Introduction
The parasite fauna of freshwater fishes in Middle America comprises typically Nearctic taxa in the north, a mixture of Nearctic and Neotropical taxa in transitional areas of southern Mexico and mostly Neotropical taxa south of the Isthmus of Panama (see Choudhury et al., Reference Choudhury, García-Varela and Pérez-Ponce de León2017 and references therein). Trematodes, with approximately 67 species, exhibit the highest diversity among the freshwater fish helminth fauna in the area, rendering this area a biodiversity hotspot. In particular, the allocreadiids account for most of the diversity, comprising ten genera and 20 species (Ponce de León et al., Reference Pérez-Ponce de León, Razo-Mendivil, Mendoza-Garfias, Rubio-Godoy and García-Varela2015a; Choudhury et al., Reference Choudhury, Aguirre-Macedo, Curran, Ostrowski de Núñez, Overstreet, Pérez-Ponce de León and Portes Santos2016; Pérez-Ponce de León & Hernández-Mena et al., Reference Pérez-Ponce De León and Hernández-Mena2019). In recent years, three genera and 11 species of these trematodes have been described and phylogenetic hypotheses generated based on molecular data (Curran et al., Reference Curran, Tkach and Overstreet2011; Razo-Mendivil et al., Reference Razo-Mendivil, Mendoza-Garfias, Pérez-Ponce de León and Rubio-Godoy2014; Pérez-Ponce de León et al., Reference Pérez-Ponce de León, Razo-Mendivil, Mendoza-Garfias, Rubio-Godoy and García-Varela2015a, Reference Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury and García-Varela2016; Hernández-Mena et al., Reference Hernández-Mena, Lynggaard, Mendoza-Garfias and Pérez-Ponce de León2016, Reference Hernández-Mena, Pinacho-Pinacho, García-Varela, Mendoza-Garfias and Pérez-Ponce de León2019). These and ongoing studies on the diversity of these trematodes suggest a complex history of diversification resulting from the interplay of host biogeography, co-phylogeny and host shifting (Choudhury et al., Reference Choudhury, Aguirre-Macedo, Curran, Ostrowski de Núñez, Overstreet, Pérez-Ponce de León and Portes Santos2016, Reference Choudhury, García-Varela and Pérez-Ponce de León2017). One genus in particular, Creptotrema Travassos, Artigas & Pereira, 1928 stands out in this milieu for its wide and enigmatic distribution that reportedly spans across the Americas (Curran et al., Reference Curran, Pulis, Hugg, Brown, Manuel and Overstreet2012; Choudhury et al., Reference Choudhury, García-Varela and Pérez-Ponce de León2017).
The genus Creptotrema currently contains eight species of allocreadiid trematodes characterized by two ventrolateral muscular lobes on the oral sucker, a uterus that is predominantly pre- or intertesticular and mainly lateral vitelline follicles that extend from the level of the pharynx to the posterior end of body (Curran, Reference Curran2008). Six species are distributed in South America – namely, Creptotrema creptotrema Travassos, Artigas & Pereira, 1928; Creptotrema lynchi Brooks, Reference Brooks1976; Creptotrema paraensis Vicente, Dos Santos & de Souza, 1978; Creptotrema pati Lunaschi, 1985; Creptotrema lamothei Curran, Reference Curran2008; and Creptotrema sucumbiosa Curran, Reference Curran2008 (Kohn et al., Reference Kohn, Fernandes and Cohen2007; Ostrowski de Nuñez et al., Reference Ostrowski de Núñez, Arredondo and Gil de Pertierra2017); one, Creptotrema agonostomi Salgado-Maldonado, Cabañas-Carranza & Caspeta-Mandujano, 1978, in Middle America; and one, Creptotrema funduli Mueller, Reference Mueller1934, in North America. In addition, Choudhury et al. (Reference Choudhury, García-Varela and Pérez-Ponce de León2017) reported what appears to be a new species of Creptotrema sp., in the heptapterid catfish, Pimelodella sp., from the Chagres River in Panama, although the small number of specimens prevented a taxonomic description. Thus far, DNA sequence data are only available for C. funduli, collected from the blackstripe minnow, Fundulus notatus (Rafinesque, 1820), from the Biloxi River, Mississippi, USA (Curran et al., Reference Curran, Pulis, Hugg, Brown, Manuel and Overstreet2012), although the trematode was originally described from Fundulus diaphanus Jordan & Copeland, 1817 in Oneida Lake, New York State (Mueller, Reference Mueller1934).
Creptotrema agonostomi was described from the mountain mullet, Dajaus monticola (Bancroft, 1834), and the Balsas catfish, Ictalurus balsanus (Jordan & Snyder, 1899), in three localities of eastern and western Mexico (Salgado-Maldonado et al., Reference Salgado-Maldonado, Cabañas-Carranza and Caspeta-Mandujano1998). The species was diagnosed by having a ventral sucker twice the size of the oral sucker and directed posteriorly, with a longitudinal opening. The original description stated: ‘oral sucker subterminal, with two dorsolateral papillae, difficult to observe in many specimens’. We recently collected allocreadiid trematodes from the intestines of mountain mullets from several river basins across Mexico and other countries of Central America (Guatemala, El Salvador and Costa Rica). Sequences of the 28S ribosomal RNA (rRNA) gene were obtained from these trematodes to explore their genetic diversity and determine their relationships with other allocreadiids; in addition, their morphology was studied using light and scanning electron microscopy (SEM). The present study uses these data to address the following specific objectives: (1) to test whether the genus Creptotrema is monophyletic; (2) to determine whether C. agonostomi represent a species complex; and (3) to describe a new species following an integrative taxonomy approach.
Materials and methods
Sample collection
In total, 337 trematodes initially identified as C. agonostomi were collected between April 2013 and January 2018 from the intestines of mountain mullet from eight locations across eastern and western Mexico (n = 209), one in Guatemala (n = 34), two in El Salvador (n = 92) and one in Costa Rica (n = 2) (table 1 and fig. 1). Fish were collected using seine nets and electrofishing, kept alive until they were killed by pithing and necropsied. The gastrointestinal tract was removed and examined under a stereomicroscope. Trematodes were cleaned in 6.5% saline; some specimens were fixed in hot (nearly boiling) tap water and kept in vials with 4% formalin for subsequent morphological and SEM study. Other specimens (including paragenophores, sensu Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) were placed directly in vials with 100% molecular-grade ethanol for molecular analyses.

Fig. 1. Map of the localities where samples of ‘Creptotrema’ agonostomi were obtained in Mexico, Guatemala, El Salvador and Costa Rica. Number of localities correspond to data shown in table 1.
Table 1. Specimens of allocreadiids from Dajous monticola used for morphological and molecular analyses in this study; GenBank accessions numbers and the localities correspond with those given in fig. 1.

Molecular work
Twenty-three specimens were individually digested overnight at 56°C in a solution containing 10 mM tris hydrochloride (pH 7.6), 20 mM sodium chloride, 100 mM Disodium ethylenediaminetetraacetate dihydrate (Na2 EDTA) (pH 8.0), 1% Sarkosyl and 0.1 mg/ml proteinase K. DNA was extracted using DNAzol (Molecular Research Center, Cincinnati, Ohio, USA) following the manufacturer's protocol. The domains D1–D3 of the 28S rRNA gene were amplified using the forward primer 391 (5′-AGCGGAGGAAAAGAAACTAA-3′; Nadler & Hudspeth, Reference Nadler and Hudspeth1998) and the reverse primer 536 (5′-CAGCTATCCTGAGGGAAAC-3′; García-Varela & Nadler, Reference García-Varela and Nadler2005). Polymerase chain reaction (PCR) reactions (25 μl) consisted of 10 μM of each primer, 2.5 μl of 10× buffer, 2 mM magnesium chloride (1.5 μl), 10 μM of deoxynucleotide triphosphates (0.5 μl), 2 μl of the genomic DNA and 1 U of Taq DNA polymerase (0.125 μl) (Platinum Taq, Invitrogen Corporation, São Paulo, Brazil). PCR cycling parameters for amplifications included denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 1 min, annealing at 50°C for 1 min and extension at 72°C for 1 min, followed by a final post-amplification elongation at 72°C for 10 min. Sequencing reactions were performed using the initial primers and two internal primers 503 5′-CCTTGGTCCGTGTTTCAAGACG-3′ and 504 5′-CGTCTTGAAACACGGACTAAGG-3′ (García-Varela & Nadler, Reference García-Varela and Nadler2005), using ABI Big Dye (Applied Biosystems, Boston, Massachusetts, USA) terminator sequencing chemistry, and reaction products were separated and detected using an ABI 3730 capillary DNA sequencer. Contigs were assembled and base-calling differences resolved using Codoncode Aligner version 5.0.2 (Codoncode Corporation, Dedham, Massachusetts, USA). Sequences were deposited in GenBank (table 1).
Phylogenetic analyses
The newly generated sequences were aligned with available sequences of 33 species of allocreadiids and three callodistomids using ClustalW (Thompson et al., Reference Thompson, Higgins and Gibson1997), implemented in the web site http://www.genome.jp/tools/clustalw/. Based on previously published phylogenetic trees of Allocreadiidae Looss, 1902 (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury and García-Varela2016; Hernández-Mena et al., Reference Hernández-Mena, Pinacho-Pinacho, García-Varela, Mendoza-Garfias and Pérez-Ponce de León2019), sequences of species of Prosthenhystera Travassos, 1922 (Callodistomidae Odhner, 1910) were used for rooting the trees. Phylogenetic analyses were run under maximum likelihood (ML) and Bayesian inference (BI), employing GTR + G + I as the most appropriate model of nucleotide evolution as estimated with jModelTest v2 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012). ML inference (20 replicates), model parameters and bootstrap (Bt) support (1000 repetitions) were estimated with RAxML v7.0.4 (Stamatakis, Reference Stamatakis2006). BI analysis was carried out using MrBayes v3.2 (Ronquist et al., Reference Ronquist2012), running two independent Markov chain Monte Carlo runs of two chains each run (a heating parameter value of 0.5) for ten million generations and sampling tree topologies every 1000 generations (printfreq = 1000 samplefreq = 1000 diagnfreq = 10,000). Burn-in periods were set to the first 500 generations. A 50% majority-rule consensus tree and nodal support estimated as posterior probability (PP) values were calculated from the remaining trees. The phylogenetic trees obtained from both analyses were visualized in FigTree v1.4.2. (Rambaut, Reference Rambaut2006). Genetic distances for the 28S dataset were calculated as uncorrected p-distance using MEGA v6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013).
Morphological study
Specimens were stained with Mayer's paracarmine and Gomori's trichrome, dehydrated in a graded ethanol series, cleared in methyl salicylate and examined as permanent mounts in Canada balsam. Specimens were examined using an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan) with differential interference contrast optics. Measurements were taken using the Olympus Quick-Photo Image-Program, and are presented in micrometres (μm) with the range followed by the mean in parentheses. Drawings were made with a drawing tube attached to the microscope. Specimens were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México (UNAM) (table 1). For morphological comparison, the following type specimens were studied: C. agonostomi ex D. monticola, Río La Palma, Veracruz (CNHE 5288, 15 specimens, 5314, two specimens); ex D. monticola, Río Ayuquila, Jalisco (CNHE 4793, 13 specimens).
Twelve individuals of C. agonostomi from different localities were studied through SEM. The specimens were dehydrated through a graded ethanol series and critical-point dried with carbon dioxide. Specimens were mounted on metal stubs with carbon adhesive tabs, gold coated and examined at 15 kV in a Hitachi Stereoscan Model SU1510 SEM (Hitachi Ltd, Tokyo, Japan).
Results
Of the 337 mountain mullets collected between 2013 and 2018, around 106 specimens of trematodes morphologically similar to C. agonostomi were collected from the intestine of 37 fish, in 12 locations across Middle America, including the type locality, Río Cuitzmala, Jalisco, western Mexico (see also table 1). Twenty-three specimens were sequenced for the 28S rRNA gene, and the remaining specimens were processed for morphological analysis through light electron microscopy and SEM.
Phylogenetic analyses and genetic divergence
The partial 28S rRNA alignment consisted of 1399 nucleotide positions. No regions of alignment ambiguity were detected. BI and ML analyses of this dataset generated trees with identical topologies (fig. 2). Our phylogenetic analyses corroborated that Allocreadiidae is a well-supported monophyletic group. The genus Creptotrema was paraphyletic, considering the current morphological concept of the genus, and based on the presumption that C. funduli, the only member of the genus for which sequence data was available until now, is a member of the genus (fig. 2, in bold, see Discussion). Most notably, the phylogenetic analyses showed that trematodes that in the first instance were morphologically identified as C. agonostomi represent three separate species. The three new species formed a well-supported monophyletic group with two species of the genus Pseudoparacreptotrema (fig. 2). Two well-supported monophyletic groups are formed, one consisting of Pseudoparacreptotrema profundulusi + one of the new species from several localities along the Pacific coast of Middle America. A second group contained a new species from Río Axtla, San Luis Potosí, as the sister taxa to the clade comprising Pseudoparacreptotrema macracetabulata + another new species collected from mountain mullets in several river drainages along the Gulf of Mexico slope, although the last clade had low nodal support from both ML and BI analyses (fig. 2). Applying the reciprocal monophyly criteria for the three lineages comprising ‘C.’ agonostomi (with relatively high Bt and PP support values) nested along with Pseudoparacreptotrema macroacetabulata and Pseudoparacreptotrema profundulisi warrants the proposal that the specimens from mountain mullets should be allocated into the genus Pseudoparacreptotrema. We concluded that the species ‘C.’ agonostomi, for which 28S DNA sequences have not been generated yet, should be also placed in Pseudoparacreptotrema, as P. agonostomi (Salgado-Maldonado, Cabañas-Carranza & Caspeta-Mandujano, Reference Salgado-Maldonado, Cabañas-Carranza and Caspeta-Mandujano1998) n. comb. Needless to say, specimens of trematodes from mountain mullets from the type locality were sampled, although none of them possessed muscular oral lobes. Additionally, genetic divergence values for the 28S rRNA gene among species forming the genus Pseudoparacreptotrema further support the contention that they represent separate evolutionary units. Genetic divergence among all the species of the genus varied from 1.17% to 3.18%, which represents between 16 and 44 nucleotide positions. The molecular results prompted us to conduct a detailed morphological analysis on the specimens we sampled.

Fig. 2. Bayesian 50% majority-rule consensus phylogram inferred of analysis of 1399 nucleotide positions of 28S rRNA sequences of species of allocreadiids. Phylogram rooted with Prosthenhystera spp. Bayesian posterior probabilities and bootstrap support are given above the internode.
Morphological analyses
Very slight morphological and morphometrical differences were found among the species of allocreadiids from mountain mullets (table 2). Based on the morphological comparison and the molecular data, an amended diagnosis of the genus and the description of three new species are presented in the following.
Table 2. Morphometric data for species of Pseudoparacreptotrema from killifishes and mountain mullets in Middle America, including three new species described in this study; measurements are expressed in micrometres as the range and mean in parentheses.

B, body; L, length; W, width; OS, oral sucker; VSL/OSL, ventral sucker/oral sucker length (ratio); VSW/OSW, ventral sucker/oral sucker length (ratio); Ph, pharynx; VS, ventral sucker; LT, left testis; RT, right testis; PTSL, post-testicular space length; PTS(%), post-testicular space (%); O, ovary; BL/OL, body length/ovary length; SR, seminal receptacle; CS, cirrus sac; CSL/BL, cirrus sac length/body length; EGG, eggs. Mex, Mexico; Gua, Guatemala; Sal, El Salvador; CR, Costa Rica.
a Measurements taken from Salgado-Maldonado et al. (Reference Salgado-Maldonado, Cabañas-Carranza and Caspeta-Mandujano1998); those specimens are flattened.
Family: Allocreadiidae Looss
Pseudoparacreptotrema Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury & García-Varela, 2016
Amended diagnosis of Pseudoparacreptotrema
Modified from Pérez-Ponce de León et al. (Reference Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury and García-Varela2016). Body elongate, aspinose, squat, widest in mid-region of body, with bluntly rounded ends. Two muscular oral lobes present or absent; when present, lobes sickle-shaped or rounded, on lateral edges of oral sucker. Scattered eyespots on dorsal surface, on either side of pharynx. Oral sucker smaller than ventral sucker. Fifteen prominent dome-like papillae on oral sucker, four on the inner edge and 11 on the outer edge. Prepharynx lacking. Pharynx relatively well developed. Oesophagus relatively inconspicuous. Caeca extending to level of testes or slightly beyond. Testes entire, symmetrical or oblique. Cirrus sac voluminous, from posterior end of pharynx to anterior, mid or posterior end of ventral sucker; containing a folded seminal vesicle, pars prostatica and unarmed ejaculatory duct. Genital pore median, in anterior region of body, pre-bifurcal or post-bifurcal. Ovary entire, dextral, posterolateral to ventral sucker. Seminal receptacle postovarian. Laurer's canal present. Uterus mostly pre-testicular. Vitelline fields extending from pharynx level to posterior end of body, overlapping caeca ventrally. Eggs relatively few, large, operculate. Excretory bladder I-shaped, extending anteriorly to testes level. Parasites in the intestine of freshwater fishes of the families Profundulidae and Mugilidae, Middle America.
Taxonomic summary
Type species. Pseudoparacreptotrema profundulusi (Salgado-Maldonado, Caspeta-Mandujano & Martínez Ramírez, 2011).
Type host. Profundulus punctatus (Günther), Oaxaca killifish (Cyprinodontiformes: Profundulidae).
Other hosts. Profundulus balsanus Ahl; Profundulus oaxacae (Meek).
Infection site. Stomach.
Type locality. Río Templo, Tehuantepec River basin, Oaxaca, México (16°53′56.3″N, 96°09′57.3″W).
Other localities. Ojo de Agua Creek, Tehuantepec River basin, Oaxaca (16°39′38.6″N, 95°49′36.6″W). Rıo San José de las Flores, Atoyac-Verde River basin, Oaxaca (16°24′21.5″N, 97°44′22.6″W). Río Cahoapán, Guerrero (17°16′37.8″N, 99°35′04.7″W); Arroyo los Sabinos (16°25′39.9″N, 97°4′28.9″W); Río Chacalapa (15°55′54.8″N, 95°56′00.3″W); Río Chico (16°55′34.50″N, 96°12′27.42″W), Oaxaca.
Remarks
Members of the family Allocreadiidae are characterized as having an aspinose tegument, unarmed cirrus, well-developed cirrus sac, lacking an external seminal vesicle, and by the disposition of the vitelline follicles and gonads (Caira & Bogéa, Reference Caira, Bogéa, Jones, Bray and Gibson2005). The genus Pseudoparacreptotrema belongs in the Allocreadiidae (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury and García-Varela2016). The fact that the amended diagnosis of the genus now shows that their species may or may not have muscular lobes on the oral sucker implies that the genus needs further comparison with all genera of allocreadiids. Eight genera possess muscular lobes on the oral sucker, including Creptotrema, Creptotrematina Yamaguti, 1954, Bunoderella Schell, 1964, Crepidostomum Braun, 1900, Bunodera Railliet, 1896, Megalogonia Surber, 1928 and the recently resurrected Acrolichanus Ward, 1917 (Atopkin et al., Reference Atopkin, Sokolov, Vainutis, Voropaeva, Shedko and Choudhury2020). For these members of the Allocreadiidae, the term ‘papillose allocreadiids’ was coined by Hopkins (Reference Hopkins1933), and later used by Caira (Reference Caira1989). The muscular lobes in species of Pseudoparacreptotrema are numerically and morphologically different from most of the genera of ‘papillose allocreadiids’, except those in species of Creptotrema and Creptotrematina. These two latter genera possess two muscular lateral lobes on the outer edge of the oral sucker, either well developed as in C. sucumbiosa and C. lamothei, or small and inconspicuous as in C. funduli and Creptotrematina aguirrepequenoi Jiménez-Guzman, Reference Jiménez-Guzman1973; likewise, oral lobes in other species in the genus Creptotrema are sickle-shaped and transversally elongated along the anterior lateral edges of the oral sucker, as in C. creptotrema (Surber, Reference Surber1928; Travassos et al., Reference Travassos, Artigas and Pereira1928; Jiménez-Guzman, Reference Jiménez-Guzman1973; Curran, Reference Curran2008).
However, Pseudoparacreptotrema differs from species in these genera by the combination of other morphological traits such as the testes position, the length ratio between oral and ventral suckers and the extent of the vitelline field. It is admittedly unusual for an allocreadiid genus to contain species with or without oral lobes, although an exceptional reduction of oral lobes is seen in Bunodera inconstans (Choudhury & León-Règagnon, Reference Choudhury and León-Règagnon2005). We have taken a conservative position in our study and have chosen not to establish a separate genus for each of the genetic lineages uncovered through our phylogenetic analyses. We posit that adding three additional monotypic genera would provide no better understanding of the taxonomy or interrelationships of allocreadiids at this time.
The two species of Pseudoparacreptotrema lacking oral lobes – namely, P. macroacetabulata and P. profundulusi – differ from other genera of allocreadiids lacking oral lobes such as Allocreadium Looss, 1900 by the position of testes (symmetrical or oblique instead of tandem), and by the extent of vitelline fields (restricted to the region between ventral sucker and posterior end instead of along the entire body). Pseudoparacreptotrema macroacetabulata and P. profundulusi differ from species in Margotrema Lamothe, 1970 and Wallinia Pearse, 1920 by having a uterus that is entirely pre-testicular (and not extending to the posterior end of body), and by the vitelline fields reaching the posterior end of body. Finally, these two species differ from Paracreptotrema Choudhury, Pérez-Ponce de León, Brooks & Daverdin, 2006 and Paracreptotrematoides Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury & García-Varela, 2016, by a combination of traits such as the extent of vitelline fields, the ratio between the oral and ventral suckers, the position of the genital pore and the shape of the cirrus sac. Thus, the current composition of the genus includes six species, four of them as parasites of mountain mullets, and two as parasites of killifishes. Three new species are described herein.
Pseudoparacreptotrema pacificum Sereno-Uribe & García-Varela n. sp. (figs 3a, b)
ZooBank Life Science Identifier: urn:lsid:zoobank.org: act:urn:lsid:zoobank.org:act:91CCB7DF-98DC-469A-97E2-FD21A4E99F20.

Fig. 3. Pseudoparacreptotrema pacificum Sereno-Uribe & García-Varela n. sp. ex Dajaus monticola. (A) Whole worm (holotype, CNHE 11230); (B) detail of the terminal genitalia from a paratype. Scale bars: (A) 200 μm; (B) 50 μm.
Taxonomic summary
Type host. Dajaus monticola (Bancroft) Mountain mullet (Perciformes: Mugulidae).
Type locality. Puente Novillero, Chiapas (15°30′06″N, 92°56′34″W).
Additional localities. Costa Rica, Río Lajas (9°43′58.7″N, 85°13′58.23″W); El Salvador, Río Sunza (13°38′14″N, 89°50′41″W); Río Banderas (13°35′34″N, 89°49.07′W); Guatemala, Río Achihuate (14°16′16″N, 90°53′59″W); Mexico, Río Copalita, Oaxaca (15°53′44″N, 96°11′17″W); Río Santa María, Huatulco, Oaxaca (15°50′10″N, 96°19′47″W); Río Cuitzmala, Jalisco (19°27′35.8″N, 104°56′11.4″W).
Site of infection. Intestine.
Prevalence. One of one (100%), three of three (100%), six of seven (85%), one of five (20%), four of seven (57%), five of nine (55%), five of seven (71%), five of 45 (11%).
Type material. Holotype (CNHE 11230), 32 paratypes (CNHE 11231–11236).
Representative DNA sequences. 28S ribosomal DNA (rDNA) (MT180810–MT180823).
Etymology. The specific epithet refers to the distribution of the new species along the Pacific coast of Middle America.
Description
Based on 26 whole mounts and eight worms using SEM. Measurements given in table 2.
Body small, plump. Tegument smooth. Anterior end rounded, posterior end slightly tapered; maximum width at hindbody at testes level. Muscular oral lobes lacking. Scattered eyespots on ventral surface, around pharynx. Oral sucker subterminal, bearing 11 dome-like papillae along outer edge and four papillae on inner surface ). Ventral sucker well-developed, strongly muscular, with a vertical slit-like opening; ventral sucker larger and wider than oral sucker. Prepharynx absent. Pharynx muscular. Oesophagus inconspicuous. Intestinal bifurcation immediately posterior to pharynx. Caeca terminate blind at level of posterior margin of testes.
Testes two, oblique or slightly symmetrical, subspherical, in posterior third of body; cirrus sac long, ovoid, median, dorsal to ventral sucker, extending to mid-level of ventral sucker; cirrus sac containing a convoluted seminal vesicle, and pars prostatica. Genital pore median, pre-bifurcal. Ovary spherical, entire, dextral, overlapping with posterior margin of ventral sucker. Mehlis’ gland and Laurer's canal not observed. Seminal receptacle lateral to ovary, rounded. Uterus entirely pretesticular; metraterm weakly developed. Vitellaria follicular, in two ventrolateral fields; fields extending from mid-level between intestinal bifurcation and ventral sucker to slightly past the posterior border of testes; follicles confluent in post-testicular space. Eggs few, ovoid, operculate. Excretory vesicle I-shaped, tubular. Excretory pore terminal. In intestine of Dajous monticola in river basins along the Pacific coast of Middle America (Costa Rica, El Salvador, Mexico).
Taxonomic remarks
The species C. agonostomi (= Pseudoparacreptotrema agonostomi) was described by Salgado-Maldonado et al. (Reference Salgado-Maldonado, Cabañas-Carranza and Caspeta-Mandujano1998) from specimens recovered from the intestine of mountain mullets from three localities of Mexico – Río Cuitzmala, Jalisco, from Río La Palma, Veracruz, and also from the Balsas catfish, I. balsanus from Río Balsas, in the Pacific Ocean slope. The type series consisted of a holotype (specimen from D. monticola from Río Cuitzmala, CNHE 3195) plus 29 paratypes corresponding to specimens from the same host and locality (CNHE 3196); additionally, 13 voucher specimens from D. monticola from Río La Palma, Veracruz, were later deposited in the CNHE (5288–5289). All specimens are flattened, and some are in poor condition. These specimens were studied, and we discovered that six out of the 29 specimens from the type locality lack muscular lobes on the oral sucker. Of the 13 specimens from Río La Palma, only three showed evidence of inconspicuous lobes. The original description of the species stated, ‘Oral sucker subterminal, with 2 small dorsolateral papillae, difficult to observe in many specimens’ (Salgado-Maldonado et al., Reference Salgado-Maldonado, Cabañas-Carranza and Caspeta-Mandujano1998), and the figures of the holotype and paratype show the presence of oral lobes. The 26 specimens we studied from several localities along the Pacific Ocean slope between Costa Rica and Mexico (including the type locality), all of which form a highly supported monophyletic clade in the molecular phylogenetic analyses (fig. 2), lack oral lobes; this result was further corroborated with SEM microphotographs of several specimens. Based on all these facts, we concluded that two morphotypes are present in the type locality, one with oral lobes, considered herein as P. agonostomi n. comb., and one without oral lobes, described here as P. pacificum Sereno-Uribe & García-Varela n. sp. The new species differs from the other three species from mountain mullets in the lack of muscular oral lobes on the oral sucker. The new species resembles two species – P. profundulusi and P. macroacetabulata – in lacking oral lobes. However, P. pacificum Sereno-Uribe & García-Varela n. sp. differs from P. profundulusi by the testes position (symmetrical vs. oblique), by the position of the genital pore (prebifurcal vs. postbifurcal) and by host association (profundulids vs. mugilids). Pseudoparacreptotrema pacificum Sereno-Uribe & García-Varela n. sp. differs from P. macroacetabulata by the position of the genital pore, the size of oesophagus and the ratio between the oral and ventral suckers.
Pseudoparacreptotrema falciformis Hernández-Mena & Pinacho-Pinacho n. sp. (figs 4a, b)
ZooBank Life Science Identifier: urn:lsid:zoobank.org:act:8AC4EEE8-A829-455C-90F2-77129D18C59C.
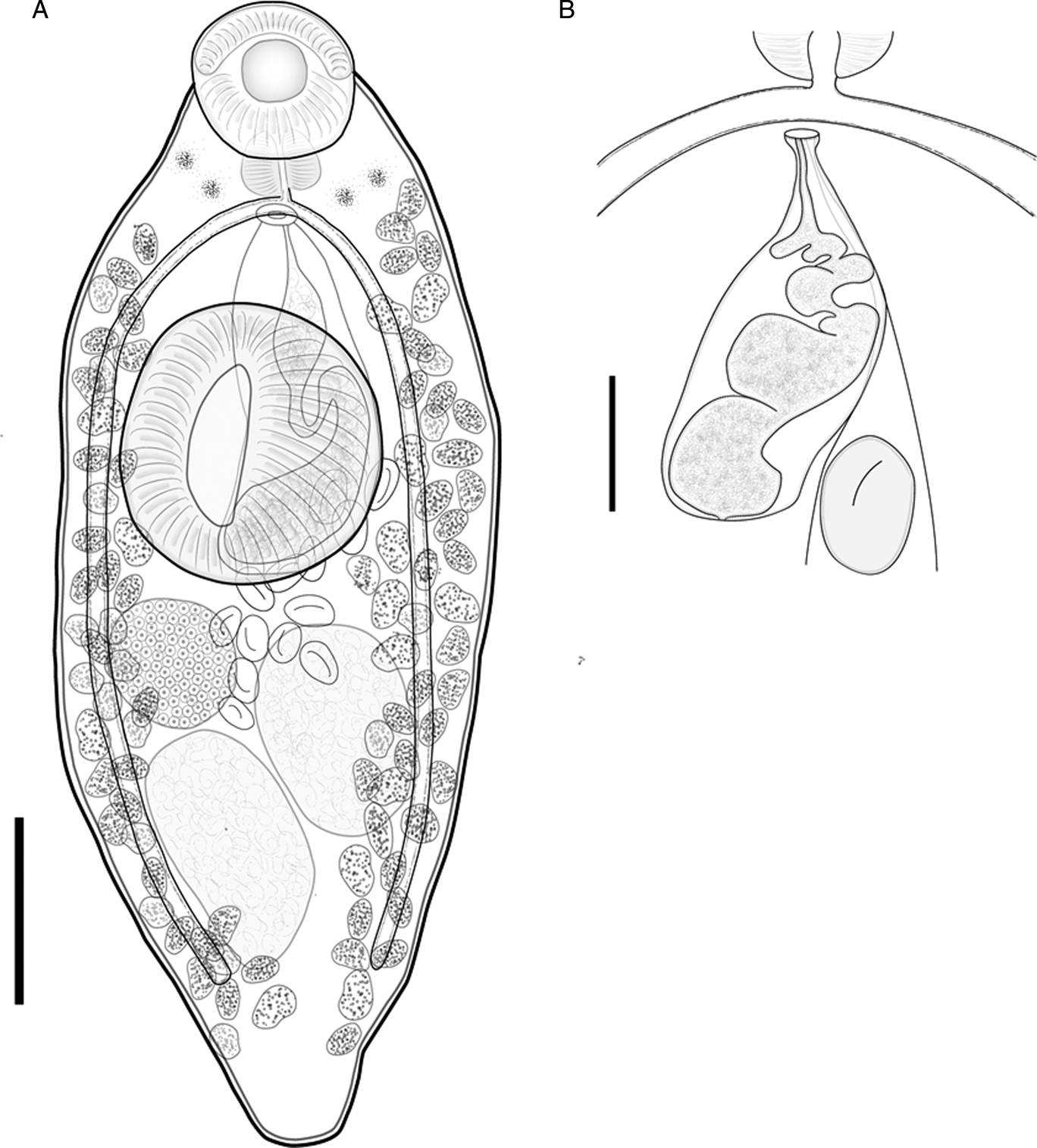
Fig. 4. Pseudoparacreptotrema falciformis Hernández-Mena & Pinacho-Pinacho n. sp. ex Dajaus monticola. (A) Whole worm (holotype, CNHE 11239); (B) detail of the terminal genitalia from a paratype. Scale bars: (A) 200 μm; (B) 50 μm.
Taxonomic summary
Type host. Dajaus monticola (Bancroft) Mountain mullet (Perciformes: Mugilidae).
Type locality. River at Matías Romero, Oaxaca, a tributary of the Río Coatzacoalcos (16°45″46.25′N, 95°01″27.9′W).
Other localities. Río La Palma, Veracruz (18°36′40.50″N, 95°39′43.96″W), and Río Tecolutla, Veracruz (20°27′52.58″N, 97°9′14.49″W).
Site in host. Intestine.
Prevalence. Matías Romero: one of one (100%); Río La Palma: two of two (100%); Río Tecolutla: one of one (100%).
Type material. Holotype (CNHE 11239), 18 paratypes (CNHE 11240–11241).
Representative DNA sequences. 28S rDNA (MT180824–MT180830).
Etymology. The specific epithet falciformis derives from the Latin words falcis (sickle) and forme (form) and refers as a neuter adjective of the sickle-shaped form of the muscular oral lobes.
Description
Based on 20 specimens. Measurements given in table 2.
Body small, plump. Tegument smooth. Anterior end rounded, posterior end slightly tapered; maximum width at ventral sucker level. Muscular oral lobe sickle-shaped, extending along anterolateral border of oral sucker; difficult to observe in some mounted specimens, but evident in SEM microphotographs (fig. 6f, g). Scattered eyespots on ventral surface, around pharynx. Oral sucker subterminal, bearing 11 dome-like papillae along outer edge, and four papillae on inner surface (fig. 7f, g); ventral sucker well-developed, strongly muscular, with elongate opening (figs 4a and 5f, g); ventral sucker larger and wider than oral sucker. Prepharynx lacking. Pharynx muscular, wider than long. Oesophagus inconspicuous. Intestinal bifurcation immediately posterior to pharynx. Caeca terminate blind at level of posterior margin of testes.
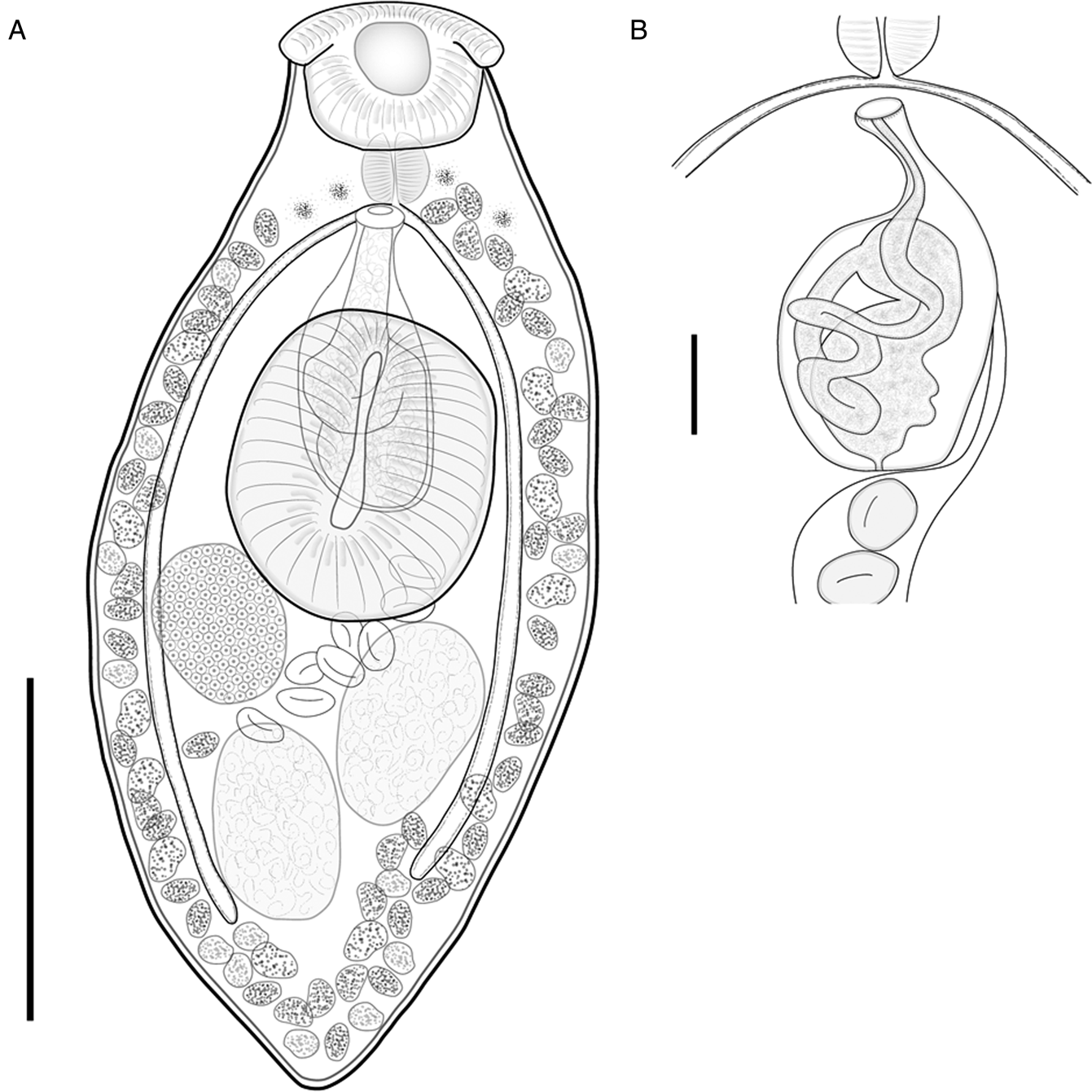
Fig. 5. Pseudoparacreptotrema axtlaensis Mendoza-Garfias & Choudhury n. sp. ex Dajaus monticola. (A) Whole worm (holotype, CNHE 11237); (B) detail of the terminal genitalia from a paratype. Scale bars: (A) 200 μm; (B) 50 μm.
Testes two, oblique, entire, subspherical, in posterior third of body; cirrus sac very long, ovoid, median, dorsal to ventral sucker, extending to posterior margin of ventral sucker; cirrus sac contains a convoluted seminal vesicle, and pars prostatica. Genital pore median, at level of intestinal bifurcation. Ovary spherical, entire, dextral, between right testis and ventral sucker. Mehlis’ gland and Laurer's canal not observed. Seminal receptacle lateral to ovary, rounded. Uterus pretesticular; metraterm weakly developed. Vitellaria follicular, in two ventrolateral fields; fields extend from posterior margin of pharynx to posterior end of body, half distance between right testis and posterior end of body; follicles not confluent in post-testicular space. Eggs relatively few, ovoid, thin-shelled, operculate. Excretory vesicle I-shaped. Excretory pore not observed. In intestine of D. monticola in Río Tecolutla, Veracruz, Río La Palma, Veracruz and Matías Romero, Oaxaca, south-eastern Mexico, all in the Gulf of Mexico slope.
Taxonomic remarks
The new species is included in a group of three species in the genus Pseudoparacreptotrema possessing muscular oral lobes, and can be readily distinguished from them by having sickle-shaped muscular structures on the anterior border of ventral sucker; the edges of these muscular structures form poorly developed muscular lobes. In addition, the large size of the testes and ovary, and the length of the cirrus sac in the new species set it apart from all other congeneric species (see table 2). In the molecular phylogenetic tree, P. falciformis Hernández-Mena & Pinacho-Pinacho n. sp. was recovered as the sister species of P. macroacetabulata. The latter species lacks any evidence of muscular oral lobes, possesses a relatively long oesophagus and prebifurcal genital pore, parasitizes profundulids and is found in river basins along the Pacific Ocean slope, whereas the new species occurs in mugilids in river basins along the Gulf of Mexico slope.
Pseudoparacreptotrema axtlaensis Mendoza-Garfias & Choudhury n. sp. (figs 5a, b)
ZooBank Life Science Identifier: urn:lsid:zoobank.org:act:36BFFAF1-278D-48A3-9022-A64C2F60F15A.
Taxonomic summary
Type host. Dajaus monticola (Bancroft), mountain mullet (Perciformes: Mugulidae).
Type locality. Río Axtla, Axtla de Terrazas, San Luis Potosí (21°28′2.7″N, 98°57′11.3″W).
Site in host. Intestine.
Prevalence. Three of three (100%).
Type material. Holotype (CNHE 11237), 20 paratypes (CNHE 11238).
Representative DNA sequences. 28S rDNA (MT180831–MT180832).
Etymology. The specific epithet refers to type locality where the new species was found, Río Axtla.
Description
Based on 25 specimens. Measurements given in table 2.
Body small, plump to elongate. Tegument smooth. Anterior end rounded, posterior end slightly tapered; maximum width at midbody. Two clearly discernible and well-developed muscular oral lobes present on anterolateral sides of oral sucker (fig. 6h). Scattered eyespots on ventral surface, between pharynx and intestinal bifurcation. Oral sucker subterminal, bearing 11 papillae along outer edge, and four papillae on inner surface (fig. 7h). Ventral sucker well-developed, strongly muscular, with longitudinal, narrow, incision-like opening (figs 5a and 6h). Ventral sucker larger and wider than oral sucker. Prepharynx absent. Pharynx muscular. Oesophagus inconspicuous; apparently pharynx leads to intestinal bifurcation. Caeca terminating blindly at level of posterior margin of posterior testis.

Fig. 6. Scanning electron microscopy of whole specimens of three new species of Pseudoparacreptotrema (A–H). (A–E) Pseudoparacreptotrema pacificum Sereno-Uribe & García-Varela n. sp.; (F, G) P. falciformis Hernández-Mena & Pinacho-Pinacho n. sp.; (H) P. axtlaensis Mandoza-Garfias & Choudhury n. sp. Scale bars: 200 μm.

Fig. 7. Scanning electron microscopy of the detail of the oral sucker showing dome-like papillae on the oral sucker of three new species of Pseudoparacreptotrema (A–H). (A–E) Pseudoparacreptotrema pacificum Sereno-Uribe & García-Varela n. sp.; (F, G) P. falciformis Hernández-Mena & Pinacho-Pinacho n. sp.; (H) P. axtlaensis Mandoza-Garfias & Choudhury n. sp. Arrows point to the position of 11 papillae on oral sucker. Scale bars: 50 μm.
Testes two, subspherical to ovoid, in posterior half of body, short distance from posterior end of body; cirrus sac relatively long, ovoid, median, dorsal to ventral sucker, extending to mid-level of ventral sucker; cirrus sac contains a highly convoluted seminal vesicle and pars prostatica. Genital pore median, at level of intestinal bifurcation. Ovary ovoid, entire, dextral. Mehlis’ gland and Laurer's canal not observed. Seminal receptacle lateral to ovary, rounded. Uterus short, pretesticular, with short loop between testes in some specimens; metraterm weakly developed. Eggs few, ovoid. Vitellarium follicular, in two ventrolateral fields; fields extend from pharynx to posterior end of body; follicles confluent in post-testicular space. Excretory vesicle I-shaped. Excretory pore not observed. In intestine of D. monticola in Río Axtla, San Luis Potosí, Pánuco River Basin, north-eastern Mexico.
Taxonomic remarks
Pseudoparacreptotrema axtlaensis Mendoza-Garfias & Choudhury n. sp., along with P. agonostomi and P. falciformis Hernández-Mena & Pinacho-Pinacho n. sp., possess muscular lobes on the oral sucker. However, the new species can be readily distinguished from P. agonostomi and P. falciformis Hernández-Mena & Pinacho-Pinacho n. sp. mainly by the shape of these oral lobes. In P. axtlaensis Mendoza-Garfias & Choudhury n. sp., the oral lobes are prominent and lateral on the edge of the oral sucker; the oral lobes in P. falciformis Hernández-Mena & Pinacho-Pinacho n. sp. are sickle-shaped and lateral lobes are inconspicuous. Furthermore, P. axtlaensis Mendoza-Garfias & Choudhury n. sp. can be differentiated by possessing smaller testes and ovary, and a shorter cirrus sac. Interestingly, these two species were found in D. monticola, and they are not each other's sister species. In the phylogenetic trees (fig. 2), P. axtlaensis Mendoza-Garfias & Choudhury n. sp. was recovered as the sister species of P. macroacetabulata + P. falciformis, albeit supported by low Bt and PP values. From P. agonostomi, P. axtlaensis Mendoza-Garfias & Choudhury n. sp. differs by the position of the genital pore, the distribution of vitelline follicles in the posterior end of body and geographical distribution. In the new species, the genital pore is located at the caecal bifurcation level, vitelline follicles are confluent and the species is distributed in the highlands of the Gulf of Mexico slope, whereas in P. agonostomi the genital pore opens at the pharynx level, vitelline follicles are not confluent at the posterior end and the species is distributed in a river basin of the Pacific Ocean slope.
Discussion
The paraphyly of Creptotrema
If we follow the morphological concept of the genus Creptotrema (see Caira & Bogéa, Reference Caira, Bogéa, Jones, Bray and Gibson2005), the results of this study suggest that the genus Creptotrema is not monophyletic. Furthermore, if we accept that the only species of the genus for which molecular data are available, C. funduli, from fundulids in USA, is actually a member of the genus (see contrary opinions in Manter, Reference Manter1962; Brooks, Reference Brooks1976), then molecular phylogenetic analyses shed light on the paraphyly of Creptotrema. Other studies have already demonstrated, through molecular analyses of the 28S rRNA gene, cases of paraphyletic genera within Allocreadiidae, which contrast with the morphological concept on which these genera were erected (see Atopkin & Shedko, Reference Atopkin and Shedko2014; Pérez-Ponce de León et al., Reference Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury and García-Varela2016; Petkevičiūtė, Reference Petkevičiūtė, Stunžėnas, Zhokhov, Poddubnaya and Stanevičiūtė2018). Recent molecular phylogenetic analyses of the Allocreadiidae show that the ‘papillose allocreadiids’ (sensu Hopkins, 1937) do not form a monophyletic assemblage; oral lobes apparently either arose or were secondarily lost several times during the evolutionary history of the group, depending on the plesiomorphic condition (see Hernández-Mena et al., Reference Hernández-Mena, Lynggaard, Mendoza-Garfias and Pérez-Ponce de León2016, Reference Hernández-Mena, Pinacho-Pinacho, García-Varela, Mendoza-Garfias and Pérez-Ponce de León2019; Pérez-Ponce de León et al., Reference Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury and García-Varela2016; Atopkin et al., Reference Atopkin, Sokolov, Vainutis, Voropaeva, Shedko and Choudhury2020). Clearly, more DNA sequences are needed to test the hypothesis of the paraphyly of Creptotrema, especially from congeners in South America where the largest species diversity of the genus is found, and where the type species was reported. Our study also showed that ‘Creptotrema’ agonostomi does not belong in the genus Creptotrema, but in Pseudoparacreptotrema. Three additional molecularly well-defined lineages were recognized through the criteria of reciprocal monophyly and genetic divergence values. We then used an integrative taxonomy approach (Dayrat, Reference Dayrat2005; Padial et al., Reference Padial, Miralles, de la Riva and Vences2010) to further evaluate these results; morphology, based on light and SEM of the body surface, molecular phylogenetic analyses, host association and geographical data were considered in the analysis. All three lineages were recovered as parasites of mountain mullets occurring in river basins of Middle America. Our results also demonstrated the importance of using SEM to better depict and understand the nature of important morphological traits within Allocreadiidae, such as the muscular lobes on the oral sucker (figs. 6a–h; 7a–h); several character states are found among these structures and future studies would reveal whether or not they can be regarded as homologous characters. Additionally, SEM is very useful for the description of the shape and distribution pattern of dome-like papillae on the body surface, and on the oral and ventral sucker surfaces. These characters have proven to be useful for future species delimitation and phylogenetic analyses, to solve taxonomic problems and to define more accurately the systematics of taxa, especially if used in combination with molecular data (e.g. Pérez-Ponce de León et al., Reference Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias and García-Varela2015b, Reference Pérez-Ponce de León, Martínez-Aquino and Mendoza-Garfiasc). In the case of allocreadiid trematodes, several authors have shown that SEM is an invaluable tool to accurately discern the presence and shape of structures such as muscular lobes on the oral sucker and papillae distribution (e.g. Caira, Reference Caira1989; Choudhury & Nelson, Reference Choudhury and Nelson2000; Razo-Mendivil et al., Reference Razo-Mendivil, Mendoza-Garfias, Pérez-Ponce de León and Rubio-Godoy2014; Hernández-Mena et al., Reference Hernández-Mena, Lynggaard, Mendoza-Garfias and Pérez-Ponce de León2016; Petkevičiūtė, Reference Petkevičiūtė, Stunžėnas, Zhokhov, Poddubnaya and Stanevičiūtė2018).
Historical biogeography and host association patterns
The history of diversification of allocreadiids in Middle American freshwater fishes does not support the hypothesis of a simple northward dispersal of allocreadiid taxa from South America, even though Middle America is considered as a ‘hot-spot’ of trematode diversification (Choudhury et al., Reference Choudhury, Aguirre-Macedo, Curran, Ostrowski de Núñez, Overstreet, Pérez-Ponce de León and Portes Santos2016, Reference Choudhury, García-Varela and Pérez-Ponce de León2017). Until the present study, only two genera of allocreadiids exhibited a reportedly trans-American distribution, Creptotrema and Creptotrematina (see also Dias et al., Reference Dias, Pérez-Ponce de León, de Almeida Camargo, Müller, da Silva, de Azevedo and Abdallah2020). Still, a molecular phylogenetic analysis that includes representative species of the genus Creptotrema from South America, and particularly the type species, is still pending. We predict that the South American species will nest together and then a new genus will be required to include the Nearctic species, C. funduli, for allocradiids of fundulids in USA, because ‘C.’ agonostomi does not belong to the genus anymore, and that creates a gap in the distribution of the species in the genus.
The host associations in species of Pseudoparacreptotrema do not follow a well-defined pattern. The two previously known species of Pseudoparacreptotrema are exclusively found in killifishes of the family Profundulidae, a group of primary Middle American freshwater cyprinodontiforms (Morcillo et al., Reference Morcillo, Ornelas-García, Alcaraz, Matamoros and Doadrio2016), and are part of the biogeographical ‘core parasite fauna’ (sensu Pérez-Ponce de León & Choudhury, Reference Pérez-Ponce de León and Choudhury2005) of killifishes. The three new species described in this study, all parasites of the mountain mullet, D. monticola, do not form a monophyletic assemblage. Instead, they belong to two clades, each clade also containing a species that infects killifishes as their sister taxa (fig. 2). The mountain mullet is a diadromous fish species widely distributed throughout Middle America (McMahan et al., Reference McMahan, Davis, Domínguez-Domínguez, García de León, Doadrio and Piller2013). Along the Atlantic slope D. monticola occurs from North Carolina south to Venezuela, while along the Pacific slope it extends from Baja California to Colombia (see Miller et al., Reference Miller, Minckley and Norris2005). Because of this distribution along the Atlantic and Pacific slopes, and the West Indies, several authors have hypothesized that this fish species may represent more than one taxon. McMahan et al. (Reference McMahan, Davis, Domínguez-Domínguez, García de León, Doadrio and Piller2013) found evidence for four distinct and divergent lineages corresponding largely with oceanic basins; these lineages of mountain mullet show no signs of gene flow, and apparently diverged during the Miocene (c. 14.7–7.0 Ma). The phylogeographic patterns of D. monticola uncovered by McMahan et al. (Reference McMahan, Davis, Domínguez-Domínguez, García de León, Doadrio and Piller2013) may have played a major role in shaping the diversification of their helminth parasite fauna, as perhaps in the case of the allocreadiids in this study.
Our molecular phylogenetic analyses of the 28S rRNA gene of the trematodes associated with mountain mullets revealed that these allocreadiids constitute three genetic lineages, in addition to P. agonostomi for which no sequence data is yet available. One of these species, P. pacificum Sereno-Uribe & García-Varela n. sp., is distributed in mountain mullets along the Pacific slope of Middle America, from Western Mexico southwards to Costa Rica; the other two species, P. falciformis Hernández-Mena & Pinacho-Pinacho n. sp. and P. axtlaensis Mendoza-Garfias & Choudhury n. sp., are distributed in mountain mullets along the Gulf of Mexico slope, the first one in river basins of south-eastern Mexico, and the second one in the Panuco River Basin, in north-eastern Mexico. However, the biogeographical associations between host and parasite lineages are not totally congruent; two lineages of mountain mullets occur along the Pacific slope. Interestingly, one geographically restricted and one widely distributed species of Pseudoparacreptotrema are found in them, whereas a single lineage of mountain mullet occurs in the Gulf of Mexico drainage with two species of Pseudoparacreptotrema. Apparently, Pseudoparacreptotrema has undergone drainage-mediated diversification along the Gulf of Mexico slope without apparent host diversification. More broadly though, species of the genus occur mainly along the Pacific slope, since P. profundulusi and P. macroacetabulata are found in profundulids from Oaxaca and Chiapas (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias, Choudhury and García-Varela2016).
Biogeographically, the Chortís block, one of the main geological structures in nuclear Middle America, played a role in the present-day distribution of freshwater fishes (Matamoros et al., Reference Matamoros, Kreiser and Schaefer2012). Apparently, the block had some influence in the diversification of D. monticola, in addition to freshwater availability and ocean currents and despite the lack of obvious geological barriers among lineages of this diadromous fish species (McMahan et al., Reference McMahan, Davis, Domínguez-Domínguez, García de León, Doadrio and Piller2013). However, the extent to which the Chortís block influenced the diversification of the trematodes associated with D. monticola is uncertain due to sampling limitations. In Middle America, three species of nematodes of mountain mullets have been reported – Spinitectus agonostomi Moravec & Barus, Reference Moravec and Barus1971, Paracapillaroides agonostomi Moravec, Salgado-Maldonado & Caspeta-Mandujano, 1999 and Procamallanus jalisciensis Salgado-Maldonado & Caspeta-Mandujano, 2000, as parasites of mountain mullets of the Cuitzmala River basin, in western Mexico (Garrido-Olvera et al., Reference Garrido-Olvera, García-Prieto and Pérez-Ponce de León2006); additionally, the cestode Symbranchiella magacirrus Scholz, Choudhury & Brooks, 2019 was described from mountain mullets in north-western Costa Rica (Scholz et al., Reference Scholz, Choudhury and Brooks2019). However, S. agonostomi stands out probably as the only member of the core parasite fauna of D. monticola (sensu Pérez-Ponce de León and Choudhury, Reference Pérez-Ponce de León and Choudhury2005); apparently, this nematode possesses a wide geographical range; the species was originally described in mountain mullets of Cuba and later reported in Guadeloupe and Puerto Rico (in the Caribbean region), and has been reported in Mexico in the Río Ayuquila, Jalisco, and in Rio La Palma, Veracruz, on the Pacific Ocean and Gulf of Mexico slopes, respectively (Moravec & Barus, Reference Moravec and Barus1971; Dyer et al., Reference Dyer, Bunckley-Williams and Williams1998; Garrido-Olvera et al., Reference Garrido-Olvera, García-Prieto and Pérez-Ponce de León2006). No genetic study of these nematodes has been conducted yet, but based on the diversification of the host is seems plausible to postulate that they will represent a species complex. Three of the four lineages of mountain mullets uncovered by McMahan et al. (Reference McMahan, Davis, Domínguez-Domínguez, García de León, Doadrio and Piller2013) occur in these three areas (Pacific, Gulf of Mexico and Caribbean). Clearly, the study of other components of the parasite fauna of this mugilid, through molecular phylogenetic analyses, will shed light on the evolutionary and biogeographical history of both parasites and the host.
Acknowledgements
We are indebted to Leopoldo Andrade and Eduardo Hernández for their help during fieldwork, and to Arturo Angulo, Universidad de Costa Rica, for his support in sampling fish in Costa Rica. We thank Luis García for providing specimens from the CNHE for comparison, and Laura M. Márquez and Nelly López for sequencing services.
Financial support
This project was partially funded by the Consejo Nacional de Ciencia y Tecnología (CONACyT) (G.P.P.L., grant number A1-S-21694), and by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT-UNAM) (M.G.V., grant number IN207219). A.C. wishes to thank St Norbert College for their support through Faculty Development grants and professional development funds.
Conflicts of interest
None.
Ethical standards
Specimens in Mexico were collected under the Cartilla Nacional de Colector Científico (FAUT 0202 and 0057) issued by the Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT), to M.G.V. and G.P.P.L., respectively. Specimens in Costa Rica were obtained under the collector permit issued by the Costa Rican government to Arturo Angulo from the Universidad de Costa Rica.











