borrelli (Boulenger, 1900) in the Paraná River, Brazil (Machado et al., Reference Machado, Pavanelli and Takemoto1996), a member of the Anostomidae Günther, 1864. Erythrinids and anostomids are both Characiforms, although they are not closely related (see Oliveira et al., Reference Oliveira2011). In this sense, I. dimorphum seems to be restricted to these particular groups of characiforms in South America. In contrast, our specimens are host-specific to cichlids and are apparently restricted to Middle America; they have only been found unencysted in the body cavity of their hosts.
Taking together all the pieces of information discussed above, it is possible that the specimens from cichlids represent an undescribed species; however, sampling adults from their definitive hosts is a requirement to present a complete species description for this potentially new species. The single specimen reported in Aguirre-Macedo et al. (Reference Aguirre-Macedo2001) as Clinostomum sp. 2, from the body cavity of Parachromis managuensis (Günther, 1867), in the South Atlantic area of Nicaragua, in Central America, corresponds fully with the morphology of the metacercariae we describe in this study (see table 2), even though the single specimen collected by those authors from the cichlid is larger (39 mm long); therefore, these specimens have to be further considered as Ithyoclinostomum sp. Interestingly, Belei et al. (Reference Belei2013) reported the presence of the metacercariae of I. dimorphum in H. malabaricus from the Parque Estadual do Rio Doce, Brazil and, as their specimens were hardened due to fixation in formalin, the body was macerated and those authors reported the presence of eggs in the metacercariae, arguing the possibility that the digenean larval stage acquired sexual maturity due to permanent absence of the definitive hosts. In our specimens, even though gonads were well developed, there was no evidence of the development of the uterine sac.
Adults of Ithyoclinostomum have been found in the buccal cavity of fish-eating birds in some locations of Brazil. The original record (as Clinostomum dimorphum Diesing, 1850) was made as a parasite of Ardea cocoi. According to Lent & Freitas (Reference Lent and Freitas1937) the species was transferred to Ithyoclinostomum by Witenberg (Reference Witenberg1925), but as a generic diagnosis was not provided, Lent & Freitas formally described the genus for the first time. Since it was first described, I. dimorphum has also been reported as a parasite of Ardea alba (Linnaeus, 1758), Nycticorax nycticorax (Linnaeus, 1758) and Tigrisoma lineatum (Boddaert, 1783) (see Benigno et al., Reference Benigno2014; Fernandes et al., Reference Fernandes2015). The genus Ithyoclinostomum is unique among clinostomids because of the species’ body size: they are ‘the largest species among the so far known Clinostomidae, reaching sizes which are rarely encountered among the trematodes: 60–100 mm’ (Braun, Reference Braun1901). Clinostomum heluans Braun, 1899, a species widely distributed in the Americas as a parasite of fish-eating birds (Briosio-Aguilar et al., Reference Briosio-Aguilar2018), is also characterized by its larger size, although it is not as large as I. dimorphum; adults of C. heluans are usually 15–20 mm long (see Werneck et al., Reference Werneck, Bacco-Mannina and Santos-Costa2017 and references therein); likewise, the specimens of C. heluans reported by Bravo-Hollis (Reference Bravo-Hollis1947) from the buccal cavity of Ardea herodias (Linnaeus, 1758) in northern Mexico are probably the largest reported, reaching 20.7–26.1 mm. The report by Bravo-Hollis (Reference Bravo-Hollis1947) led us to consider, initially, that our specimens corresponded with C. heluans, as they were 11.6–29.5 mm long; also, our samples coincided with the geographical range of the species (Briosio-Aguilar et al., Reference Briosio-Aguilar2018). However, morphologically, our specimens resembled more closely Ithyoclinostomum than C. heluans; the molecular evidence gathered in this study confirmed unequivocally that our specimens did not nest within the clade formed by Clinostomum species. Metacercariae of I. dimorphum are variable in length, reaching 15–50 mm (table 2). On average, our specimens lie within the length range of that species, although in the lower limit (18.7 mm), and as mentioned above, the body length/width ratio is much greater in I. dimorphum, which means that specimens of that species are more elongated.
The definitive hosts of the metacercariae characterized herein still remain unknown. Considering the most parsimonious explanation of sister-group relationships among clinostomids shown by the concatenated phylogenetic analysis (fig. 7), the possibility that crocodilians are the definitive hosts of this trematode cannot be ruled out, especially as we have analysed for helminths c. 377 fish-eating bird individuals in the last few years, including species of ardeids, pelicans, cormorants, anhingids and threskiornithids, and we have been unable to recover adult parasites corresponding with the species we characterize in this study. We have to keep looking at a wider diversity of birds and whenever possible at crocodilians to try to obtain adult forms of this trematode species. Previous classification schemes of the family Clinostomidae considered Ithyoclinostomum to be part of the subfamily Clinostominae (see Skrjabin, Reference Skrjabin1947; Feizullaev & Mirzoeva, Reference Feizullaev and Mirzoeva1983) or the monotypic subfamily Ithyoclinostominae (see Yamaguti, Reference Yamaguti1971); these classifications included only species whose adults were found in birds. The current classification scheme (Kanev et al., Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002) also includes the subfamily Nephrocephalinae in the family Clinostomidae; their members are parasites of crocodilians. Our molecular phylogenetic analysis provides additional support to the classification scheme proposed by Kanev et al. (Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002), which is based solely on morphology.
As our specimens did not conform entirely to the diagnosis of I. dimorphum and C. heluans, we compared our material with the other genera included in separate subfamilies of the family Clinostomidae (see Kanev et al., Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002). Molecularly, our specimens were closely related to the genera Euclinostomum and Odhneriotrema, the only members other than Clinostomum for which sequences are available in GenBank; genetic divergence values and reciprocal monophyly indicated that our specimens were independent evolutionarily significant units. Morphologically, species included in these genera are different. Euclinostomum is the only genus in the subfamily Euclinostominae (Kanev et al., Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002); adults are parasites of ardeids and metacercariae are commonly found in cichlids in the old world (see Caffara et al., Reference Caffara2016); adults and metacercariae of species of Euclinostomum are characterized by having 6–15 blind diverticula extending latero-posteriorly to the main caeca (Kanev et al., Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002; Caffara et al., Reference Caffara2016); this character, and the body size of the organisms are the main traits that distinguish our specimens of Ithyoclinostomum sp. from the species in that genus. Species of the genus Odhneriotrema, a member of the Nephrocephalinae along with Nephrocephalus, are parasites of the buccal cavity and oesophagus of crocodilians in the Americas. They differ from Ithyoclinostomum sp. in the small size of the oral sucker, the position of the ovary and cirrus sac with respect to testes, the size of the cirrus sac, and the large inter-testicular space.
The genus Clinostomatopsis Dollfus, 1932 belongs to the Clinostomatinae (Kanev et al., Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002), and shows some resemblance to the metacercariae from the present study because of the lobated nature of the testes. Unfortunately, no sequence data were available for this species, and the comparison relied solely on morphology. Currently, the genus contains two species known to parasitize the oesophagus of Neotropical birds (Lunaschi & Drago, Reference Lunaschi and Drago2009): Clinostomatopsis intermedialis Lamont, 1920, recorded as a parasite of the cormorants Phalacrocorax brasilianus (Gmelin, 1789) from Venezuela (Lamont, Reference Lamont1920), and P. penicillatus (Brandt, 1837) from north-eastern Mexico (Bravo-Hollis, Reference Bravo-Hollis1947). The second species, Clinostomatopsis sorbens (Braun, 1899) Dollfus, 1932, is a parasite of Mycteria americana (Linnaeus, 1758), Ardea cocoi and Jabiru mycteria (Lichtenstein, 1819) in Brazil (see Benigno et al., Reference Benigno2014; Fernandes et al., Reference Fernandes2015, and references therein) and has been found in Tigrisoma lineatum in Argentina (Lunaschi & Drago, Reference Lunaschi and Drago2009). Metacercariae were recorded from the mesentery of the erythrinids H. malabaricus and H. unitaeniatus in Brazil by Benigno et al. (Reference Benigno2014), who included SEM micrographs of the specimens. The metacercariae of C. sorbens are different from the ones we characterize in this study because they are relatively smaller (< 10 mm), they have an inter-testicular cirrus sac and genital pore (which is a diagnostic trait for the genus), and although they possess deeply lobed testes, the shape of the testes is very different: the anterior testis is formed by five irregular lobes, and the posterior testis by six lobes, two directed anteriorly and four directed posteriorly (see Figure 1 in Benigno et al., Reference Benigno2014). Finally, the metacercariae of Ithyoclinostomum sp. are also different from the monotypic Clinostomoides. Clinostomoides brieni Dollfus, 1950 infects the oesophagus of herons and their metacercariae are more commonly found in siluriform fishes in Asia and Africa (Kanev et al., Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002); this species is characterized by an elongated body, with gonads also located in the posterior fourth of the body; however, it can be easily distinguished from our metacercariae because the cirrus sac is inter-testicular and the genital pore is post-testicular according to the diagnosis by Kanev et al. (Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002), although a recent description of the metacercariae from Clarias gariepinus (Burchell, 1822) from Botswana, Africa (van Rensburg et al., Reference van Rensburg, van As and King2013) refers to a ‘genital pore just submedian to right, immediately anterior to posterior testis’. The fact that the sequenced specimen of this species (from a metacercaria obtained from a siluriform fish in India – KF781299 – as a direct submission) is nested within a group of species of Clinostomum that includes the cosmopolitan C. complanatum indicates a possible misidentification, and requires further verification.
In conclusion, the metacercariae characterized in this study were included in the genus Ithyoclinostomum because they share some morphological traits; however, the inclusion of our specimens in the genus is tentative until sequences of the adults or metacercariae of the species I. dimorphum are provided from specimens sampled from their natural distributional range in South America. Molecular data will provide confirmation or rejection of this hypothesis. Even if our specimens belong to a different genus, they represent an undescribed species; to accomplish the proper species description, and naming of the species, we need to collect adult forms from their definitive hosts, either fish-eating birds or crocodilians, and characterize them morphologically and molecularly, to establish a link between the larval forms and the adults in their definitive hosts.
Acknowledgements
Thanks are due to Luis García for the loan of specimens from the Colección Nacional de Helmintos (CNHE, Mexico City). We also thank Susana Guzmán and Berenit Mendoza-Garfias for their assistance with image acquisition through light microcopy and scanning electron microscopy, respectively. Laura Márquez provided technical assistance with the sequencer. We also thank Arturo Angulo from the Universidad de Costa Rica for support in sampling freshwater fish in Costa Rica.
Financial support
This work has been supported by the Programa de Apoyo a Proyectos de Investigación e Inovación Tecnológica (PAPIIT-UNAM) through the grants IN202617 and IN206716 to GPPL and MGV, respectively.
Conflict of interest
None.
Ethical standards
In Mexico, specimens were collected under the Cartilla Nacional de Colector Científico (FAUT 0202 and 0057) issued by the Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT), to MGV and GPPL, respectively. In Costa Rica, specimens were obtained under the collector permit issued to Arturo Angulo from the Universidad de Costa Rica.











