Introduction
The transmission of parasites is characterized by aggregated distributions within host populations and marked spatial heterogeneities of infection (Morrill & Forbes, Reference Morrill and Forbes2012). The rate of acquisition and loss of parasites can be modulated by several ecological features of the host, including geographical ranges, body size, population density, social behaviour, lifespan and diet (Morand & Poulin, Reference Morand and Poulin1998; Arneberg, Reference Arneberg2002; Poulin, Reference Poulin2004; Poulin & George-Nascimento, Reference Poulin and George-Nascimento2007). In addition, variations in environmental conditions may operate as specific sources of selection pressures. Many wildlife hosts live in fragmented habitats in which the spatial heterogeneity is recognized to influence the dynamics of host–parasite interactions (Behnke et al., Reference Behnke, Harris, Bajer, Barnard, Sherif, Cliffe, Hurst, Lamb, Rhodes, James, Clifford, Gilbert and Zalat2004; Deter et al., Reference Deter, Chaval, Galan, Berthier, Ribas Salvador, Casanova Garcia, Morand, Cosson and Charbonnel2007).
Human activities have led to alterations and fragmentation of the environments, exposing the isolated or semi-isolated subpopulations of animals and their helminth communities to different selection pressures (Behnke et al., Reference Behnke, Harris, Bajer, Barnard, Sherif, Cliffe, Hurst, Lamb, Rhodes, James, Clifford, Gilbert and Zalat2004). Changes in human demography, behaviour, land-use practices and environment exert a marked influence on the emergence and proliferation of parasites, many of which are recognized as zoonotic (Patz et al., Reference Patz, Graczyk, Geller and Vittor2000; Colwell et al., Reference Colwell, Dantas-Torres and Otranto2011). The absence of detailed studies on key aspects of the ecology of many zoonotic parasites is a major constraint for the design and implementation of effective prevention and control measures against these pathogens (Polley, Reference Polley2005).
The concentration and expansion of urban environments and human activities triggers, in many cases, a cascade of factors that alter the composition of host species and can change the ecology of infectious disease agents (Patz et al., Reference Patz, P., Tabor, A., Pearl, Epsten, Wolfe, Kilpatrick, Foufopoulos, Molyneux and Bradley2004). Uncontrolled urban growth in developing countries has resulted in large health inequities and inadequate sanitation, which can promote the transmission of zoonotic helminthiases (Alirol et al., Reference Alirol, Getaz, Stoll, Chappuis and Loutan2011). Many urban-adapted species occur at much higher densities in urban environments, where human activities provide resources not prone to seasonal fluctuations, than in less-disturbed areas, thus supporting these populations and affecting the parasite transmission rates (Bradley & Altizer, Reference Bradley and Altizer2006). Some of the best urban-adapted animals distributed worldwide are rats from the genus Rattus. The adaptation capacity and opportunistic behaviour of Rattus spp. make them one of the main urban pests, whose presence is mostly correlated with inadequate disposal of anthropogenic waste (Traweger et al., Reference Traweger, Travnitzky, Moser, Walzer and Bernatzky2006).
According to Cavia et al. (Reference Cavia, Cueto and Suárez2009), the rodent community composition in the city of Buenos Aires (Argentina) is associated with environmental characteristics. Black rats (Rattus rattus) dominate sites with high proportions of constructions, while brown rats (Rattus norvegicus) are associated with areas consisting of highly vegetated urban cover. The latter conditions are abundant in shantytowns and parklands close to bodies of water. This heterogeneity in habitats and host characteristics might lead to variations in the infection patterns of parasites with both species of rats as potential hosts.
The cestode Hymenolepis diminuta, which is distributed worldwide, is primarily a parasite of Rattus spp. (Mafiana et al., Reference Mafiana, Osho and Sam-Wobo1997; Battersby et al., Reference Battersby, Parsons and Webster2002; Zain et al., Reference Zain, Behnke and Lewis2012), but human infections, principally of children, are not uncommon (Marangi et al., Reference Marangi, Zechini, Fileti, Quaranta and Aceti2003; Mowlavi et al., Reference Mowlavi, Mobedi, Mamishi, Rezaeian, Haghi Ashtiani and Kashi2008). Particularly in Argentina, H. diminuta has been found in rats captured in poultry farms and in a shantytown of Buenos Aires City (Gómez Villafañe et al., Reference Gómez Villafañe, Robles and Busch2008; Hancke et al., Reference Hancke, Navone and Suárez2011). Hymenolepis diminuta has an indirect life cycle; the adult cestodes live in the host's gut and require an arthropod as an intermediate host (Roberts & Janovy, Reference Roberts and Janovy2009). More than 90 species of arthropods can serve as suitable intermediate hosts, but stored-grain beetles (Tribolium spp.) are commonly involved, and infection occurs when the intermediate host is ingested by the definitive host (Roberts & Janovy, Reference Roberts and Janovy2009).
For the design of disease control programmes, it is increasingly important to understand the ecology of pathogens in urban environments and the mechanisms shaping the host–parasite communities (Bradley & Altizer, Reference Bradley and Altizer2006; Pedersen & Fenton, Reference Pedersen and Fenton2007). In this study, we recorded the abundance of H. diminuta in rats captured in different environments of the city of Buenos Aires, and studied host and environmental variables that influenced the establishment of this parasite.
Materials and methods
Collection and examination of rats
Fieldwork was conducted in the city of Buenos Aires, Argentina (34°37′S, 58°24′W), which covers an area of around 200 km2 and has around 2,891,100 inhabitants. The climate is temperate with a mean annual temperature of 17.4°C, seasonal amplitude of 13.2°C and mean annual precipitation of 1014 mm. The matrix of the city is formed by buildings and paved streets, while parks and open green areas form patches (Cavia et al., Reference Cavia, Cueto and Suárez2009). Rat samples were examined from four landscape units including four parklands, five shantytowns, two scrap-metal yards and three industrial–residential sites. The sites were not contiguous and were spaced by at least 1 km and separated by barriers such as railroad tracks, avenues, highways, etc.
Parklands refer to public areas of recreation, where areas of spontaneous vegetation and woodlots with planted species are included in a matrix of grass or ornamental lawn (Cavia et al., Reference Cavia, Cueto and Suárez2009). The parklands studied were located on the banks of the Río de la Plata River and the Riachuelo River, both of which allow the entry of native flora and fauna to an urban ecosystem (Cavia et al., Reference Cavia, Cueto and Suárez2009).
Shantytowns refer to areas inhabited by a very low-income population that lives in precarious dwellings with an inadequate supply of basic urban services, such as garbage removal, sanitation networks, electricity, telephones and plumbing (Fernández et al., Reference Fernández, Cavia, Cueto and Suárez2007; Hancke & Suárez, Reference Hancke and Suárez2014).
Industrial–residential areas refer to neighbourhoods where buildings and pavement are the dominant elements in the landscape unit. In these neighborhoods, the dominant types of construction are houses of no more than two stories, but in some sections there are also industries and apartment block buildings. Different kinds of stores may be found along avenues (Cavia et al., Reference Cavia, Cueto and Suárez2009).
Scrap-metal yards refer to fields that serve as deposits of vehicles affected by lawsuits, or abandoned on public roads, and of old and unused ships. In these sites, great amounts of scrap are piled, and heaps of garbage and standing water are also present, creating a suitable environment for the establishment of R. norvegicus.
Rats were collected from surveys carried out as part of a rodent control programme in the city of Buenos Aires. The disadvantage of working with urban rodents is that they are very difficult to capture and quantify during field research (Himsworth et al., Reference Himsworth, Jardine, Parsons, Feng and Patrick2014). So, to achieve an acceptable number of samples, it was necessary to consider an extended period of time, from 2004 to 2011. Rats were captured using live cage traps, which were wire-mesh traps of 15 × 16 × 31 cm with a door that is locked open with a pin connected to a trigger device holding the bait. Traps were placed inside houses and in their yards, in stores or factories, or on lines in sites dominated by vegetation, baited with carrot and raw meat and monitored every morning for three consecutive days. Captured animals were carried to a field-processing station, where they were killed with anaesthetic, sexed, measured and weighed. All animals were fixed in formaldehyde and a week later preserved in 70% ethanol and deposited in the collection of the Laboratory of Urban Rodent Ecology of the University of Buenos Aires.
The majority of rats trapped from some landscape units were mature individuals and, since juvenile are less likely to harbour parasites due to reduced times of exposure to infection, only sexually mature rats were kept for this study. Rats from the four landscape sites were collected during the spring and summer, between October and March, when mean temperature was 17°C or more and mean precipitation above 100 ml. Rats were also trapped during the autumn and winter, between April and September, when mean temperature was below 17°C and mean precipitation 100 ml or less.
In total, 28 R. rattus and 141 R. norvegicus were analysed for parasitological screening. Rats were selected according to the number of adult animals available in each landscape unit with no signs of decomposition. The entire alimentary tract was removed and examined. Specimens of H. diminuta were subsequently stained in Semichon's acetocarmine, dehydrated in ethanol, cleared in xylene, mounted in Canada balsam and observed under a microscope (400 × ). Identification was done according to the characteristics of the rostellum, the location of genital pores and the number of testes per proglottid (Khalil et al., Reference Khalil, Jones and Bray1994).
Data analysis
Chi-square tests of homogeneity were performed, to study whether the relationship of males/females of the rodents in each landscape unit differed from 1:1.
The following infection parameters recommended by Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997) were used to describe H. diminuta distribution: prevalence, mean intensity (I) and abundance (A). The degree of aggregation in the worm counts was calculated by the index of discrepancy (D), where a value of 0 indicates an even distribution and a value of 1 indicates that all parasites aggregated in a single host. These terms were estimated for the total sample and for each landscape unit by using the software Quantitative Parasitology 3.0 (Rózsa et al., Reference Rózsa, Reiczigel and Majoros2000). Fisher's exact tests were performed to study differences in the prevalence of H. diminuta between sampling sites in each landscape unit (Rózsa et al., Reference Rózsa, Reiczigel and Majoros2000).
Macroparasite distributions are empirically best described by the negative binomial distribution, but parasite count data usually contain excessive zero counts (Wilson & Grenfell, Reference Wilson and Grenfell1997; Munroe et al., Reference Munroe, Avery, Shutler and Dadswell2011; Pilosof et al., Reference Pilosof, Dick, Korine, Patterson and Krasnov2012). Ignoring these zeros can have two consequences: the estimated parameters and standard errors may be biased, and the excessive number of zeros can cause overdispersion (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009). Mixed models commonly used for these kinds of distributions are the zero-inflated (ZI) models (Nødtvedt et al., Reference Nødtvedt, Dohoo, Sanchez, Conboy, DesCĵteaux, Keefe, Leslie and Campbell2002; Minami et al., Reference Minami, Lennert-Cody, Gao and Roman-Verdesoto2007; Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009). ZI distributions can be viewed as a two-part model, in which the probability of species presence and abundance are modelled from the same data (Wenger & Freeman, Reference Wegner and Freeman2008). Particularly in zero-inflated negative binomial (ZINB) models, the probability of a zero outcome is modelled by logistic regression, while the continuous count is modelled by using a negative binomial error structure. The separate occurrence and abundance terms can represent different mechanisms that give rise to observed patterns of species abundance due to factors operating at different temporal or spatial scales (Wenger & Freeman, Reference Wegner and Freeman2008).
A ZINB model was used to evaluate the factors that affected the abundance of H. diminuta. The number of worms per rat was considered as the dependent variable, whereas factors such as landscape units, season, sex, year and body length (covariable) were tested in both the logistic and the negative binomial parts of the model as potential independent variables. Also, data were fitted to other distributions (Poisson (P), negative binomial (NB), zero-inflated Poisson (ZIP)) and the overdispersion in each was tested. Likelihood ratio tests were used to compare pairs of models (P vs. NB; ZIP vs. ZINB). Overdispersion was calculated from Pearson residuals, where values >1 indicate overdispersion. The ZINB model and the NB regression model are not nested, and thus, the likelihood ratio test cannot be applied. Instead, Vuong's test was applied to assess the usefulness of a ZINB versus a regular NB model, with a highly positive value favouring the zero-inflated version. The ZINB model was fitted manually, testing the significance of factors, covariable and interactions, first of the binomial part and then of the count part. The model was then progressively simplified by backward deletion and only significant terms (P< 0.05) were left in the final model. The model was selected with likelihood ratio tests and Akaike information criteria (AIC).
All calculations were performed using the R version 2.15.1 (R Development Core Team, 2013). ZINB models were created with the package pscl and all likelihood ratio tests were carried out in package lmtest.
Results
Table 1 summarizes the rat samples studied by landscape unit, season and sex. The relationship of males/females was homogeneous except for scrap-metal yards (χ2= 3.90; df = 1; P< 0.05).
Table 1 The number of Rattus norvegicus and R. rattus examined relative to host sex, season and landscape unit.

The overall prevalence of H. diminuta was 21.3% (95% confidence limits (CL): 15.4–28.3%). The mean abundance of the sample (N= 169) was 2.04 parasites/rat (95% CL: 1.34–3.09 parasites/rat) and the mean intensity of the 36 infected hosts was 9.56 parasites/rat (95% CL: 7.19–13.19 parasites/rat). With respect to the measure of aggregation, the index of discrepancy (D) was 0.88, suggesting that parasite distribution was aggregated across the sample. We detected no differences between sampling sites regarding H. diminuta prevalence, except for shantytowns where prevalence ranged from 10 to 70% (Fisher's exact test: P< 0.05). In shantytowns and parklands, in each season, the prevalence did not vary between samples from different years (Fisher's exact test: P>0.05 each for the warm and the cold season). In industrial–residential areas and scrap-metal yards data were only available for 1 year for each season. Table 2 shows the prevalence, mean intensity and mean abundance values for each landscape unit.
Table 2 Infection levels (with 95% confidence limits in brackets) and indices of discrepancy of Hymenolepis diminuta in Rattus norvegicus and R. rattus relative to landscape unit.
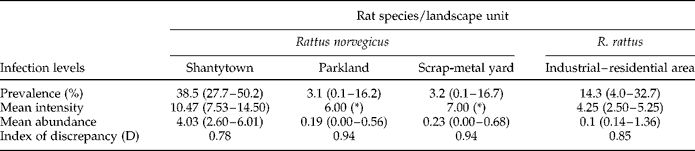
* Not calculated.
Data were fitted to regular Poisson and negative binomial models, but both models showed overdispersion (P model = 10.09 and NB model = 1.25). The likelihood ratio test suggested that data fitted better to a negative binomial distribution than to Poisson (χ2= 575.04, df = 1, P< 0.05). Similarly, by fitting data to ZI models, the ZINB model provided a better fit than ZIP (χ2= 90.99, df = 1, P< 0.05). Finally, the Vuong's test had a high positive value, indicating that a ZINB model of the abundance of H. diminuta is a significant improvement over a standard NB model (Vuong's non-nested hypothesis test-statistic = 3.71, P< 0.05).
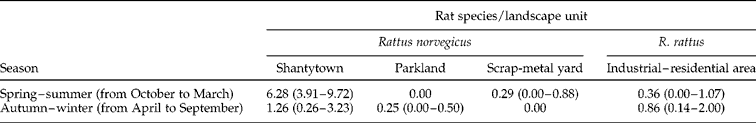
After progressively deleting the variables of the binomial part and then the count part, the final ZINB was as follows: in the logistic part, the interaction between season and landscape units was the only significant predictor of H. diminuta occurrence (χ2= 10.87, df = 3, P< 0.05). Since the probability of a zero count is what was modelled, the interpretation of this part of the model is that the difference in the probability of having rats with zero abundance of H. diminuta between trapping seasons varied with the landscape unit. In parklands, industrial–residential areas and scrap-metal yards, the abundance of the parasite was close to zero in both trapping seasons, but in shantytowns the number of parasites per host was significantly higher in spring–summer than in the colder months (P< 0.05) (table 3). This means that rats have a smaller likelihood of having a zero count of H. diminuta in the warm season, but only for those captured in the shantytowns.
Table 3 Mean abundance of infection (with 95% confidence limits in brackets) of Hymenolepis diminuta in Rattus norvegicus from three landscape units – shantytowns, parklands and scrap metal yards – and in R. rattus from industrial–residential areas, relative to season defined as autumn–winter and spring–summer.
In the negative binomial or count part of the model, the only significant coefficient in the final model was the hosts' body length (χ2= 6.78, df = 1, P< 0.05). As it was a positive coefficient, larger rats had higher expected number of H. diminuta specimens.
Discussion
Hymenolepis diminuta has a worldwide distribution with a wide range of prevalence values, ranging from over 80% to close to zero. This parasite has been reported in R. norvegicus and R. rattus from different types of environments, but mainly close to human settlements (Mafiana et al., Reference Mafiana, Osho and Sam-Wobo1997; Battersby et al., Reference Battersby, Parsons and Webster2002; Iannacone Oliver & Alvariño Flores, Reference Iannacone Oliver and Alvariño Flores2002; Abu-Madi et al., Reference Abu-Madi, Behnke, Mikhail, Lewis and Al-Kaabi2005; Easterbrook et al., Reference Easterbrook, Kaplan, Vanasco, Reeves, Purcell, Kosoy, Glass, Watson and Klein2007; Kataranovski et al., Reference Kataranovski, Mirkov, Belij, Popov, Petrovic, Gaci and Kataranovski2011; Zain et al., Reference Zain, Behnke and Lewis2012). In the present study, the overall prevalence of H. diminuta in adult rats was 21.3% and was strongly influenced by environmental factors within the urban matrix.
Cities like Buenos Aires represent artificial ecosystems composed of a number of microenvironments where isolated or semi-isolated subpopulations of animals may experience different selection pressures, and are exposed to particular threats of helminth infection (Tarsitano, Reference Tarsitano2006). Here, we analysed the abundance of H. diminuta in rats from four different landscape units of the city of Buenos Aires and found a heterogeneity in the intensity of infection among environments, with a high aggregation of the parasite in the total host sample and a high proportion of zeros.
The variable that affected the occurrence of H. diminuta in rats captured in Buenos Aires city was the interaction between the landscape unit and the time of the year. Thus, the probability of presence of uninfected rats was strongly influenced by environmental conditions. Both the type of environment and climatic conditions seem to play an important role in the establishment of this parasite in an urban area like the city of Buenos Aires.
Although R. norvegicus and R. rattus are two species highly adapted to coexisting with human populations, they are not easy to capture during fieldwork. Even though an urban environment represents an artificial ecosystem particulary vulnerable to colonization by synanthropic rodents, cities are complex and limited (Tarsitano, Reference Tarsitano2006). Human activities, construction of buildings, paved streets and demographic changes may induce variations in the microenvironment of a city block, particularly as it pertains to resource availability for rodents (Himsworth et al., Reference Himsworth, Jardine, Parsons, Feng and Patrick2014). However, even though cities are in a constant state of transformation, these changes seem not to affect the occurrence of H. diminuta. In fact, we found no differences in the occurrence of this parasite during a period of 8 years
In shantytowns, the density of rats is higher than in other landscape units (Cavia et al., Reference Cavia, Cueto and Suárez2009). Here, the environmental and social characteristics generate the appropriate conditions for the development of R. norvegicus (Fernández et al., Reference Fernández, Cavia, Cueto and Suárez2007). The inadequate garbage removal and existence of unpaved areas give rats an almost unlimited food source and suitable conditions to build ground burrows, typical of this species, and to form large colonies (Timm, Reference Timm, Hyngstrom, Timm and Larson1994; Cavia et al., Reference Cavia, Cueto and Suárez2009; Hancke & Suárez, Reference Hancke and Suárez2014). A positive relationship between host density and parasite abundance has been detected for parasites with direct life cycles (Arneberg, Reference Arneberg2001). In the case of H. diminuta, transmission is mediated by an arthropod and this is a crucial factor in transmission dynamics of this parasite. Two of the main species mentioned as intermediate hosts for H. diminuta are the flour beetles Tribolium castaneum and T. confusum. These beetles occur principally in houses, as accidental invaders, and infest a wide range of products related to exposed food storage (Robinson, Reference Robinson2005). In shantytowns, the living conditions of their inhabitants are precarious, with inadequate general hygiene, organic waste disposal and/or food storage. These conditions are favourable for arthropods like stored-grain and flour beetles.
In parklands and scrap-metal yards, the dominant rodent species was also R. norvegicus. These areas present different environmental conditions. since there are no dwellings or occupied buildings. The common intermediate hosts for H. diminuta, grain and flour beetles, are described as household pests (Robinson, Reference Robinson2005). Thus, a lower abundance of these arthropods is expected in uninhabited open spaces, which might be negatively affecting the life cycle of H. diminuta. Besides, we detected the presence of the acanthocephalan Moniliformis moniliformis in 19% of the rats captured in parklands (Hancke, pers. comm.). The life cycle of this parasite is similar to that of H. diminuta. Interspecific effects between these two parasites have been described in laboratory rats, decreasing their weight in the presence of each other (Holland, Reference Holland1987). In the present study, some kind of competitive effect may be occurring between the two parasites in parklands, but other studies are required to test this hypothesis.
In industrial–residential areas of Buenos Aires city, the dominant rat species is R. rattus. In fact, R. rattus was mainly restricted to these types of landscape units, being almost the only rodent species present. Therefore, it is necessary to consider the rat's specific composition and its characteristics. Rattus rattus has the ability to climb and build nests with artificial materials, being more aerial than R. norvegicus (Marsh, Reference Marsh, Hyngstrom, Timm and Larson1994; Cavia et al., Reference Cavia, Cueto and Suárez2009). This aerial behaviour could prevent these animals from coming into contact with a high number of infection sources. On the other hand, the density of R. rattus, based on trap success, is lower than that recorded for R. norvegicus in shantytowns (Cavia et al., Reference Cavia, Cueto and Suárez2009). A decrease in the host's density has been mentioned as a limiting factor in parasite transmission (Morand & Poulin, Reference Morand and Poulin1998). Zain et al. (Reference Zain, Behnke and Lewis2012) conducted a parasitological study of R. norvegicus and R. rattus, both captured in two contrasting urban sites in Kuala Lumpur, Malaysia, and detected no differences in the abundance of H. diminuta between sites or hosts. In the present study, we detected differences regarding host species, but our study area shows a marked spatial segregation of R. norvegicus and R. rattus, which do not co-occur simultaneously. The composition of the rodent community responds to an urban gradient, leading to changes in H. diminuta abundance.
The probability of having zero abundance of H. diminuta in shantytowns was not homogeneous throughout the year. While the prevalence in the warm season reached 60%, it only reached the 10% in autumn and winter. This effect has been seen previously in R. norvegicus captured in a shantytown of Buenos Aires, where H. diminuta had higher abundance in summer (Hancke et al., Reference Hancke, Navone and Suárez2011). It is likely that unfavourable environmental conditions affect negatively the intermediate host populations in colder months.
In the negative binomial part of the ZINB model, the host body length had a significant positive coefficient, meaning that the abundance of H. diminuta increases with rat body size. The body mass of hosts is positively correlated with factors that affect parasite transmission rates, such as host movement rates and food intake (Arneberg, Reference Arneberg2002), and in R. norvegicus up to 5 months old, body size was mentioned as a function of age (Kataranovski et al., Reference Kataranovski, Kataranovski, Savic, Cakic, Soldatovic and Matic1994). Probably, larger rats had more opportunities for infection and accumulated a greater amount of parasites.
This study was conceived due to the fact that H. diminuta is a zoonotic parasite whose presence in humans has been documented, especially in children living in poor socio-economic conditions. Even if infections occur by accidental ingestion of an infected arthropod with H. diminuta larvae, the presence of rats in the area is essential as reservoir of the pathogen. Our results showed that shantytowns in the warm months had the highest abundance of H. diminuta. Shantytowns are overcrowded urban marginal settlements with most of their inhabitants living in precarious conditions and with inadequate sanitary practices, supporting a high abundance of synanthropic rodents. The combination of these factors exposes their inhabitants to a higher risk of infection by zoonotic endoparasites, such as H. diminuta. For sustainable control of infectious diseases with zoonotic origin, including parasitic diseases, a holistic approach is essential to identify the factors affecting their occurrence and to gain detailed understanding of the disease ecology.
Acknowledgements
We are especially grateful to the team of Laboratorio de Ecología de Poblaciones for their assistance during the field sampling and Lic. Mariel Trípodi for her help during the parasitological examination.
Financial support
Financial support was provided by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and the University of Buenos Aires.
Conflict of interest
None.





