1 Introduction
The migration of droplets over a solid substrate is a long-standing topic of interest in academia and crucial in several situations of practical importance (Oron, Davis & Bankoff Reference Oron, Davis and Bankoff1997; Craster & Matar Reference Craster and Matar2009). This scenario is commonly encountered in a variety of industrial applications, such as coating, ink-jet printing, microfluidics and micro-electronics, and medical diagnostics (Huebner et al. Reference Huebner, Sharma, Srisa-Art, Hollfelder, Edel and Demello2008; Casadevall i Solvas & DeMello Reference Casadevall i Solvas and DeMello2011; Choi et al. Reference Choi, Ng, Fobel and Wheeler2012; Campana et al. Reference Campana, Ubal, Giavedoni, Saita and Carvalho2016). These technological processes make use of different physical principles to displace the liquids, which includes electro-osmotic forces, electrohydrodynamic, thermocapillarity, chemical gradients, centrifugation and magneto-hydrodynamics (Cachile et al. Reference Cachile, Schneemilch, Hamraoui and Cazabat2002; Stone, Stroock & Ajdari Reference Stone, Stroock and Ajdari2004; Ubal et al. Reference Ubal, Campana, Giavedoni and Saita2008). In particular, the thermocapillary effect, which concentrates our attention here, has been employed in a large number of microfluidic devices to actuate droplets and bubbles given that the change of the surface tension with temperature is observable in most liquids (Karbalaei, Kumar & Cho Reference Karbalaei, Kumar and Cho2016).
Among the wide variety of possible configurations proposed in the literature for the displacement of droplets, we focus on droplets on horizontal surfaces that are driven by thermocapillary forces. A sessile drop over a non-uniformly heated substrate undergoes a shear stress along the surface of the liquid that moves the droplet from warmer to colder regions. The motion is quite complex and disagreement in the current knowledge of the topic clearly indicates the need for more detailed understanding of the dynamics of these flows (Karbalaei et al. Reference Karbalaei, Kumar and Cho2016).
There is a large volume of published studies describing the thermocapillary motion of droplets. Brochard-Wyart (Reference Brochard-Wyart1989) analysed the motion of a wedge-shape drop in the presence of both chemical and thermal gradients. The droplet may move to hot or cold regions depending on the selected liquid–substrate system. The result is analogous to the Soret effect in binary mixtures, where it is well known that a solute can move toward either the cold or the hot region. Ford & Nadim (Reference Ford and Nadim1994) extended the study to a steady drop with an arbitrary but fixed shape, and derived a general analytical expression for the velocity using the Navier slip condition. They assumed different contact angles at the rear and leading fronts, and found a threshold depinning force that depends on the applied temperature gradient. Smith (Reference Smith1995) also assumed slipping at the liquid solid interface, but introduced a dynamic boundary condition that relates the contact line speed with the contact angle. He treated the steady state case and found two possible regimes: either a droplet moving with a constant velocity and fixed profile or a pinned (cylindrical) droplet with an inner circulation flow.
Gomba & Homsy (Reference Gomba and Homsy2010) studied the problem using a precursor film model at the contact line. One crucial difference with previous works is that they solve an initial value problem for the displacement of the droplet, without imposing restrictions on neither the shape of the interface nor the velocity of the contact lines. This approach allowed the authors to find other solutions different from the steady ones reported before. They carried out a parametric study using the contact angle and they distinguished three different flow regimes. For small contact angles, the drop spreads in the direction of motion, while for large contact angles the liquid migrates steadily preserving its shape. For intermediate contact angles, the authors alluded to a transient complex dynamics where the droplet breaks up into smaller droplets but did not investigate this regime exhaustively. One of our goals here is to shed light on this rich flow regime.
More recently, Karapetsas, Sahu & Matar (Reference Karapetsas, Sahu and Matar2013) also solved an initial value problem and analysed the spreading of droplets initially far from equilibrium and cases where the surface energies depend on the temperature. They showed that for constant equilibrium contact angles, the substrate temperature gradients combined with gravity give rise to enhanced spreading rates, characterized by exponents as large as
![]() $2/3$
, larger than those predicted by Tanner’s law. For cases where the surface energy changes with temperature, they observed a rather complex dynamics that includes the non-monotonic displacements of drops and stick–slip movements of the contact line. Chaudhury & Chakraborty (Reference Chaudhury and Chakraborty2015) compared ‘normal’ and ‘self-rewetting’ fluids. In the first the surface tension presents a linear dependence with temperature, while in the second the relationship is quadratic. They found power laws for the width of small-contact-angle droplets with time, that are in good agreement with the results of Gomba & Homsy (Reference Gomba and Homsy2010) and Karapetsas et al. (Reference Karapetsas, Sahu, Sefiane and Matar2014), respectively. Diametrically opposed, Nguyen & Chen (Reference Nguyen and Chen2010) focused on large-contact-angle droplets and found that non-wetting liquids (i.e. contact angles larger than
$2/3$
, larger than those predicted by Tanner’s law. For cases where the surface energy changes with temperature, they observed a rather complex dynamics that includes the non-monotonic displacements of drops and stick–slip movements of the contact line. Chaudhury & Chakraborty (Reference Chaudhury and Chakraborty2015) compared ‘normal’ and ‘self-rewetting’ fluids. In the first the surface tension presents a linear dependence with temperature, while in the second the relationship is quadratic. They found power laws for the width of small-contact-angle droplets with time, that are in good agreement with the results of Gomba & Homsy (Reference Gomba and Homsy2010) and Karapetsas et al. (Reference Karapetsas, Sahu, Sefiane and Matar2014), respectively. Diametrically opposed, Nguyen & Chen (Reference Nguyen and Chen2010) focused on large-contact-angle droplets and found that non-wetting liquids (i.e. contact angles larger than
![]() $90^{\circ }$
) are faster than those with contact angles just below
$90^{\circ }$
) are faster than those with contact angles just below
![]() $90^{\circ }$
. They explained this difference in terms of the large internal convection observed for the smaller angle case, that opposes to the Marangoni stress.
$90^{\circ }$
. They explained this difference in terms of the large internal convection observed for the smaller angle case, that opposes to the Marangoni stress.
The theoretical models and their predictions are partially validated by the available experimental literature. Brzoska, Brochard-Wyart & Rondelez (Reference Brzoska, Brochard-Wyart and Rondelez1993) and Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005) found a threshold in the temperature gradient at which the droplet migrates with a fixed shape or, below the threshold, the drop may not move due to the contact-angle hysteresis. Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) also concluded that above this critical value, the velocity of the droplet is proportional to the temperature gradient and inversely proportional to the viscosity, in agreement with Brochard-Wyart (Reference Brochard-Wyart1989) and Gomba & Homsy (Reference Gomba and Homsy2010). Additionally, Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005) compared the experimental results with the predictions of a theoretical expression derived by Ford & Nadim (Reference Ford and Nadim1994) and found that the fitting of the experimental values is more sensitive to contact angle hysteresis than to the magnitude of the slip length. Pratap, Moumen & Subramanian (Reference Pratap, Moumen and Subramanian2008) reported experiments of decane droplets on a polydimethylsiloxane (PDMS)-coated surface. In this case, the significant distortion observed in the footprint of the drop from a circular shape is compatible with the modelling of Karapetsas et al. (Reference Karapetsas, Sahu and Matar2013), where the surface energies (and contact angles) vary with the local temperature. The evolution into a film predicted by Gomba & Homsy (Reference Gomba and Homsy2010) for vanishingly small contact angles resembles the profiles obtained in the experiments of Sur, Bertozzi & Behringer (Reference Sur, Bertozzi and Behringer2003), while the dependence of the velocity on the thermal gradient and viscosity, for droplets that move keeping their shape, are compatible with experiments reported by Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005). Interestingly, Dai et al. (Reference Dai, Khonsari, Shen, Huang and Wang2017) showed experiments where a thin film fluid is deposited behind the droplet, as observed in the simulations of Gomba & Homsy (Reference Gomba and Homsy2010).
Other theoretical predictions seem to lack correlated experimental support. For instance, the occurrence of the breakups for intermediate contact angles, which is one of main goals of this article. In the experiments of Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993), where the authors employ PDMS on hydrophobic surfaces and the contact angle is
![]() $\unicode[STIX]{x1D703}=13^{\circ }$
, the larger volumes should break up into smaller droplets according to Gomba & Homsy (Reference Gomba and Homsy2010). Nevertheless, the experiments show that the droplets enlarge and displace as a single droplet. Another open issue is the relationship between the displacement velocity
$\unicode[STIX]{x1D703}=13^{\circ }$
, the larger volumes should break up into smaller droplets according to Gomba & Homsy (Reference Gomba and Homsy2010). Nevertheless, the experiments show that the droplets enlarge and displace as a single droplet. Another open issue is the relationship between the displacement velocity
![]() $U$
and the footprint
$U$
and the footprint
![]() $R$
. The model of Brzoska predicts a linear dependence for small droplets that it is not observed in Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005) experiments for alkane hydrocarbons, despite the volumes are comparable with the smallest ones of Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993). On the other hand, the modelling of Gomba & Homsy (Reference Gomba and Homsy2010), under non-gravity conditions, predicts that
$R$
. The model of Brzoska predicts a linear dependence for small droplets that it is not observed in Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005) experiments for alkane hydrocarbons, despite the volumes are comparable with the smallest ones of Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993). On the other hand, the modelling of Gomba & Homsy (Reference Gomba and Homsy2010), under non-gravity conditions, predicts that
![]() $U\propto A(R)^{0.4}$
,
$U\propto A(R)^{0.4}$
,
![]() $A$
being the cross-sectional area of the droplet.
$A$
being the cross-sectional area of the droplet.
In particular, Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993), who performed experiments for a wider range of volumes than Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005), presented results that triggered some of the questions that motivated the present work. For example, the experiments show a change in the curve
![]() $U$
versus
$U$
versus
![]() $R$
, from a linear law for the smallest droplets to a weaker dependence with
$R$
, from a linear law for the smallest droplets to a weaker dependence with
![]() $R$
for larger volumes, suggesting a saturation for
$R$
for larger volumes, suggesting a saturation for
![]() $R\rightarrow \infty$
. Also, larger droplets present an elongation in the direction of displacement. Brzoska and collaborators attribute the saturation in
$R\rightarrow \infty$
. Also, larger droplets present an elongation in the direction of displacement. Brzoska and collaborators attribute the saturation in
![]() $U$
and the elongation of droplets to the effect of gravity. The elongation of droplets in their experiments is also observed in the simulations by Gomba & Homsy (Reference Gomba and Homsy2010). Nevertheless, the theoretical results predict that the elongation in the experiments of Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) with the largest areas and the strongest thermal gradients (
$U$
and the elongation of droplets to the effect of gravity. The elongation of droplets in their experiments is also observed in the simulations by Gomba & Homsy (Reference Gomba and Homsy2010). Nevertheless, the theoretical results predict that the elongation in the experiments of Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) with the largest areas and the strongest thermal gradients (
![]() $\unicode[STIX]{x1D735}T=1~\text{C}~\text{mm}^{-1}$
) must be followed by a breakup into smaller droplets. The breakup has not been reported in experiments.
$\unicode[STIX]{x1D735}T=1~\text{C}~\text{mm}^{-1}$
) must be followed by a breakup into smaller droplets. The breakup has not been reported in experiments.
After review of the available experimental data, we observe that all the experiments were performed with non-polar liquids (polydimethylsiloxane and alkane hydrocarbons), while the modelling employed by Gomba & Homsy (Reference Gomba and Homsy2010) is adequate for polar liquids (Starov, Velarde & Radke Reference Starov, Velarde and Radke2007; Mac Intyre, Gomba & Perazzo Reference Mac Intyre, Gomba and Perazzo2016). The disagreement between theory and experiments, and also the differences in the experimental results, suggest that the dynamics may depend on the polarity of the liquid. Furthermore, the experiments were made under gravity conditions and thus, it should be included in the modelling to evaluate its role in the saturation of
![]() $U$
and the elongation of the droplets.
$U$
and the elongation of the droplets.
Summarizing, our ultimate goal here is to understand the origin of the mentioned disagreements by exploring the effects of the polarity of liquids and the gravity on the velocity of the travelling droplets and the occurrence of breakups. In the present work different flow regimes are identified, the drop velocity for each regime is determined, and the non-occurrence of breakup in the large volume experiments is explained. In addition, simulations with gravity included are presented in order to ascertain its effect on morphology, velocity and stability of the drop.
The article is organized as follows. In § 2, we present the mathematical modelling for the droplet evolution. In § 3, we describe the flow of droplets with small contact angles and demonstrate the independence of the dynamics from the molecular potential. In § 4, we present the behaviour of the droplets for intermediate contact angles. We show that, depending on the polar nature of the liquid, the droplets migrate with a steady profile or break up into a smaller droplets. A study of the stability of the thickness profile sheds light on whether or not breakup is observed. In § 5, we focus on the large-contact-angle regime of flow. There, we show that droplets migrate keeping their initial shape for both potentials and when we include the gravity, the shape of the droplets modifies only slightly from their initially steady shape. Finally, in § 6 we present the discussions and conclusions.
2 Mathematical model
We consider a two-dimensional droplet of density
![]() $\unicode[STIX]{x1D70C}$
and viscosity
$\unicode[STIX]{x1D70C}$
and viscosity
![]() $\unicode[STIX]{x1D707}$
deposited on a non-uniformly heated surface, as shown in figure 1. Under the assumptions of lubrication approximation, the Navier–Stokes equations are reduced to a single partial differential equation for the thickness
$\unicode[STIX]{x1D707}$
deposited on a non-uniformly heated surface, as shown in figure 1. Under the assumptions of lubrication approximation, the Navier–Stokes equations are reduced to a single partial differential equation for the thickness
![]() $h$
of the droplet. Although this approximation assumes small slope of the liquid–gas interface, it is usually employed to describe partial wetting systems. Its applicability was studied and small differences of a few per cent were found when compared with solutions of the Navier–Stokes equation for contact angles as large as
$h$
of the droplet. Although this approximation assumes small slope of the liquid–gas interface, it is usually employed to describe partial wetting systems. Its applicability was studied and small differences of a few per cent were found when compared with solutions of the Navier–Stokes equation for contact angles as large as
![]() $40^{\circ }$
(Goodwin & Homsy Reference Goodwin and Homsy1991; Perazzo & Gratton Reference Perazzo and Gratton2004).
$40^{\circ }$
(Goodwin & Homsy Reference Goodwin and Homsy1991; Perazzo & Gratton Reference Perazzo and Gratton2004).

Figure 1. Sketch of the problem: a two-dimensional droplet of thickness
![]() $h(x,t)$
resting on a non-uniformly heated surface. The temperature induces a gradient of the surface tension which drives the liquid towards the region with the highest surface energy. The droplet is surrounded by a constant film thickness with an initial (equilibrium) thickness
$h(x,t)$
resting on a non-uniformly heated surface. The temperature induces a gradient of the surface tension which drives the liquid towards the region with the highest surface energy. The droplet is surrounded by a constant film thickness with an initial (equilibrium) thickness
![]() $h_{film}$
(Mac Intyre et al.
Reference Mac Intyre, Gomba and Perazzo2016).
$h_{film}$
(Mac Intyre et al.
Reference Mac Intyre, Gomba and Perazzo2016).
The flow field
![]() $u(x,z,t)$
is governed by the momentum balance equation (Oron et al.
Reference Oron, Davis and Bankoff1997; Craster & Matar Reference Craster and Matar2009)
$u(x,z,t)$
is governed by the momentum balance equation (Oron et al.
Reference Oron, Davis and Bankoff1997; Craster & Matar Reference Craster and Matar2009)
and the flow continuity
where
![]() $h=h(x,t)$
is the thickness profile of the drop. The second term in (2.1) is given by
$h=h(x,t)$
is the thickness profile of the drop. The second term in (2.1) is given by
![]() $\unicode[STIX]{x1D6FE}$
being the surface tension and
$\unicode[STIX]{x1D6FE}$
being the surface tension and
![]() $g$
the gravity. The term
$g$
the gravity. The term
![]() $\unicode[STIX]{x1D6F1}(h)$
represents the disjoining–conjoining pressure defined by (Oron et al.
Reference Oron, Davis and Bankoff1997; Schwartz & Roy Reference Schwartz and Roy2004)
$\unicode[STIX]{x1D6F1}(h)$
represents the disjoining–conjoining pressure defined by (Oron et al.
Reference Oron, Davis and Bankoff1997; Schwartz & Roy Reference Schwartz and Roy2004)
The thickness of the energetically favoured molecular film is represented by
![]() $h_{\ast }$
and
$h_{\ast }$
and
![]() $\unicode[STIX]{x1D705}$
is related to the static contact angle
$\unicode[STIX]{x1D705}$
is related to the static contact angle
![]() $\unicode[STIX]{x1D703}$
by (Schwartz & Eley Reference Schwartz and Eley1998)
$\unicode[STIX]{x1D703}$
by (Schwartz & Eley Reference Schwartz and Eley1998)
The contact angle
![]() $\unicode[STIX]{x1D703}$
is defined as the angle at the inflection point for large drops (Schwartz & Eley Reference Schwartz and Eley1998; Gomba & Homsy Reference Gomba and Homsy2009). Once
$\unicode[STIX]{x1D703}$
is defined as the angle at the inflection point for large drops (Schwartz & Eley Reference Schwartz and Eley1998; Gomba & Homsy Reference Gomba and Homsy2009). Once
![]() $\unicode[STIX]{x1D703}$
is chosen, the value of
$\unicode[STIX]{x1D703}$
is chosen, the value of
![]() $\unicode[STIX]{x1D705}$
is determined and, therefore
$\unicode[STIX]{x1D705}$
is determined and, therefore
![]() $\unicode[STIX]{x1D703}$
quantifies the strength of the molecular interaction between the liquid and the substrate (the relationship among
$\unicode[STIX]{x1D703}$
quantifies the strength of the molecular interaction between the liquid and the substrate (the relationship among
![]() $\unicode[STIX]{x1D705}$
, the Hammaker constants and
$\unicode[STIX]{x1D705}$
, the Hammaker constants and
![]() $\unicode[STIX]{x1D703}$
is discussed in Solomentsev & White (Reference Solomentsev and White1999) and Mac Intyre et al. (Reference Mac Intyre, Gomba and Perazzo2016)).
$\unicode[STIX]{x1D703}$
is discussed in Solomentsev & White (Reference Solomentsev and White1999) and Mac Intyre et al. (Reference Mac Intyre, Gomba and Perazzo2016)).
The definition in (2.4) has been largely employed to model the effects of molecular interactions. Here, we focus on two different types of molecular potential. On the one hand, the case
![]() $(n,m)=(3,2)$
that represents the competition of London–van der Waals (
$(n,m)=(3,2)$
that represents the competition of London–van der Waals (
![]() $n=3$
) and ionic–electrostatics forces (
$n=3$
) and ionic–electrostatics forces (
![]() $m=2$
) (Schwartz & Eley Reference Schwartz and Eley1998; Gotkis et al.
Reference Gotkis, Ivanov, Murisic and Kondic2006). The dependence with
$m=2$
) (Schwartz & Eley Reference Schwartz and Eley1998; Gotkis et al.
Reference Gotkis, Ivanov, Murisic and Kondic2006). The dependence with
![]() $m=2$
, valid for thickness much lower than the Debye length, was verified in experiments of water (polar liquid) films on glass, quartz and mica (Derjaguin & Churaev Reference Derjaguin and Churaev1974). The term with
$m=2$
, valid for thickness much lower than the Debye length, was verified in experiments of water (polar liquid) films on glass, quartz and mica (Derjaguin & Churaev Reference Derjaguin and Churaev1974). The term with
![]() $m=3$
is an exact classical result obtained by summing individual London–van der Waals interactions between molecules. On the other hand, the case
$m=3$
is an exact classical result obtained by summing individual London–van der Waals interactions between molecules. On the other hand, the case
![]() $(n,m)=(4,3)$
that correctly describes retarded and non-retarded effects in London–van der Waals interactions, as in experiments with tetradecane on quartz and hexane on metals (Derjaguin & Churaev Reference Derjaguin and Churaev1974; Derjaguin, Rabinovich & Churaev Reference Derjaguin, Rabinovich and Churaev1978; Starov et al.
Reference Starov, Velarde and Radke2007). The term with
$(n,m)=(4,3)$
that correctly describes retarded and non-retarded effects in London–van der Waals interactions, as in experiments with tetradecane on quartz and hexane on metals (Derjaguin & Churaev Reference Derjaguin and Churaev1974; Derjaguin, Rabinovich & Churaev Reference Derjaguin, Rabinovich and Churaev1978; Starov et al.
Reference Starov, Velarde and Radke2007). The term with
![]() $n=4$
is an exact expression that results from considering retardation effects in London–van der Waals interactions (Oron & Bankoff Reference Oron and Bankoff2001; Glasner & Witelski Reference Glasner and Witelski2003; Starov et al.
Reference Starov, Velarde and Radke2007). Summarizing, the pairs
$n=4$
is an exact expression that results from considering retardation effects in London–van der Waals interactions (Oron & Bankoff Reference Oron and Bankoff2001; Glasner & Witelski Reference Glasner and Witelski2003; Starov et al.
Reference Starov, Velarde and Radke2007). Summarizing, the pairs
![]() $(3,2)$
and
$(3,2)$
and
![]() $(4,3)$
model the molecular interactions for polar and non-polar liquids, respectively.
$(4,3)$
model the molecular interactions for polar and non-polar liquids, respectively.
Equation (2.1) is subject to two boundary conditions: no slip at the solid substrate and tangential Marangoni stress along the liquid–air interface (Ehrhard & Davis Reference Ehrhard and Davis1991); that is
The surface tension
![]() $\unicode[STIX]{x1D6FE}$
is linear in the temperature
$\unicode[STIX]{x1D6FE}$
is linear in the temperature
![]() $T(x,h)$
at the air–liquid interface (Eötvös Reference Eötvös1886; Guggenheim Reference Guggenheim1945)
$T(x,h)$
at the air–liquid interface (Eötvös Reference Eötvös1886; Guggenheim Reference Guggenheim1945)
where
![]() $\unicode[STIX]{x1D6FE}_{0}$
is the surface tension at
$\unicode[STIX]{x1D6FE}_{0}$
is the surface tension at
![]() $T=T_{0}$
and
$T=T_{0}$
and
![]() $\unicode[STIX]{x1D70E}$
a positive constant. Based on experimental data (Brzoska et al.
Reference Brzoska, Brochard-Wyart and Rondelez1993; Chen et al.
Reference Chen, Troian, Darhuber and Wagner2005), it is reasonable to assume that the conduction is the main heat transfer mechanism within the drop (i.e. Péclet number
$\unicode[STIX]{x1D70E}$
a positive constant. Based on experimental data (Brzoska et al.
Reference Brzoska, Brochard-Wyart and Rondelez1993; Chen et al.
Reference Chen, Troian, Darhuber and Wagner2005), it is reasonable to assume that the conduction is the main heat transfer mechanism within the drop (i.e. Péclet number
![]() $Pe=HU/\unicode[STIX]{x1D6FF}\ll 1$
) and the conductivity of the liquid is high (i.e. Biot number
$Pe=HU/\unicode[STIX]{x1D6FF}\ll 1$
) and the conductivity of the liquid is high (i.e. Biot number
![]() $Bi=Hq_{\infty }/\unicode[STIX]{x1D705}_{t}\ll 1$
). Here,
$Bi=Hq_{\infty }/\unicode[STIX]{x1D705}_{t}\ll 1$
). Here,
![]() $H$
is the maximum droplet thickness,
$H$
is the maximum droplet thickness,
![]() $U$
the migration velocity,
$U$
the migration velocity,
![]() $\unicode[STIX]{x1D6FF}$
the liquid thermal diffusivity,
$\unicode[STIX]{x1D6FF}$
the liquid thermal diffusivity,
![]() $q_{\infty }$
the interfacial heat transfer coefficient and
$q_{\infty }$
the interfacial heat transfer coefficient and
![]() $\unicode[STIX]{x1D705}_{t}$
the liquid thermal conductivity. Consequently, the unknown temperature
$\unicode[STIX]{x1D705}_{t}$
the liquid thermal conductivity. Consequently, the unknown temperature
![]() $T$
is related to the linear temperature profile at the substrate
$T$
is related to the linear temperature profile at the substrate
![]() $T_{s}(x)$
by means of
$T_{s}(x)$
by means of
![]() $T(x,h)=T_{s}(x)$
(Ehrhard & Davis Reference Ehrhard and Davis1991; Gomba & Homsy Reference Gomba and Homsy2010) and the boundary condition at the drop air interface becomes
$T(x,h)=T_{s}(x)$
(Ehrhard & Davis Reference Ehrhard and Davis1991; Gomba & Homsy Reference Gomba and Homsy2010) and the boundary condition at the drop air interface becomes
![]() $\unicode[STIX]{x1D70F}$
being a positive constant and
$\unicode[STIX]{x1D70F}$
being a positive constant and
![]() $L$
the distance of application of the two temperature.
$L$
the distance of application of the two temperature.
We set the scale in both space directions as
![]() $h_{c}=x_{c}=a$
, where
$h_{c}=x_{c}=a$
, where
![]() $a=\sqrt{\unicode[STIX]{x1D6FE}_{0}/\unicode[STIX]{x1D70C}g}$
is the capillary length, and define the characteristic time as
$a=\sqrt{\unicode[STIX]{x1D6FE}_{0}/\unicode[STIX]{x1D70C}g}$
is the capillary length, and define the characteristic time as
![]() $t_{c}=3\unicode[STIX]{x1D707}a/\unicode[STIX]{x1D6FE}_{0}$
. Equation (2.2) for the thickness
$t_{c}=3\unicode[STIX]{x1D707}a/\unicode[STIX]{x1D6FE}_{0}$
. Equation (2.2) for the thickness
![]() $h(x,t)$
becomes
$h(x,t)$
becomes
where the constants are
and
![]() $h_{\ast }$
is in units of
$h_{\ast }$
is in units of
![]() $a$
. The term which models the gravity effect is multiplied by the constant
$a$
. The term which models the gravity effect is multiplied by the constant
![]() $G=x_{c}^{2}\unicode[STIX]{x1D70C}g/\unicode[STIX]{x1D6FE}_{0}$
, which in the selected scale results in
$G=x_{c}^{2}\unicode[STIX]{x1D70C}g/\unicode[STIX]{x1D6FE}_{0}$
, which in the selected scale results in
The evolution equation (2.10) includes capillary, gravity, disjoining and Marangoni effects. This fourth-order differential equation is solved using finite element technique in the COMSOL Multiphysics environment, and is subject to periodic boundary conditions at both extreme of the domain for the height of the droplet
![]() $h$
and pressure
$h$
and pressure
![]() $p$
. A convergence analysis was carried out to check the accuracy of the calculations, and the results were satisfactory compared with those obtained using a finite difference technique (Gomba & Homsy Reference Gomba and Homsy2010).
$p$
. A convergence analysis was carried out to check the accuracy of the calculations, and the results were satisfactory compared with those obtained using a finite difference technique (Gomba & Homsy Reference Gomba and Homsy2010).
The initial condition in the following simulations corresponds to the profile of a static droplet of area
![]() $A$
when the temperature gradient is zero. We employ the static profiles reported by Mac Intyre et al. (Reference Mac Intyre, Gomba and Perazzo2016) for a molecular potential with
$A$
when the temperature gradient is zero. We employ the static profiles reported by Mac Intyre et al. (Reference Mac Intyre, Gomba and Perazzo2016) for a molecular potential with
![]() $(n,m)=(4,3)$
and by Gomba & Homsy (Reference Gomba and Homsy2009), Perazzo, Mac Intyre & Gomba (Reference Perazzo, Mac Intyre and Gomba2014) for
$(n,m)=(4,3)$
and by Gomba & Homsy (Reference Gomba and Homsy2009), Perazzo, Mac Intyre & Gomba (Reference Perazzo, Mac Intyre and Gomba2014) for
![]() $(n,m)=(3,2)$
. Those analytic solutions are scaled using molecular constant
$(n,m)=(3,2)$
. Those analytic solutions are scaled using molecular constant
![]() $\unicode[STIX]{x1D705}$
and, in order to change the scale appropriately, we have to set the thermodynamic contact angle
$\unicode[STIX]{x1D705}$
and, in order to change the scale appropriately, we have to set the thermodynamic contact angle
![]() $\unicode[STIX]{x1D703}$
in the parameter
$\unicode[STIX]{x1D703}$
in the parameter
![]() $K$
in (2.11) (Mac Intyre et al.
Reference Mac Intyre, Gomba and Perazzo2016). The profile of the droplet is allowed to change dynamically over time and the contact angle
$K$
in (2.11) (Mac Intyre et al.
Reference Mac Intyre, Gomba and Perazzo2016). The profile of the droplet is allowed to change dynamically over time and the contact angle
![]() $\unicode[STIX]{x1D703}$
plays the role of parametric value. Given that the scale in
$\unicode[STIX]{x1D703}$
plays the role of parametric value. Given that the scale in
![]() $x$
and
$x$
and
![]() $z$
axes is
$z$
axes is
![]() $a$
,
$a$
,
![]() $\unicode[STIX]{x1D703}$
has the same value in both dimensional and dimensionless variables.
$\unicode[STIX]{x1D703}$
has the same value in both dimensional and dimensionless variables.
Table 1 summarize the relevant physical parameters, together with the typical values found in experiments. Based on these parameters, we estimate the range for
![]() $B$
as
$B$
as
![]() $0.002\leqslant B\leqslant 0.05$
. A word about the value of the precursor film thickness is appropriate. When the ratio of the maximum height to the precursor film thickness increases, the use of realistic values for
$0.002\leqslant B\leqslant 0.05$
. A word about the value of the precursor film thickness is appropriate. When the ratio of the maximum height to the precursor film thickness increases, the use of realistic values for
![]() $h_{\ast }$
in problems with moving contact lines is impractical because it requires cell sizes of the order of
$h_{\ast }$
in problems with moving contact lines is impractical because it requires cell sizes of the order of
![]() $h_{\ast }$
, as shown in Gaskell et al. (Reference Gaskell, Jimack, Sellier and Thompson2004). Then, most of the simulations are solved using
$h_{\ast }$
, as shown in Gaskell et al. (Reference Gaskell, Jimack, Sellier and Thompson2004). Then, most of the simulations are solved using
![]() $h_{\ast }\geqslant 5\times 10^{-3}\,a$
.
$h_{\ast }\geqslant 5\times 10^{-3}\,a$
.

Figure 2. Evolution of the thickness profile for a droplet with cross-sectional area
![]() $A=10$
, contact angle
$A=10$
, contact angle
![]() $\unicode[STIX]{x1D703}=4^{\circ }$
, thermal gradient
$\unicode[STIX]{x1D703}=4^{\circ }$
, thermal gradient
![]() $B=0.01$
and
$B=0.01$
and
![]() $(n,m)=(4,3)$
. The corresponding profiles for
$(n,m)=(4,3)$
. The corresponding profiles for
![]() $(n,m)=(3,2)$
are almost identical to these ones. Here
$(n,m)=(3,2)$
are almost identical to these ones. Here
![]() $h_{\ast }=0.01$
. The dashed blue line is the analytic solution for the rear profile given by (3.2).
$h_{\ast }=0.01$
. The dashed blue line is the analytic solution for the rear profile given by (3.2).
Table 1. Orders of magnitude of the relevant physical parameters (Brochard-Wyart Reference Brochard-Wyart1989; Brzoska et al. Reference Brzoska, Brochard-Wyart and Rondelez1993; Schwartz & Eley Reference Schwartz and Eley1998; Chen et al. Reference Chen, Troian, Darhuber and Wagner2005; Pratap et al. Reference Pratap, Moumen and Subramanian2008).

3 Small contact angle: film regime
3.1 Absence of gravity,
 $G=0$
$G=0$
The film regime occurs for small contact angles. Therefore, we have that
![]() $\tan ^{2}\unicode[STIX]{x1D703}\sim 0$
, which implies that the molecular action is negligible (Chaudhury & Chakraborty Reference Chaudhury and Chakraborty2015). Experimental results for Marangoni films by Fote, Slade & Feuerstein (Reference Fote, Slade and Feuerstein1977) have shown that non-polar liquids under microgravity develop a linear profile. Similarly, Sur et al. (Reference Sur, Bertozzi and Behringer2003) have shown experimentally a linear profile connecting the advancing ridges with the fluid in the container. Gomba & Homsy (Reference Gomba and Homsy2010) have reported a linear profile numerically and demonstrated an analytical expression for this evolution for polar liquids.
$\tan ^{2}\unicode[STIX]{x1D703}\sim 0$
, which implies that the molecular action is negligible (Chaudhury & Chakraborty Reference Chaudhury and Chakraborty2015). Experimental results for Marangoni films by Fote, Slade & Feuerstein (Reference Fote, Slade and Feuerstein1977) have shown that non-polar liquids under microgravity develop a linear profile. Similarly, Sur et al. (Reference Sur, Bertozzi and Behringer2003) have shown experimentally a linear profile connecting the advancing ridges with the fluid in the container. Gomba & Homsy (Reference Gomba and Homsy2010) have reported a linear profile numerically and demonstrated an analytical expression for this evolution for polar liquids.
Figure 2 shows the numerical solution of (2.10) for a non-polar liquid with
![]() $\unicode[STIX]{x1D703}=4^{\circ }$
and
$\unicode[STIX]{x1D703}=4^{\circ }$
and
![]() $G=0$
. After a short transient stage, the front develops a characteristic capillary ridge and the bulk adopts a linear profile which slope decreases in time. Note that the bump forms because the contact line moves slower than the fluid being pumped in from behind by the Marangoni stress. Due to independence of molecular interaction, non-polar liquids exhibits almost identical behaviour to polar liquids. As time increases, the droplet elongates, its maximum height decreases and the volume under the linear profile grows.
$G=0$
. After a short transient stage, the front develops a characteristic capillary ridge and the bulk adopts a linear profile which slope decreases in time. Note that the bump forms because the contact line moves slower than the fluid being pumped in from behind by the Marangoni stress. Due to independence of molecular interaction, non-polar liquids exhibits almost identical behaviour to polar liquids. As time increases, the droplet elongates, its maximum height decreases and the volume under the linear profile grows.
The numerical solutions of the differential equation retaining all terms show that the long rear part of the drop develops a linear profile. To describe it analytically, we assume null curvature in this region so that the capillary effect can be neglected and, due to the fact that the molecular forces are negligible, equation (2.10) becomes
Then, the thickness profile is constant along of the characteristic
![]() $\text{d}x/\text{d}t=2Bh$
, which means that a point with height
$\text{d}x/\text{d}t=2Bh$
, which means that a point with height
![]() $h$
moves to the right with speed
$h$
moves to the right with speed
![]() $2Bh$
. As a result, the linear profile is (Gomba & Homsy Reference Gomba and Homsy2010)
$2Bh$
. As a result, the linear profile is (Gomba & Homsy Reference Gomba and Homsy2010)
where
![]() $x_{0}$
is a constant related to the initial position of the rear contact line. Figure 2 shows the comparison between the numerical solution of the full equation (2.10) with (3.2). The high agreement justifies the omission of surface tension and disjoining pressure terms in (3.1). Figure 3 shows the evolution of the front
$x_{0}$
is a constant related to the initial position of the rear contact line. Figure 2 shows the comparison between the numerical solution of the full equation (2.10) with (3.2). The high agreement justifies the omission of surface tension and disjoining pressure terms in (3.1). Figure 3 shows the evolution of the front
![]() $x_{f}$
for several values of
$x_{f}$
for several values of
![]() $\unicode[STIX]{x1D703}$
and
$\unicode[STIX]{x1D703}$
and
![]() $B$
with both polar and non-polar liquids. At long times, the curves converge to the asymptotic curve
$B$
with both polar and non-polar liquids. At long times, the curves converge to the asymptotic curve
![]() $x_{f}/h_{m}\sim t^{1/2}$
independently of the molecular interaction. Note that as the value of
$x_{f}/h_{m}\sim t^{1/2}$
independently of the molecular interaction. Note that as the value of
![]() $B$
is increased, the duration of the transient stage is reduced.
$B$
is increased, the duration of the transient stage is reduced.

Figure 3. Evolution of the front
![]() $x_{f}$
for several values of
$x_{f}$
for several values of
![]() $\unicode[STIX]{x1D703}$
and
$\unicode[STIX]{x1D703}$
and
![]() $B$
with both molecular potentials. Here,
$B$
with both molecular potentials. Here,
![]() $A=10$
,
$A=10$
,
![]() $h_{\ast }=0.01$
,
$h_{\ast }=0.01$
,
![]() $G=0$
and
$G=0$
and
![]() $h_{m}$
is the maximum of the profile.
$h_{m}$
is the maximum of the profile.
The bulk region of the droplet can be approximated by a triangular profile, and then its area
![]() $A$
is (Gomba & Homsy Reference Gomba and Homsy2010)
$A$
is (Gomba & Homsy Reference Gomba and Homsy2010)
with
![]() $x_{a}$
the location of the receding contact line and
$x_{a}$
the location of the receding contact line and
![]() $w=x_{f}-x_{a}$
the droplet width. Then, the width is
$w=x_{f}-x_{a}$
the droplet width. Then, the width is
![]() $w=(4ABt)^{1/2}$
and, therefore, the footprint of the droplet increases with time, as shown in figure 4. In addition, we can see that while the slope of the linear profile is independent of
$w=(4ABt)^{1/2}$
and, therefore, the footprint of the droplet increases with time, as shown in figure 4. In addition, we can see that while the slope of the linear profile is independent of
![]() $A$
, as predicted by (3.2), the velocity of the front increases with
$A$
, as predicted by (3.2), the velocity of the front increases with
![]() $A$
and the position
$A$
and the position
![]() $x_{a}$
is almost constant.
$x_{a}$
is almost constant.

Figure 4. Evolution of the thickness profile for several values of
![]() $A$
at two different times:
$A$
at two different times:
![]() $t=9000$
(dashed lines) and
$t=9000$
(dashed lines) and
![]() $t=19\,000$
(solid lines). The contact angle is
$t=19\,000$
(solid lines). The contact angle is
![]() $\unicode[STIX]{x1D703}=3^{\circ }$
,
$\unicode[STIX]{x1D703}=3^{\circ }$
,
![]() $B=0.01$
,
$B=0.01$
,
![]() $h_{\ast }=0.01$
,
$h_{\ast }=0.01$
,
![]() $G=0$
and
$G=0$
and
![]() $(n,m)=(4,3)$
. The linear profile is given by (3.2).
$(n,m)=(4,3)$
. The linear profile is given by (3.2).
3.2 Effect of gravity,
 $G=1$
$G=1$
Figure 5 shows the evolution of the thickness profile under the same conditions as in figure 2 but with
![]() $G=1$
. The initial profile, compressed in the
$G=1$
. The initial profile, compressed in the
![]() $z$
direction due to gravity, evolves to the asymptotic linear profile (3.2). Nevertheless, gravity has a role at the leading front: comparing with the evolution shown in figure 2, we can see that the presence of gravity decreases the height of the capillary ridge and smooths the valley immediately behind it.
$z$
direction due to gravity, evolves to the asymptotic linear profile (3.2). Nevertheless, gravity has a role at the leading front: comparing with the evolution shown in figure 2, we can see that the presence of gravity decreases the height of the capillary ridge and smooths the valley immediately behind it.

Figure 5. Evolution of the thickness profile for a droplet with
![]() $A=10$
,
$A=10$
,
![]() $\unicode[STIX]{x1D703}=4^{\circ }$
,
$\unicode[STIX]{x1D703}=4^{\circ }$
,
![]() $B=0.01$
,
$B=0.01$
,
![]() $G=1$
,
$G=1$
,
![]() $(n,m)=(4,3)$
and
$(n,m)=(4,3)$
and
![]() $h_{\ast }=0.01$
. The dashed blue line is the analytic solution (3.2). Similar behaviour is observed for a molecular potential with
$h_{\ast }=0.01$
. The dashed blue line is the analytic solution (3.2). Similar behaviour is observed for a molecular potential with
![]() $(n,m)=(3,2)$
.
$(n,m)=(3,2)$
.
The most remarkable effect of gravity is on the velocity of the front, as shown in figure 6. Given that the initial profiles for
![]() $G=1$
and
$G=1$
and
![]() $G=0$
have the same initial front position, in figure 6(a) we observe that the profile with
$G=0$
have the same initial front position, in figure 6(a) we observe that the profile with
![]() $G=1$
takes more time to reach the asymptotic linear shape than the case
$G=1$
takes more time to reach the asymptotic linear shape than the case
![]() $G=0$
. Figure 6(b) shows the evolution of the front position for
$G=0$
. Figure 6(b) shows the evolution of the front position for
![]() $G=1$
and
$G=1$
and
![]() $G=0$
. The final velocity,
$G=0$
. The final velocity,
![]() $\text{d}x_{f}/\text{d}t=2Bh(x_{f})$
, is monotonically reached in both cases, but it decreases with time for
$\text{d}x_{f}/\text{d}t=2Bh(x_{f})$
, is monotonically reached in both cases, but it decreases with time for
![]() $G=0$
, while the velocity increases for
$G=0$
, while the velocity increases for
![]() $G=1$
. Similarly to the case with
$G=1$
. Similarly to the case with
![]() $G=0$
, the velocity for
$G=0$
, the velocity for
![]() $G=1$
is independent of the molecular potential and the speed increases with
$G=1$
is independent of the molecular potential and the speed increases with
![]() $A$
and
$A$
and
![]() $B$
.
$B$
.

Figure 6. (a) Comparison of the thickness evolution with gravity
![]() $G=1$
(dashed blue line) and without gravity
$G=1$
(dashed blue line) and without gravity
![]() $G=0$
(black line). The position of the initial front is the same in both droplets. (b) Position of the front
$G=0$
(black line). The position of the initial front is the same in both droplets. (b) Position of the front
![]() $x_{f}$
versus time
$x_{f}$
versus time
![]() $t$
for droplets with
$t$
for droplets with
![]() $\unicode[STIX]{x1D703}=3^{\circ }$
,
$\unicode[STIX]{x1D703}=3^{\circ }$
,
![]() $A=10$
,
$A=10$
,
![]() $h_{\ast }=0.01$
,
$h_{\ast }=0.01$
,
![]() $B=0.01$
and several combination of
$B=0.01$
and several combination of
![]() $(n,m)$
and
$(n,m)$
and
![]() $G$
. For the dotted line,
$G$
. For the dotted line,
![]() $A=50$
.
$A=50$
.
4 Intermediate contact angle: transition regime
4.1 Absence of gravity,
 $G=0$
$G=0$
The transition regime appears for intermediate values of the contact angle
![]() $\unicode[STIX]{x1D703}$
. In contrast with previous section, here the effects of the molecular potential and the Marangoni stress are of the same order. We observe two different dynamics: a droplet breaks into two or more smaller volumes, as reported Gomba & Homsy (Reference Gomba and Homsy2010), or it evolves to a steady profile that migrates with a constant velocity without breakups.
$\unicode[STIX]{x1D703}$
. In contrast with previous section, here the effects of the molecular potential and the Marangoni stress are of the same order. We observe two different dynamics: a droplet breaks into two or more smaller volumes, as reported Gomba & Homsy (Reference Gomba and Homsy2010), or it evolves to a steady profile that migrates with a constant velocity without breakups.
Figure 7 shows the evolution of the thickness profile for
![]() $(n,m)=(3,2)$
,
$(n,m)=(3,2)$
,
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
and
$\unicode[STIX]{x1D703}=15^{\circ }$
and
![]() $G=0$
. As described in Gomba & Homsy (Reference Gomba and Homsy2010), the fluid breaks up into smaller droplets and the behaviour is quite complex. Nevertheless, we observe that before breakup, the droplet adopts a transient stage consisting in a linear profile followed by a (short length) almost-flat thickness
$G=0$
. As described in Gomba & Homsy (Reference Gomba and Homsy2010), the fluid breaks up into smaller droplets and the behaviour is quite complex. Nevertheless, we observe that before breakup, the droplet adopts a transient stage consisting in a linear profile followed by a (short length) almost-flat thickness
![]() $h_{e}$
(see
$h_{e}$
(see
![]() $t=4000$
in the figure). We will show that this thickness determines the later dynamics of the flow.
$t=4000$
in the figure). We will show that this thickness determines the later dynamics of the flow.

Figure 7. Evolution of the thickness profile with
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
,
$\unicode[STIX]{x1D703}=15^{\circ }$
,
![]() $G=0$
,
$G=0$
,
![]() $h_{\ast }=0.01$
,
$h_{\ast }=0.01$
,
![]() $B=0.03$
and
$B=0.03$
and
![]() $(n,m)=(3,2)$
. The droplet with initial cross-sectional area
$(n,m)=(3,2)$
. The droplet with initial cross-sectional area
![]() $A=10$
breaks into smaller droplets.
$A=10$
breaks into smaller droplets.
In figure 8, we keep all the parameters of the previous figure but we change the pair of the molecular potential to
![]() $(n,m)=(4,3)$
. Here, instead of the rupture observed for polar liquids, we observe that an steady profile is adopted after a transition stage. The initial shape evolves into a flat droplet with a capillary ridge at the front, which migrates with a constant velocity. This droplet shape has not been reported before.
$(n,m)=(4,3)$
. Here, instead of the rupture observed for polar liquids, we observe that an steady profile is adopted after a transition stage. The initial shape evolves into a flat droplet with a capillary ridge at the front, which migrates with a constant velocity. This droplet shape has not been reported before.

Figure 8. Evolution of the thickness profile with
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
,
$\unicode[STIX]{x1D703}=15^{\circ }$
,
![]() $G=0$
,
$G=0$
,
![]() $h_{\ast }=0.01$
,
$h_{\ast }=0.01$
,
![]() $(n,m)=(4,3)$
,
$(n,m)=(4,3)$
,
![]() $B=0.03$
and
$B=0.03$
and
![]() $A=10$
. The change of the molecular potential stabilizes the profile.
$A=10$
. The change of the molecular potential stabilizes the profile.
Thus, in the transition regime a constant height
![]() $h_{e}$
emerges, and the dynamics of the flow depends on the stability of that thickness. In the next two subsections, we analyse the parametric dependence of
$h_{e}$
emerges, and the dynamics of the flow depends on the stability of that thickness. In the next two subsections, we analyse the parametric dependence of
![]() $h_{e}$
and also the occurrence of breakups. Finally, we explore the effect of gravity.
$h_{e}$
and also the occurrence of breakups. Finally, we explore the effect of gravity.
4.1.1 Migration of flat droplets
We first analyse flat droplets, i.e. steady solutions that do not break up, move with a constant velocity and present a characteristic flat thickness
![]() $h_{e}$
. Figure 9(a) shows the profiles for
$h_{e}$
. Figure 9(a) shows the profiles for
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
,
$\unicode[STIX]{x1D703}=15^{\circ }$
,
![]() $A=10$
and several values of
$A=10$
and several values of
![]() $B$
at
$B$
at
![]() $t=2.4\times 10^{4}$
, when the steady profile is completely developed. As
$t=2.4\times 10^{4}$
, when the steady profile is completely developed. As
![]() $B$
increases, the width of the profile
$B$
increases, the width of the profile
![]() $w$
and the velocity of the droplet
$w$
and the velocity of the droplet
![]() $U$
increases, while the thickness
$U$
increases, while the thickness
![]() $h_{e}$
decreases. Note that the area below the capillary ridge enlarges as
$h_{e}$
decreases. Note that the area below the capillary ridge enlarges as
![]() $B$
diminishes and, when most of the fluid is within the capillary ridge, the profile does not present a flat film, such as in the case with
$B$
diminishes and, when most of the fluid is within the capillary ridge, the profile does not present a flat film, such as in the case with
![]() $B=0.01$
. In figure 9(b) we set
$B=0.01$
. In figure 9(b) we set
![]() $\unicode[STIX]{x1D703}=18^{\circ }$
. If we compare these profiles with those with
$\unicode[STIX]{x1D703}=18^{\circ }$
. If we compare these profiles with those with
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
, we observe that for the same values of
$\unicode[STIX]{x1D703}=15^{\circ }$
, we observe that for the same values of
![]() $B$
, a larger
$B$
, a larger
![]() $\unicode[STIX]{x1D703}$
implies a shorter
$\unicode[STIX]{x1D703}$
implies a shorter
![]() $w$
, and larger values for
$w$
, and larger values for
![]() $h_{e}$
and
$h_{e}$
and
![]() $U$
.
$U$
.

Figure 9. Profiles at
![]() $t=2.4\times 10^{4}$
in the evolution of a droplet with
$t=2.4\times 10^{4}$
in the evolution of a droplet with
![]() $A=10$
,
$A=10$
,
![]() $G=0$
,
$G=0$
,
![]() $h_{\ast }=0.01$
,
$h_{\ast }=0.01$
,
![]() $(n,m)=(4,3)$
and initial contact angle (a)
$(n,m)=(4,3)$
and initial contact angle (a)
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
, and (b)
$\unicode[STIX]{x1D703}=15^{\circ }$
, and (b)
![]() $\unicode[STIX]{x1D703}=18^{\circ }$
. We observe that the thickness
$\unicode[STIX]{x1D703}=18^{\circ }$
. We observe that the thickness
![]() $h_{e}$
, the width
$h_{e}$
, the width
![]() $w$
and the velocity
$w$
and the velocity
![]() $U$
depends on the thermal gradient
$U$
depends on the thermal gradient
![]() $B$
.
$B$
.
Simulations show that
![]() $w$
increases with
$w$
increases with
![]() $A$
, while
$A$
, while
![]() $h_{e}$
is independent of
$h_{e}$
is independent of
![]() $A$
. Dimensional analysis and parametric studies on
$A$
. Dimensional analysis and parametric studies on
![]() $K$
,
$K$
,
![]() $B$
and
$B$
and
![]() $A$
allows us to determine how the different parameters are related. We find that the dependence of
$A$
allows us to determine how the different parameters are related. We find that the dependence of
![]() $h_{e}$
on
$h_{e}$
on
![]() $K$
and
$K$
and
![]() $B$
is given by
$B$
is given by
Figure 10(a) shows a good agreement between (4.1) and the numerical data for several combinations of the parameters. To understand how
![]() $w$
depends on
$w$
depends on
![]() $A$
,
$A$
,
![]() $K$
and
$K$
and
![]() $B$
, we approximate the area
$B$
, we approximate the area
![]() $A$
by:
$A$
by:
and therefore the width follows the power law
This expression is successfully tested in figure 10(b). Also, the numerical results show that when
![]() $A$
is increased, the width
$A$
is increased, the width
![]() $w$
increases but the thickness
$w$
increases but the thickness
![]() $h_{e}$
remains constant, as expected from (4.1) and (4.3).
$h_{e}$
remains constant, as expected from (4.1) and (4.3).

Figure 10. Dependence of (a)
![]() $h_{e}$
and (b)
$h_{e}$
and (b)
![]() $w$
on the parameters of the problem
$w$
on the parameters of the problem
![]() $B$
,
$B$
,
![]() $\unicode[STIX]{x1D703}$
and
$\unicode[STIX]{x1D703}$
and
![]() $A$
. The dashed lines are the derived analytical expressions.
$A$
. The dashed lines are the derived analytical expressions.
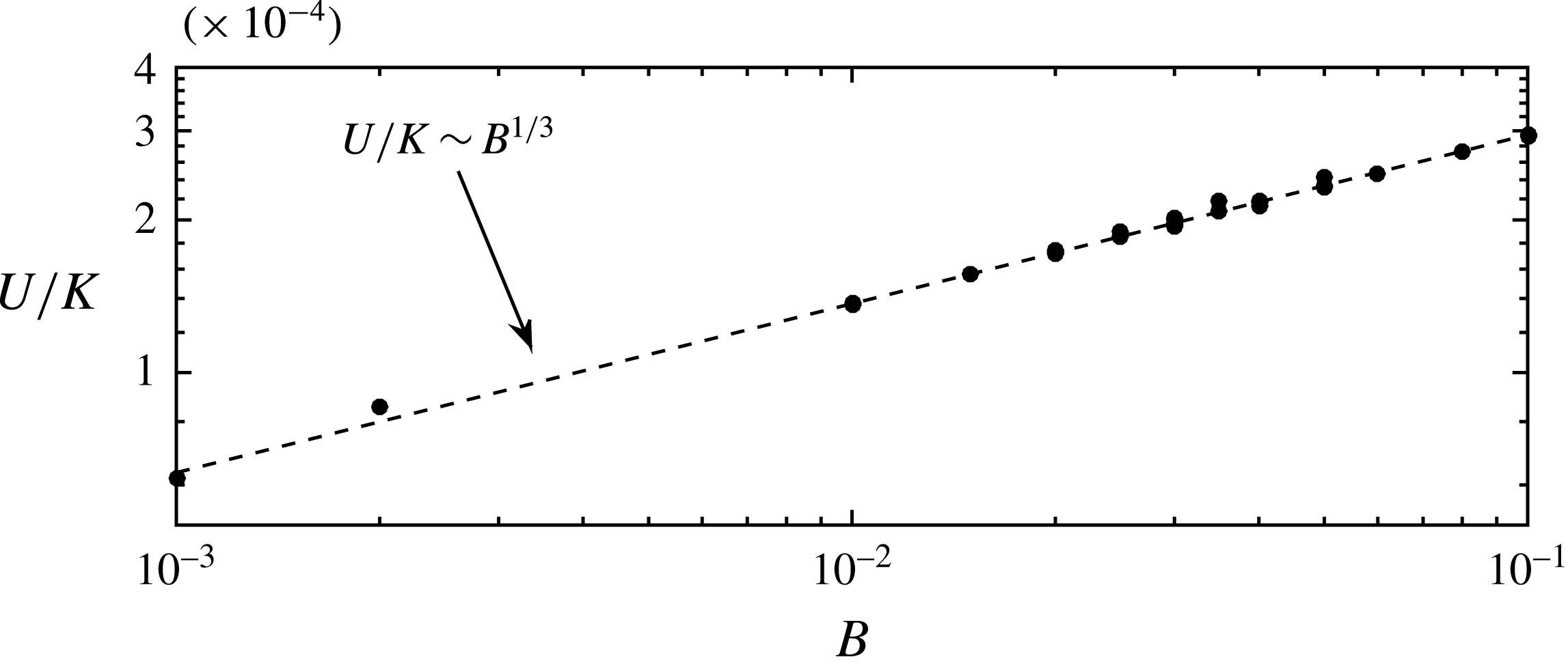
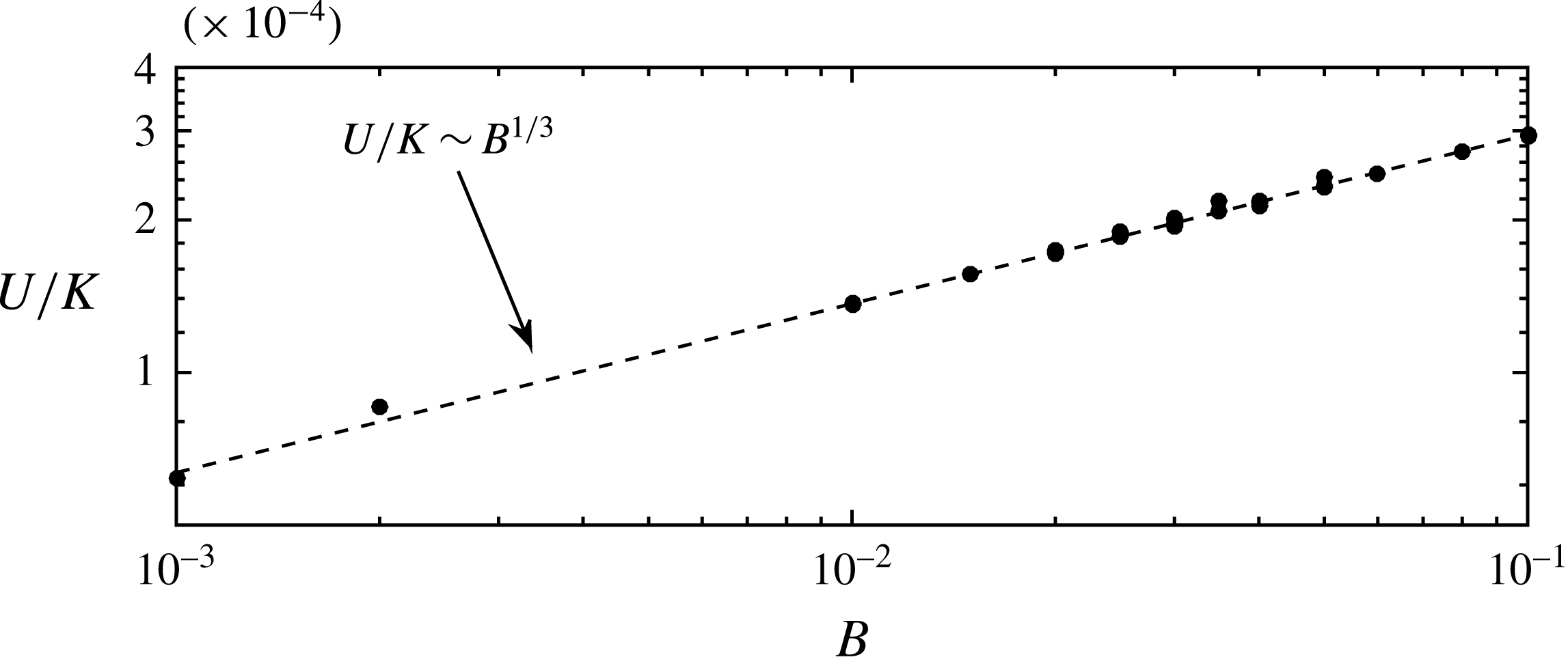
As mentioned, the droplets finally move with a constant velocity
![]() $U$
. To estimate
$U$
. To estimate
![]() $U$
, we assume that the solution is well represented by a travelling wave of the form
$U$
, we assume that the solution is well represented by a travelling wave of the form
![]() $h(x,t)\equiv h(\unicode[STIX]{x1D702})$
, with
$h(x,t)\equiv h(\unicode[STIX]{x1D702})$
, with
![]() $\unicode[STIX]{x1D702}=x-Ut$
. After integration, equation (2.10) becomes
$\unicode[STIX]{x1D702}=x-Ut$
. After integration, equation (2.10) becomes
with
![]() ${\mathcal{V}}$
a constant of integration. At the flat film, where
${\mathcal{V}}$
a constant of integration. At the flat film, where
![]() $h=h_{e}$
, the first and third derivatives are negligible, i.e.
$h=h_{e}$
, the first and third derivatives are negligible, i.e.
and then,
Similarly, at
![]() $h=h_{\ast }$
, the same conditions (4.5) apply, and (4.4) becomes
$h=h_{\ast }$
, the same conditions (4.5) apply, and (4.4) becomes
Assuming
![]() $h_{\ast }/h_{e}\ll 1$
, the velocity of migration is
$h_{\ast }/h_{e}\ll 1$
, the velocity of migration is
![]() $U=Bh_{e}$
, which using (4.1) becomes
$U=Bh_{e}$
, which using (4.1) becomes
Figure 11 confirms this dependence of
![]() $U$
on the parameters
$U$
on the parameters
![]() $K$
and
$K$
and
![]() $B$
.
$B$
.
4.1.2 Occurrence of breakup
For intermediate contact angles, most of the cases with polar liquids break up into smaller droplets during the migration, while the rupture process with non-polar liquids was only observed for the highest values of
![]() $B$
. Figure 12(a) shows the breakup of a droplet for
$B$
. Figure 12(a) shows the breakup of a droplet for
![]() $B=0.05$
and
$B=0.05$
and
![]() $(n,m)=(4,3)$
. Although rupture is present, it only occurs for late times and in few points when compared with the cases with
$(n,m)=(4,3)$
. Although rupture is present, it only occurs for late times and in few points when compared with the cases with
![]() $(n,m)=(3,2)$
. On the contrary, figure 12(b) shows a multiple droplet breakup occurring for
$(n,m)=(3,2)$
. On the contrary, figure 12(b) shows a multiple droplet breakup occurring for
![]() $B=0.01$
and
$B=0.01$
and
![]() $(n,m)=(3,2)$
. Therefore, simulations show that the molecular potentials and the Marangoni force clearly play a role in the stability of the flat film.
$(n,m)=(3,2)$
. Therefore, simulations show that the molecular potentials and the Marangoni force clearly play a role in the stability of the flat film.

Figure 12. Evolution of the thickness profile with
![]() $\unicode[STIX]{x1D703}=10^{\circ }$
,
$\unicode[STIX]{x1D703}=10^{\circ }$
,
![]() $A=10$
,
$A=10$
,
![]() $h_{\ast }=0.01$
and
$h_{\ast }=0.01$
and
![]() $G=0$
. (a) Non-polar liquid,
$G=0$
. (a) Non-polar liquid,
![]() $(n,m)=(4,3)$
and thermal gradient
$(n,m)=(4,3)$
and thermal gradient
![]() $B=0.05$
. (b) Polar liquid,
$B=0.05$
. (b) Polar liquid,
![]() $(n,m)=(3,2)$
and
$(n,m)=(3,2)$
and
![]() $B=0.01$
.
$B=0.01$
.
The molecular potential, i.e. the nature of polar or non-polar liquid, manages the stability of the flat film. In order to understand the rupture process, we implement a linear stability analysis assuming that
![]() $h=h_{e}+\unicode[STIX]{x1D709}$
, with
$h=h_{e}+\unicode[STIX]{x1D709}$
, with
![]() $\unicode[STIX]{x1D709}\ll h_{e}$
. After linearization, equation (2.10) becomes
$\unicode[STIX]{x1D709}\ll h_{e}$
. After linearization, equation (2.10) becomes
with
We replace
![]() $\unicode[STIX]{x1D709}\sim \exp (\text{i}kx+\unicode[STIX]{x1D714}t)$
and obtain the following dispersion equation
$\unicode[STIX]{x1D709}\sim \exp (\text{i}kx+\unicode[STIX]{x1D714}t)$
and obtain the following dispersion equation
A stable constant thickness
![]() $h_{e}$
has to satisfy
$h_{e}$
has to satisfy
![]() $Re[\unicode[STIX]{x1D714}]<0$
, i.e.
$Re[\unicode[STIX]{x1D714}]<0$
, i.e.
and the mode with the largest growth rate is
Figure 13 shows the dispersion relationship for different values of
![]() $\unicode[STIX]{x1D703}$
and both molecular potentials. Comparing cases with the same value of
$\unicode[STIX]{x1D703}$
and both molecular potentials. Comparing cases with the same value of
![]() $B$
and
$B$
and
![]() $A$
, the growth rate is always notably smaller for the pair
$A$
, the growth rate is always notably smaller for the pair
![]() $(n,m)=(4,3)$
than for the
$(n,m)=(4,3)$
than for the
![]() $(n,m)=(3,2)$
. Also, the minimum unstable wavelength is larger for the case
$(n,m)=(3,2)$
. Also, the minimum unstable wavelength is larger for the case
![]() $(n,m)=(4,3)$
. Thus, non-polar liquids needs longer times and larger domains to develop an instability.
$(n,m)=(4,3)$
. Thus, non-polar liquids needs longer times and larger domains to develop an instability.

Figure 13. Relation of dispersion (4.11) for different combination of the parameters. The thickness
![]() $h_{e}$
is estimated in the transition stage. Same colour is used for cases with same
$h_{e}$
is estimated in the transition stage. Same colour is used for cases with same
![]() $\unicode[STIX]{x1D703}$
and
$\unicode[STIX]{x1D703}$
and
![]() $A$
.
$A$
.
To estimate the distance between droplets, we implement a Fourier analysis of the thickness profile. Figure 14 shows the Fourier transform of the thickness profiles obtained for
![]() $(n,m)=(3,2)$
and different values of
$(n,m)=(3,2)$
and different values of
![]() $B$
. The position of the vertical line, that indicates the maximum wavelength amplitude
$B$
. The position of the vertical line, that indicates the maximum wavelength amplitude
![]() $\unicode[STIX]{x1D706}_{m}^{n}$
, agrees well with the theoretical value
$\unicode[STIX]{x1D706}_{m}^{n}$
, agrees well with the theoretical value
![]() $\unicode[STIX]{x1D706}_{m}^{t}=2\unicode[STIX]{x03C0}/k_{m}$
as shown in table 2. Thus, the number of droplets and the distance between them can be predicted from a linear stability analysis.
$\unicode[STIX]{x1D706}_{m}^{t}=2\unicode[STIX]{x03C0}/k_{m}$
as shown in table 2. Thus, the number of droplets and the distance between them can be predicted from a linear stability analysis.
Table 2. Comparison of theoretical
![]() $\unicode[STIX]{x1D706}_{m}^{t}$
, equation (4.13) and numerical
$\unicode[STIX]{x1D706}_{m}^{t}$
, equation (4.13) and numerical
![]() $\unicode[STIX]{x1D706}_{m}^{n}$
wavelengths.
$\unicode[STIX]{x1D706}_{m}^{n}$
wavelengths.


Figure 14. Fourier transform of the thickness profile with a molecular potential
![]() $(n,m)=(3,2)$
and three different values of
$(n,m)=(3,2)$
and three different values of
![]() $B$
. The vertical line points the maximum wavelength
$B$
. The vertical line points the maximum wavelength
![]() $\unicode[STIX]{x1D706}_{m}^{n}$
.
$\unicode[STIX]{x1D706}_{m}^{n}$
.
4.2 Effect of gravity,
 $G=1$
$G=1$
Figure 15 shows the evolution of the thickness profile for
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
,
$\unicode[STIX]{x1D703}=15^{\circ }$
,
![]() $B=0.01$
,
$B=0.01$
,
![]() $G=1$
and both molecular potentials. Gravity compresses the profiles in the
$G=1$
and both molecular potentials. Gravity compresses the profiles in the
![]() $z$
direction, softens the valley that appears immediately behind the capillary ridge and increases the width of the initial profile (compare with the profiles in figures 7 and 9). More interesting, under the action of gravity the breakups are not observed.
$z$
direction, softens the valley that appears immediately behind the capillary ridge and increases the width of the initial profile (compare with the profiles in figures 7 and 9). More interesting, under the action of gravity the breakups are not observed.

Figure 15. Evolution of the thickness profile for a droplet with
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
,
$\unicode[STIX]{x1D703}=15^{\circ }$
,
![]() $A=10$
,
$A=10$
,
![]() $G=1$
,
$G=1$
,
![]() $h_{\ast }=0.01$
and
$h_{\ast }=0.01$
and
![]() $B=0.01$
. (a) Non-polar liquid,
$B=0.01$
. (a) Non-polar liquid,
![]() $(n,m)=(4,3)$
. (b) Polar liquid,
$(n,m)=(4,3)$
. (b) Polar liquid,
![]() $(n,m)=(3,2)$
.
$(n,m)=(3,2)$
.
We study how gravity affects the stability of flat profiles. A linear stability analysis shows that the growth rate is now given by
and then, a uniform film
![]() $h_{e}$
is stable if the wavenumber satisfies
$h_{e}$
is stable if the wavenumber satisfies
Figure 16 shows that the effect of
![]() $G$
is to decrease the real part of the growth rate
$G$
is to decrease the real part of the growth rate
![]() $\unicode[STIX]{x1D714}$
and the mode
$\unicode[STIX]{x1D714}$
and the mode
![]() $k_{m}$
. Thus, situations where breakup is observed with
$k_{m}$
. Thus, situations where breakup is observed with
![]() $G=0$
may not present ruptures with
$G=0$
may not present ruptures with
![]() $G=1$
, as in the cases for
$G=1$
, as in the cases for
![]() $B=0.03$
and
$B=0.03$
and
![]() $(n,m)=(3,2)$
shown in figures 7 and 17. Nevertheless, figure 17 shows that breakup for
$(n,m)=(3,2)$
shown in figures 7 and 17. Nevertheless, figure 17 shows that breakup for
![]() $G=1$
and polar liquids may occur for larger values of
$G=1$
and polar liquids may occur for larger values of
![]() $B$
. Breakup of a polar liquid in the presence of gravity can be understood in the following way: assuming that the relation
$B$
. Breakup of a polar liquid in the presence of gravity can be understood in the following way: assuming that the relation
![]() $h_{e}\propto B^{-\unicode[STIX]{x1D6FD}}$
(with
$h_{e}\propto B^{-\unicode[STIX]{x1D6FD}}$
(with
![]() $\unicode[STIX]{x1D6FD}>0$
) is still valid when
$\unicode[STIX]{x1D6FD}>0$
) is still valid when
![]() $G=1$
(later this is confirmed), if the thermal gradient
$G=1$
(later this is confirmed), if the thermal gradient
![]() $B$
increases, the thickness of the stable film
$B$
increases, the thickness of the stable film
![]() $h_{e}$
decreases. Then, from (4.10)
$h_{e}$
decreases. Then, from (4.10)
![]() $K^{\prime }$
increases and finally, from (4.15), the smallest wavenumber of a stable mode becomes larger. Briefly, an increase of
$K^{\prime }$
increases and finally, from (4.15), the smallest wavenumber of a stable mode becomes larger. Briefly, an increase of
![]() $B$
is destabilizing. Added to this, when
$B$
is destabilizing. Added to this, when
![]() $B$
is increased, the region with
$B$
is increased, the region with
![]() $h=h_{e}$
widens (
$h=h_{e}$
widens (
![]() $w$
enlarges) and it is easier to fit unstable modes inside the drop.
$w$
enlarges) and it is easier to fit unstable modes inside the drop.

Figure 16. Effect of gravity on the growth rate. Here,
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
and
$\unicode[STIX]{x1D703}=15^{\circ }$
and
![]() $h_{e}=0.07$
. The maximum of
$h_{e}=0.07$
. The maximum of
![]() $\unicode[STIX]{x1D714}$
and the largest unstable
$\unicode[STIX]{x1D714}$
and the largest unstable
![]() $k$
decrease when
$k$
decrease when
![]() $G=1$
compared with the case
$G=1$
compared with the case
![]() $G=0$
.
$G=0$
.

Figure 17. Profiles at
![]() $t=2.7\times 10^{4}$
in the evolution of a droplet with initial contact angle
$t=2.7\times 10^{4}$
in the evolution of a droplet with initial contact angle
![]() $\unicode[STIX]{x1D703}=15^{\circ }$
,
$\unicode[STIX]{x1D703}=15^{\circ }$
,
![]() $A=10$
,
$A=10$
,
![]() $G=1$
,
$G=1$
,
![]() $h_{\ast }=0.01$
and
$h_{\ast }=0.01$
and
![]() $(n,m)=(3,2)$
. In contrast with the observed evolution for the case with
$(n,m)=(3,2)$
. In contrast with the observed evolution for the case with
![]() $G=0,$
here the case with
$G=0,$
here the case with
![]() $B=0.01$
does not present a breakup when gravity is considered.
$B=0.01$
does not present a breakup when gravity is considered.
Gravity also changes the dependence of
![]() $h_{e}$
on the parameters. Figure 18(a) shows
$h_{e}$
on the parameters. Figure 18(a) shows
![]() $h_{e}/K$
against
$h_{e}/K$
against
![]() $B$
for
$B$
for
![]() $G=1$
for the two explored potentials (we also include cases with
$G=1$
for the two explored potentials (we also include cases with
![]() $G=0$
to illustrate the difference in the exponent of the power law). For
$G=0$
to illustrate the difference in the exponent of the power law). For
![]() $G=1$
, the simulations show that the dependence of
$G=1$
, the simulations show that the dependence of
![]() $h_{e}$
on
$h_{e}$
on
![]() $K$
and
$K$
and
![]() $B$
is
$B$
is
Since for a given value of
![]() $\unicode[STIX]{x1D703}$
,
$\unicode[STIX]{x1D703}$
,
![]() $K$
is higher for non-polar than for polar liquids, the value of
$K$
is higher for non-polar than for polar liquids, the value of
![]() $h_{e}$
is higher for the pair
$h_{e}$
is higher for the pair
![]() $(n,m)=(4,3)$
. Then, equation (4.7) predicts that the velocity of migration is higher for non-polar liquids than for polar liquids. Effectively, figure 18(b) shows that both
$(n,m)=(4,3)$
. Then, equation (4.7) predicts that the velocity of migration is higher for non-polar liquids than for polar liquids. Effectively, figure 18(b) shows that both
![]() $h_{e}$
and
$h_{e}$
and
![]() $U$
are larger for the pair
$U$
are larger for the pair
![]() $(n,m)=(4,3)$
.
$(n,m)=(4,3)$
.

Figure 18. (a) Dependence of the thickness
![]() $h_{e}$
for several combinations of
$h_{e}$
for several combinations of
![]() $B$
and
$B$
and
![]() $\unicode[STIX]{x1D703}$
. (b) Evolution of the thickness profile with
$\unicode[STIX]{x1D703}$
. (b) Evolution of the thickness profile with
![]() $\unicode[STIX]{x1D703}=22^{\circ }$
,
$\unicode[STIX]{x1D703}=22^{\circ }$
,
![]() $A=10$
,
$A=10$
,
![]() $G=1$
,
$G=1$
,
![]() $h_{\ast }=0.01$
,
$h_{\ast }=0.01$
,
![]() $B=0.03$
and both molecular potentials.
$B=0.03$
and both molecular potentials.
5 Large contact angles: droplet regime
5.1 Absence of gravity,
 $G=0$
$G=0$
For large contact angles, the value of
![]() $K$
increases and the molecular potential becomes strong enough to keep the initial shape of the droplet during the migration. In the droplet regime, the Marangoni stress displaces the droplet with constant velocity
$K$
increases and the molecular potential becomes strong enough to keep the initial shape of the droplet during the migration. In the droplet regime, the Marangoni stress displaces the droplet with constant velocity
![]() $U$
without stretching it. Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) and Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005) show experimentally that droplets migrate keeping their initial shape. In their experiments, the velocity increases with the footprint
$U$
without stretching it. Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) and Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005) show experimentally that droplets migrate keeping their initial shape. In their experiments, the velocity increases with the footprint
![]() $R$
of the wet surface, but different liquids present differences in the dependence of
$R$
of the wet surface, but different liquids present differences in the dependence of
![]() $U$
on
$U$
on
![]() $R$
. This leads us to think that the type of the molecular force should modify the velocity of migration. Figure 19 shows the evolution of the thickness profile for a droplet with area
$R$
. This leads us to think that the type of the molecular force should modify the velocity of migration. Figure 19 shows the evolution of the thickness profile for a droplet with area
![]() $A=10$
, initial contact angle
$A=10$
, initial contact angle
![]() $\unicode[STIX]{x1D703}=25^{\circ }$
and thermal gradient
$\unicode[STIX]{x1D703}=25^{\circ }$
and thermal gradient
![]() $B=0.01$
, for both molecular potentials. Effectively, the velocity of migration changes with the polarity of the liquid.
$B=0.01$
, for both molecular potentials. Effectively, the velocity of migration changes with the polarity of the liquid.

Figure 19. Evolution of the thickness profile for (a) non-polar liquid,
![]() $(n,m)=(4,3)$
and (b) polar liquid,
$(n,m)=(4,3)$
and (b) polar liquid,
![]() $(n,m)=(3,2)$
, until
$(n,m)=(3,2)$
, until
![]() $t=4.8\times 10^{4}$
. The effect of the potential avoids drop stretching due to Marangoni stress. Here,
$t=4.8\times 10^{4}$
. The effect of the potential avoids drop stretching due to Marangoni stress. Here,
![]() $\unicode[STIX]{x0394}t=6\times 10^{3}$
,
$\unicode[STIX]{x0394}t=6\times 10^{3}$
,
![]() $\unicode[STIX]{x1D703}=25^{\circ }$
,
$\unicode[STIX]{x1D703}=25^{\circ }$
,
![]() $A=10$
,
$A=10$
,
![]() $G=0$
,
$G=0$
,
![]() $h_{\ast }=0.01$
and
$h_{\ast }=0.01$
and
![]() $B=0.01$
.
$B=0.01$
.
In this regime, front and rear contact lines move with the same constant velocity
![]() $U$
. Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) and Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005) have shown that the velocity is proportional to
$U$
. Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) and Chen et al. (Reference Chen, Troian, Darhuber and Wagner2005) have shown that the velocity is proportional to
![]() $B$
and inversely proportional to the viscosity. Gomba & Homsy (Reference Gomba and Homsy2010) demonstrated, by means of dimensional analysis, that the velocity for droplets with
$B$
and inversely proportional to the viscosity. Gomba & Homsy (Reference Gomba and Homsy2010) demonstrated, by means of dimensional analysis, that the velocity for droplets with
![]() $(n,m)=(3,2)$
supports this experimental conclusion. The latter authors constructed two
$(n,m)=(3,2)$
supports this experimental conclusion. The latter authors constructed two
![]() $\unicode[STIX]{x1D6F1}^{\ast }$
groups and, using numerical results, explored the relationship between them. They found that the velocity is given by
$\unicode[STIX]{x1D6F1}^{\ast }$
groups and, using numerical results, explored the relationship between them. They found that the velocity is given by
with
![]() $\unicode[STIX]{x1D6FD}=0.4$
for the case
$\unicode[STIX]{x1D6FD}=0.4$
for the case
![]() $(n,m)=(3,2)$
and
$(n,m)=(3,2)$
and
![]() $G=0$
. Here, we generalize the previous result considering that the pair
$G=0$
. Here, we generalize the previous result considering that the pair
![]() $(n,m)$
could change depending on the type of liquid. Then, we define the dimensionless number
$(n,m)$
could change depending on the type of liquid. Then, we define the dimensionless number
Figure 20 shows
![]() $U/Bh_{\ast }$
versus
$U/Bh_{\ast }$
versus
![]() $A\tan \unicode[STIX]{x1D703}/h_{\ast }^{2}$
for several combinations of the parameters
$A\tan \unicode[STIX]{x1D703}/h_{\ast }^{2}$
for several combinations of the parameters
![]() $(n,m)$
,
$(n,m)$
,
![]() $A$
,
$A$
,
![]() $\unicode[STIX]{x1D703}$
,
$\unicode[STIX]{x1D703}$
,
![]() $B$
and
$B$
and
![]() $h_{\ast }$
. Interestingly, the exponent in (5.1) is
$h_{\ast }$
. Interestingly, the exponent in (5.1) is
![]() $\unicode[STIX]{x1D6FD}=0.40$
, independently of the pair
$\unicode[STIX]{x1D6FD}=0.40$
, independently of the pair
![]() $(n,m)$
. Therefore, the pre-factor in (5.2) is responsible of the different velocity for each liquid.
$(n,m)$
. Therefore, the pre-factor in (5.2) is responsible of the different velocity for each liquid.

Figure 20. Dependence of the velocity
![]() $U$
for several combination of the parameters
$U$
for several combination of the parameters
![]() $(n,m)$
,
$(n,m)$
,
![]() $A$
,
$A$
,
![]() $\unicode[STIX]{x1D703}$
,
$\unicode[STIX]{x1D703}$
,
![]() $B$
and
$B$
and
![]() $h_{\ast }$
.
$h_{\ast }$
.
5.2 Effect of gravity,
 $G=1$
$G=1$
Gravity produces similar results to those observed in previous regimes. Figure 21 shows the evolution of a droplet with
![]() $A=10$
,
$A=10$
,
![]() $\unicode[STIX]{x1D703}=25^{\circ }$
,
$\unicode[STIX]{x1D703}=25^{\circ }$
,
![]() $B=0.01$
and
$B=0.01$
and
![]() $G=1$
, for both molecular potentials. Here, the initial profile is wider and has a lower height than the corresponding case with
$G=1$
, for both molecular potentials. Here, the initial profile is wider and has a lower height than the corresponding case with
![]() $G=0$
. When the thermal gradient is applied, the droplet slightly modified its shape. After a short time, the droplet migrates with a steady profile, but its velocity is smaller than the case with
$G=0$
. When the thermal gradient is applied, the droplet slightly modified its shape. After a short time, the droplet migrates with a steady profile, but its velocity is smaller than the case with
![]() $G=0$
, as shown in figures 19 and 21.
$G=0$
, as shown in figures 19 and 21.

Figure 21. Evolution of the thickness profile for (a)
![]() $(n,m)=(4,3)$
and (b)
$(n,m)=(4,3)$
and (b)
![]() $(n,m)=(3,2)$
, until
$(n,m)=(3,2)$
, until
![]() $t=6.6\times 10^{4}$
. Here,
$t=6.6\times 10^{4}$
. Here,
![]() $\unicode[STIX]{x0394}t=1.1\times 10^{4}$
,
$\unicode[STIX]{x0394}t=1.1\times 10^{4}$
,
![]() $\unicode[STIX]{x1D703}=25^{\circ }$
,
$\unicode[STIX]{x1D703}=25^{\circ }$
,
![]() $A=10$
,
$A=10$
,
![]() $G=1$
,
$G=1$
,
![]() $h_{\ast }=0.01$
and
$h_{\ast }=0.01$
and
![]() $B=0.01$
.
$B=0.01$
.
Figure 22 shows
![]() $U/Bh_{\ast }$
versus
$U/Bh_{\ast }$
versus
![]() $A\tan \unicode[STIX]{x1D703}/h_{\ast }^{2}$
for several combination of the parameters
$A\tan \unicode[STIX]{x1D703}/h_{\ast }^{2}$
for several combination of the parameters
![]() $(n,m)$
,
$(n,m)$
,
![]() $A$
,
$A$
,
![]() $\unicode[STIX]{x1D703}$
,
$\unicode[STIX]{x1D703}$
,
![]() $B$
,
$B$
,
![]() $h_{\ast }$
and
$h_{\ast }$
and
![]() $G$
. Gravity has an noticeable effect on the power law exponent. Effectively, for
$G$
. Gravity has an noticeable effect on the power law exponent. Effectively, for
![]() $G=1$
(
$G=1$
(
![]() $G=0$
), the exponent is
$G=0$
), the exponent is
![]() $\unicode[STIX]{x1D6FD}=0.45$
(
$\unicode[STIX]{x1D6FD}=0.45$
(
![]() $\unicode[STIX]{x1D6FD}=0.40$
), with
$\unicode[STIX]{x1D6FD}=0.40$
), with
![]() $\unicode[STIX]{x1D6FD}$
independent of
$\unicode[STIX]{x1D6FD}$
independent of
![]() $n$
and
$n$
and
![]() $m$
. Due to the fact that the exponents are different in each case, the results suggest that the droplet could migrate with a larger velocity for
$m$
. Due to the fact that the exponents are different in each case, the results suggest that the droplet could migrate with a larger velocity for
![]() $G=1$
than for
$G=1$
than for
![]() $G=0$
. In addition, we can see from the figure that for a given value of
$G=0$
. In addition, we can see from the figure that for a given value of
![]() $G$
, polar liquids migrate always with a velocity
$G$
, polar liquids migrate always with a velocity
![]() $U$
smaller than non-polar ones.
$U$
smaller than non-polar ones.

Figure 22. Dependence of the velocity
![]() $U$
for several combinations of the parameters
$U$
for several combinations of the parameters
![]() $(n,m)$
,
$(n,m)$
,
![]() $A$
,
$A$
,
![]() $\unicode[STIX]{x1D703}$
,
$\unicode[STIX]{x1D703}$
,
![]() $B$
,
$B$
,
![]() $h_{\ast }$
and
$h_{\ast }$
and
![]() $G$
.
$G$
.
6 Discussions and conclusions
In this paper, the fate of droplets subject to a thermocapillary driving force is investigated numerically with a particular emphasis on the effects of the form of the molecular interaction potential and gravity. The droplet dynamics model is based on the lubrication approximation and the resulting partial differential equation is solved in the finite element package COMSOL. The study confirms earlier observations that three regimes exist depending on the value of the contact angle.
For small contact angles, the film regime is observed for which the droplet stretches with the advancing contact line moving substantially faster than the receding one, leading to an increasing droplet footprint and a decreasing maximum thickness. The balance between viscous and Marangoni stresses produces a linear profile behind the capillary ridge at the front, analogous to the self-similar solution observed for falling films on solid substrates (Huppert Reference Huppert1982; Gomba et al.
Reference Gomba, Diez, González and Gratton2005, Reference Gomba, Diez, Gratton, González and Kondic2007). This regime is well described by the similarity solution given by Gomba & Homsy (Reference Gomba and Homsy2010) irrespective of the molecular interaction potential which is a consequence of the fact that for small contact angles, the contribution of the disjoining pressure term is negligible. The effect of gravity is to delay the development of this similarity solution which is still reached for larger times but with a decreased height of the capillary ridge at the advancing contact line. The asymptotic velocity of the front is given by
![]() $U=\sqrt{BA/t}$
.
$U=\sqrt{BA/t}$
.
For intermediate values of the contact angle, the transition regime occurs. In this regime, the droplet either travels with a constant profile which is different from the initial one or breaks up into a series of smaller droplets. In the former case, the droplet is found to travel with a constant velocity which scales as the
![]() $1/3$
power of the thermocapillary driving stress and is proportional to
$1/3$
power of the thermocapillary driving stress and is proportional to
![]() $K$
, i.e. small differences in the contact angle produce noticeable effects on the velocity, as shown in figure 9. In the latter case, the outcome is found to be strongly dependent of the molecular interaction potential. For polar liquids with a disjoining pressure given by the pair
$K$
, i.e. small differences in the contact angle produce noticeable effects on the velocity, as shown in figure 9. In the latter case, the outcome is found to be strongly dependent of the molecular interaction potential. For polar liquids with a disjoining pressure given by the pair
![]() $(n,m)=(3,2)$
, breakup occurs more readily than for non-polar liquids with a disjoining pressure for which
$(n,m)=(3,2)$
, breakup occurs more readily than for non-polar liquids with a disjoining pressure for which
![]() $(n,m)=(4,3)$
. In other words, it takes a smaller value of the driving thermocapillary stress to break up the initial droplets into smaller ones for polar liquids than for non-polar ones. This feature is explained by a linear stability analysis of the uniform film which develops as the droplet evolves in the intermediate regime. This linear stability analysis reveals that the maximum growth rate of the instability is larger for polar liquids,
$(n,m)=(4,3)$
. In other words, it takes a smaller value of the driving thermocapillary stress to break up the initial droplets into smaller ones for polar liquids than for non-polar ones. This feature is explained by a linear stability analysis of the uniform film which develops as the droplet evolves in the intermediate regime. This linear stability analysis reveals that the maximum growth rate of the instability is larger for polar liquids,
![]() $(n,m)=(3,2)$
than for non-polar ones and occurs for shorter wavelengths. In other words, the instability for non-polar liquids takes longer times and larger distances to develop. The stability analysis also allows a prediction of the number of droplets the parent droplet breaks up into and the distance between them. The effect of gravity on the growth of the instability is to decrease the maximum growth rate and shift it to larger wavelengths.
$(n,m)=(3,2)$
than for non-polar ones and occurs for shorter wavelengths. In other words, the instability for non-polar liquids takes longer times and larger distances to develop. The stability analysis also allows a prediction of the number of droplets the parent droplet breaks up into and the distance between them. The effect of gravity on the growth of the instability is to decrease the maximum growth rate and shift it to larger wavelengths.
For larger values of the contact angle, the model confirms earlier observations that the droplet travels with a constant profile and at constant speed towards the colder part of the substrate. The travelling velocity is found to be greater for non-polar than for polar liquids and scales linearly with the thermocapillary driving force with or without gravity. Interestingly, the exponent of the law is found to be independent of model of the disjoining pressure term, i.e. independent of
![]() $n$
and
$n$
and
![]() $m$
, but it depends on the gravity. We expect that for a different combination of parameters, the travelling velocity will be greater for the case with gravity. The effect of gravity in that case is to modify slightly the droplet profile as it travels on the substrate. Table 3 summarizes the description of the velocities of droplets for each regime, for polar and non-polar liquids and considering or not the effect of gravity.
$m$
, but it depends on the gravity. We expect that for a different combination of parameters, the travelling velocity will be greater for the case with gravity. The effect of gravity in that case is to modify slightly the droplet profile as it travels on the substrate. Table 3 summarizes the description of the velocities of droplets for each regime, for polar and non-polar liquids and considering or not the effect of gravity.
Table 3. Velocity of droplets for each regime depending on the molecular and gravitational effects. The velocities for the transition regime are for cases without breakups.

The results allow us to discuss the points raised in § 1 about experimental observations. These are: (i) the saturation in the curve
![]() $U$
versus
$U$
versus
![]() $R$
, (ii) the elongation of large droplets and (iii) the non-occurrence of breakups.
$R$
, (ii) the elongation of large droplets and (iii) the non-occurrence of breakups.
Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) show in their figure
![]() $2$
the velocity of the droplets versus the footprint,
$2$
the velocity of the droplets versus the footprint,
![]() $R$
. While for the smallest droplets in the experiments (
$R$
. While for the smallest droplets in the experiments (
![]() $1<R<4$
), the velocity follows a roughly linear dependence on
$1<R<4$
), the velocity follows a roughly linear dependence on
![]() $R$
, from
$R$
, from
![]() $R\geqslant 4$
the experimental curve suggests that the velocity starts to converge to a saturation value. The authors attribute the change in the curve to the effect of gravity, despite the fact that gravity is expected to have an effect from smaller values of
$R\geqslant 4$
the experimental curve suggests that the velocity starts to converge to a saturation value. The authors attribute the change in the curve to the effect of gravity, despite the fact that gravity is expected to have an effect from smaller values of
![]() $R$
, i.e. for
$R$
, i.e. for
![]() $R\geqslant 1$
.
$R\geqslant 1$
.
Our results show that gravity is not needed to explain the change in the behaviour of
![]() $U$
, and that gravity effectively has an effect from
$U$
, and that gravity effectively has an effect from
![]() $R=1$
. Under non-gravity conditions, it is observed that as
$R=1$
. Under non-gravity conditions, it is observed that as
![]() $A$
is increased, the velocity first follows a dependence with
$A$
is increased, the velocity first follows a dependence with
![]() $A^{0.4}$
in the droplet regime, to later saturate to a velocity
$A^{0.4}$
in the droplet regime, to later saturate to a velocity
![]() $U\propto KB^{1/3}$
when flat droplets emerge due to the presence of the Marangoni stress (§ 4.1.1). When gravity is included to match experimental conditions, droplets with
$U\propto KB^{1/3}$
when flat droplets emerge due to the presence of the Marangoni stress (§ 4.1.1). When gravity is included to match experimental conditions, droplets with
![]() $R<1$
also displace with a velocity
$R<1$
also displace with a velocity
![]() $U\propto A^{0.4}$
because the effect of gravity is negligible. Middle size droplets (
$U\propto A^{0.4}$
because the effect of gravity is negligible. Middle size droplets (
![]() $1<R<4$
) move with
$1<R<4$
) move with
![]() $U\propto A^{0.45}$
, and larger droplets migrate with a velocity
$U\propto A^{0.45}$
, and larger droplets migrate with a velocity
![]() $U\propto KB^{2/5}$
.
$U\propto KB^{2/5}$
.
Figure 23 shows
![]() $U$
versus
$U$
versus
![]() $R$
(both dimensionless), in the same fashion as in the experimental works. Let us first focus on the curve for
$R$
(both dimensionless), in the same fashion as in the experimental works. Let us first focus on the curve for
![]() $G=1$
. Notice that for
$G=1$
. Notice that for
![]() $R<1$
we have
$R<1$
we have
![]() $U\propto R^{0.72}$
. For
$U\propto R^{0.72}$
. For
![]() $1<R<4$
,
$1<R<4$
,
![]() $U\propto R^{0.45}$
which is easily explained because as gravity is not negligible,
$U\propto R^{0.45}$
which is easily explained because as gravity is not negligible,
![]() $A\propto R$
. Finally, for
$A\propto R$
. Finally, for
![]() $R>4$
the velocity saturates. The inset of figure 23 shows the same data in a regular plot: the points with
$R>4$
the velocity saturates. The inset of figure 23 shows the same data in a regular plot: the points with
![]() $1<R<4$
resembles the ‘almost linear’ dependence observed in the experiments for
$1<R<4$
resembles the ‘almost linear’ dependence observed in the experiments for
![]() $R<4$
. The saturation of
$R<4$
. The saturation of
![]() $U$
for
$U$
for
![]() $R\approx 4$
is observed for both
$R\approx 4$
is observed for both
![]() $G=1$
and
$G=1$
and
![]() $G=0$
, as occurs in experiments. Therefore, the saturation of
$G=0$
, as occurs in experiments. Therefore, the saturation of
![]() $U$
is due to the change of the dynamics from droplet to transition regimes, and not to the action of gravity.
$U$
is due to the change of the dynamics from droplet to transition regimes, and not to the action of gravity.

Figure 23. Log–log plot of the velocity against the footprint of the droplets (both are dimensionless,
![]() $B=0.008$
,
$B=0.008$
,
![]() $\unicode[STIX]{x1D703}=13^{\circ }$
). The plot clearly shows three different behaviours for
$\unicode[STIX]{x1D703}=13^{\circ }$
). The plot clearly shows three different behaviours for
![]() $U$
when
$U$
when
![]() $G=1$
. For
$G=1$
. For
![]() $R<1$
, gravity is negligible and
$R<1$
, gravity is negligible and
![]() $U\propto R^{0.72}$
. For
$U\propto R^{0.72}$
. For
![]() $1<R<4$
,
$1<R<4$
,
![]() $U\propto R^{0.45}$
due to the action of gravity. Larger droplets present a saturation with a velocity
$U\propto R^{0.45}$
due to the action of gravity. Larger droplets present a saturation with a velocity
![]() $U=Bh_{e}$
when the dynamics switches from ‘droplet’ to ‘transition’ regime. For
$U=Bh_{e}$
when the dynamics switches from ‘droplet’ to ‘transition’ regime. For
![]() $G=0$
,
$G=0$
,
![]() $U$
also presents a saturation at
$U$
also presents a saturation at
![]() $R\approx 4$
, as in the experiments. The inset shows the same data, in regular coordinates, as in figure 2 of Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993).
$R\approx 4$
, as in the experiments. The inset shows the same data, in regular coordinates, as in figure 2 of Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993).
Brzoska et al. (Reference Brzoska, Brochard-Wyart and Rondelez1993) also tracked the shape of the perimeter of the moving droplet, and observed that smaller ones almost keep their initial shape, while larger ones elongate in the direction of displacement. In figure 6 of their work, the authors present the ratio of the elongation on the initial radius against the initial radius: the relative elongation is stronger for larger volume droplets. Our simulations show, effectively, that the relative elongation is larger for
![]() $R>4$
without the need for the action of gravity. Again, the change is due to the transition from ‘droplet’ to ‘transition’ regime.
$R>4$
without the need for the action of gravity. Again, the change is due to the transition from ‘droplet’ to ‘transition’ regime.
Another important issue connected with the elongation of the droplet is the non-occurrence of the breakup in experiments, as predicted by Gomba & Homsy (Reference Gomba and Homsy2010). Here, we show that, when a non-polar liquid is considered, the droplet elongates but does not break up into smaller droplets, as shown in § 4. The instability of the bulk region behind the bump is found to be sensitive both to the effects of gravity and the polarity of the liquid. As shown in figure 16, polar liquids present a larger growth rate than non-polar ones. Furthermore, as the shorter unstable mode of a polar liquid is smaller than the corresponding one for non-polar liquids, the instability of the latter requires larger domains than the former to develop, i.e. more stretched droplets. Gravity has a similar effect, reducing both the growth rate (the maximum growth rate
![]() $\unicode[STIX]{x1D714}_{max}=h_{e}^{3}(K^{\prime }-G)^{2}/4$
), and the shorter unstable mode,
$\unicode[STIX]{x1D714}_{max}=h_{e}^{3}(K^{\prime }-G)^{2}/4$
), and the shorter unstable mode,
![]() $k_{c}=(K^{\prime }-G)^{1/2}$
. In conclusion, the polar nature of the liquid is an important parameter to consider to correctly account for the experimental results. Experimentalists should employ polar liquids or stronger thermal gradients in order to observe, under gravity conditions, the breakup of drops.
$k_{c}=(K^{\prime }-G)^{1/2}$
. In conclusion, the polar nature of the liquid is an important parameter to consider to correctly account for the experimental results. Experimentalists should employ polar liquids or stronger thermal gradients in order to observe, under gravity conditions, the breakup of drops.
Summarizing, this study provides new insights on the fate of partially wetting droplets subject to a non-uniform, linearly varying temperature field. It also provides new guidelines on how to select fluid properties and operating conditions to achieve a desired wetting outcome which may be of importance, for example, in the context of droplet actuation in open microfluidics devices.
Acknowledgements
The authors gratefully acknowledge the funding supports via the CONICET grants PIP no. 356 and PIP no. 299, the ANPCyP grant no. 2012-1707 and CICPBA.