Introduction
Many women experience various forms of psychological, social and environmental adversity, and mounting evidence indicates that the consequences can affect following generations. Exposure to stressors before or during pregnancy is referred to as maternal adversity. Maternal adversity can engender negative and positive adaptations in brain function and behavior that can be passed along to offspring through a process termed transgenerational transmission. Specifically, maternal adversity has been associated with cognitive, neurodevelopmental and psychological effects on offspring. The temporal proximity of stressors to pregnancy and delivery may alter risk; however, an empirical understanding of the effects of stress before pregnancy remains limited.
There is emerging evidence to suggest that enrichment, or positive psychosocial and environmental exposures for mothers and/or their offspring, may confer protective benefits to counter adverse transgenerational transmission. Currently, epigenetics is the most prominent explanation of transgenerational transmission.Reference Youngson and Whitelaw 1 Epigenetics describes how interactions between the genotype and environment alters the phenotype via heritable changes in gene expression. Evidence suggests that epigenetic processes can be altered by life experiences, prenatal environmental, postnatal mother–infant interactions and juvenile social rearing with long-term consequences that affect first and second-generation offspring.Reference Champagne 2
In this paper, empirical findings regarding the transgenerational transmission of maternal adversity during three critical periods – childhood, pregestational adulthood and pregnancy – will be reviewed in terms of pregnancy outcomes, maternal care, offspring behavior and development, and physiological functioning. Key findings will be summarized in Table 1. Research on the transgenerational transmission of enrichment and the implications for interventions to ameliorate the consequences of adversity will also be presented. In the final section, underlying epigenetic and environmental mechanisms that have been proposed to explain how experience is transferred across generations through transgenerational transmission will be reviewed.
Table 1 A summary of key experimental rodent models, demonstrating transgenerational transmission of early, pregestational and prenatal stress and enrichment to the next generation in terms of behavioral and physiological effects
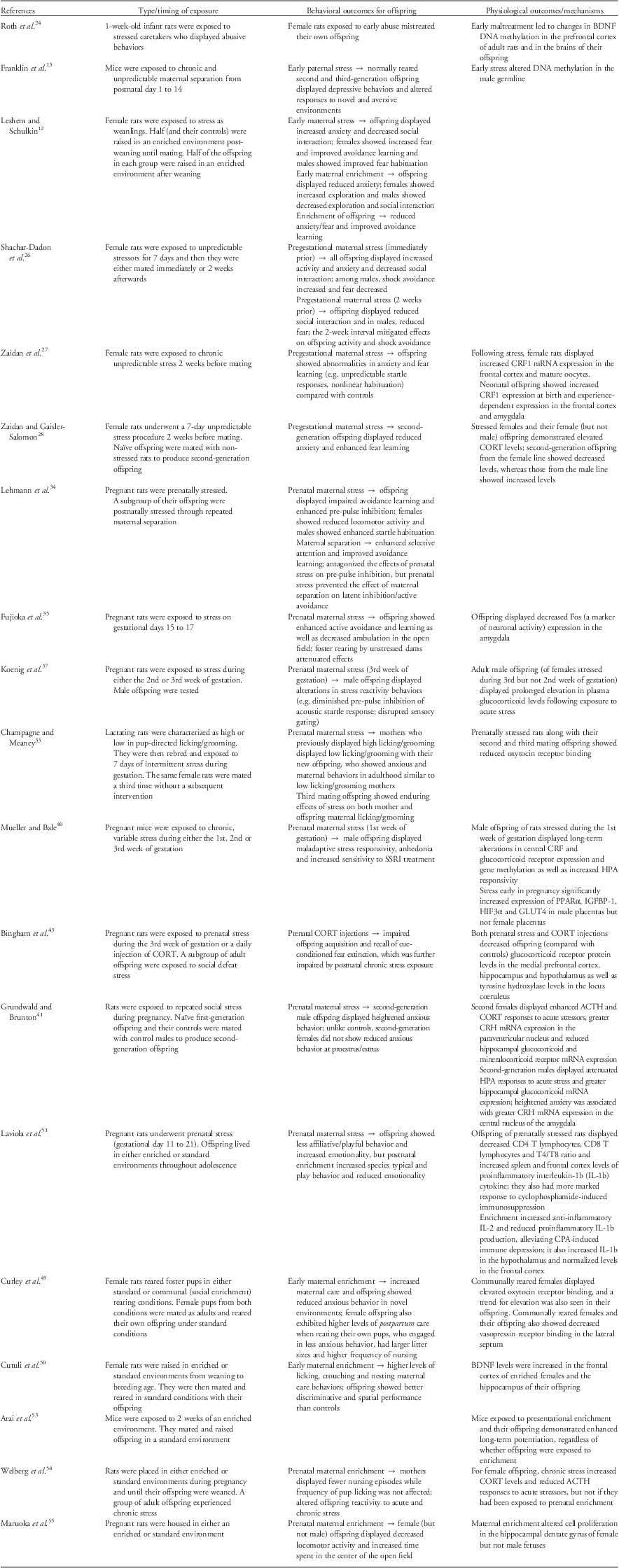
BDNF, brain-derived neurotrophic factor; CRF1, corticotropin-releasing factor type 1; mRNA, messenger RNA; CORT, corticosterone; HPA, hypothalamic–pituitary–adrenal axis; ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; SSRI: selective serotonin reuptake inhibitor; CPA: cyclophosphamide (an immunosuppressant agent).
Early adversity
Adverse experiences that occur early in development, such as abuse during childhood, are associated with long-term consequences. For instance, young children exposed to war-related trauma exhibit lasting and severe posttraumatic stress symptoms that place them at risk for future maladaptation,Reference Feldman and Vengrober 3 and childhood abuse has been found to contribute to maladaptation independent of confounding environmental and social stressors.Reference Noll 4 Childhood adversity is also a major risk factor for the development of mental disorders and behavioral dysfunctions, with numerous studies demonstrating that early abuse is associated with psychopathology (e.g. depression, anxiety, suicidality) and substance use in adulthood.Reference Brodsky, Mann and Stanley 5 – Reference MacMillan, Fleming and Streiner 8 Considering abuse and trauma survivors remain vulnerable to lasting psychological consequences, certain life events may trigger dormant symptoms. Pregnancy or the birth of a child can be particularly stressful, making it a time of increased vulnerability.Reference Noll 4 Women who were abused in childhood, for example, report a greater degree of suicidal ideation during their pregnancies.Reference Farber, Herbert and Reviere 9
Studies examining the effect of early maternal adversity on pregnancy outcomes are scarce, contradictory and seriously limited by methodological challenges.Reference Wosu, Gelaye and Williams 10 However, three studies have found significant associations, such that women with a history of childhood abuse had 2.6–4.8 increased odds of preterm delivery.Reference Wosu, Gelaye and Williams 10 Preterm delivery, in turn, increases long-term risks for the infant, including respiratory, gastrointestinal and renal problems; cerebral palsy; visual, auditory and intellectual impairments; and other neurological disorders.Reference Moster, Lie and Markestad 11 Although preterm delivery is not necessarily a result of transgenerational transmission, it is a noteworthy way in which maternal adversity may affect offspring.
There is also preliminary evidence that the long-term consequences of early maternal adversity affects the behavior and psychopathology of future offspring. In a study by Leshem and Schulkin, the offspring of female rats exposed to stress as weanlings exhibited increased anxiety and decreased social interactions.Reference Leshem and Schulkin 12 Sex-specific effects were also observed, such that males demonstrated improved fear habituation and stress coping, whereas females demonstrated increased fear responses and agitation.Reference Leshem and Schulkin 12 Another study found that mice exposed to stress from birth to 2 weeks of age displayed depressive behaviors and altered DNA methylation, both of which were transmitted across generations in a sex-specific manner such that normally reared offspring displayed similar behavioral responses and alterations in gene expression.Reference Franklin, Russig and Weiss 13 Although empirical evidence regarding the transgenerational transmission of childhood adversity remains limited, physiological alterations and epigenetic changes have been elucidated.
To begin with, childhood abuse is associated with the hyper secretion of cortisol when stressed in adulthood.Reference Field, Diego and Hernandez-Reif 14 , Reference Bremner, Vythilingam and Vermetten 15 Early adversity affects future reactivity to stress by altering the developing neural circuits controlling neuroendocrine responses. Exposure to severe and chronic stress during periods of high neuronal plasticity (as in childhood) produces lasting alterations in the hypothalamic–pituitary–adrenal (HPA) axis, a major pathway in the stress response system.Reference Austin, Leader and Reilly 16 , Reference Lupien, McEwen and Gunnar 17 It is worth noting that HPA dysregulation may explain heightened vulnerability to preterm delivery, and heightened basal cortisol in women before pregnancy has been found to be a strong predictor of preterm delivery.Reference Field, Diego and Hernandez-Reif 14 , Reference Noll, Schulkin and Trickett 18 Furthermore, early adversity may elevate corticotropin-releasing hormone (CRH) gene expression in the mother’s brain and (during pregnancy) placenta, stimulating fetal cortisol and adrenocorticotropic hormone (ACTH) and signaling premature maturation of fetal tissue.Reference Horan, Hill and Schulkin 19 , Reference Moog, Buss and Entringer 20
As these physiological alterations are not always observed, it is possible that variations in epigenetic programming influence the long-term consequences of early adversity. Indeed, the postmortem hippocampal brain tissue of adults who experienced trauma or maltreatment in childhood reveal diminished glucocorticoid receptor (NR3C1 gene) expression,Reference McGowan, Sasaki and D’Alessio 21 which may explain heightened HPA responsiveness to stress.Reference Hyman 22 Furthermore, institutionally reared children (from birth) show increased DNA methylation throughout the genome, including genes implicated in brain development, when compared with age-matched children reared by their biological parents.Reference Naumova, Lee and Koposov 23 In a rat model, early adversity produced brain-derived neurotrophic factor (BDNF) DNA methylation and reduced BDNF gene expression in the prefrontal cortex; these epigenetic alterations persisted into adulthood, were transmitted to offspring and could be ‘rescued’ with robust DNA methylation inhibitor treatment.Reference Roth, Lubin and Funk 24 It is important to note that the rodents who experienced early abuse were also more likely to mistreat their own offspring. In addition to physiological dysregulation and epigenetic alterations, behavioral learning as well as psychosocial environmental continuity between the mother and her child may account for the transgenerational transmission of the consequences of childhood abuse.Reference Collishaw, Dunn and O’connor 25
In sum, preliminary evidence indicates that the long-term adverse consequences of childhood abuse affect pregnancy and offspring. Women exposed to early adversity may be more susceptible to stress during a major psychosocial stressor like pregnancy. This may be due in part to HPA dysregulation along with elevated CRH and cortisol levels. Differences in gene expression as well as individual differences in the outcomes of early adversity suggest that epigenetic influences exist.
Pregestational adversity
In addition to early adversity, adverse events that occur in adolescence or adulthood, but precede pregnancy may engender transgenerational transmission. An important consideration is the temporal proximity of the adverse event to reproduction and whether its impact may wane over time. Indeed, if the parent generation is to impart useful environmental information, adversity early in life may be less relevant than adversity shortly before reproduction. Although there is limited research on the impact of pregestational stress during the different life stages of the future mother, findings indicate that adverse experiences before pregnancy can have long-term behavioral and physiological effects on offspring.
In one experimental model, female rats were exposed to stress for 7 days and either mated immediately or 2 weeks afterwards.Reference Shachar-Dadon, Schulkin and Leshem 26 When maternal stress occurred immediately before pregnancy, offspring (compared with controls) displayed increased activity and anxiety and decreased social interaction; males also showed enhanced shock avoidance and decreased fear.Reference Shachar-Dadon, Schulkin and Leshem 26 When maternal stress occurred 2 weeks before pregnancy, offspring exhibited enhanced social interaction, and among males reduced fear.Reference Shachar-Dadon, Schulkin and Leshem 26 On most measures, stress immediately before mating had a greater impact than stress 2 weeks earlier, suggesting a mitigation of the effects of adversity with the passage of time between stress and conception.Reference Shachar-Dadon, Schulkin and Leshem 26 Compared with a previously discussed model of weanling stress,Reference Leshem and Schulkin 12 both early and pregestational stress increased offspring activity in the explorative tests, reduced social interaction and improved avoidance learning in males, but only weanling stress increased avoidance learning in females. Furthermore, some of the behavioral effects of pregestational stress are similar to those of prenatal stress (discussed below), such as increased anxiety in the elevated maze, reduced social interaction and improved avoidance learning in males.Reference Lehmann, Stöhr and Feldon 34 , Reference Fujioka, Fujioka and Tan 35 , Reference Koenig, Elmer and Shepard 37 , Reference Weinstock 39 Finally, it is noteworthy that some of these behavioral effects of transgenerational transmission might be considered disadvantageous (e.g. anxiety, reduced social interaction) and others advantageous (e.g. improved avoidance learning, reduced fear) to the offspring.
Beyond behavioral effects, it is worth briefly mentioning that pregestational adversity may delay conception and affect reproductive outcomes. In experimental rat models, neonatal weight has been found to be increased in the offspring of females stressed immediately before mating,Reference Shachar-Dadon, Schulkin and Leshem 26 although birth weight is often reduced after pregestational stress.Reference Zaidan, Leshem and Gaisler-Salomon 27 However, such findings are mixed and are not necessarily the result of transgenerational transmission. Models that explore physiological mechanisms underlying behavioral alterations provide more compelling evidence of transgenerational transmission via epigenetic mechanisms.
One study found that exposing female rats to chronic unpredictable stress 2 weeks before conception altered the emotional, learning and social behavior of future offspring.Reference Zaidan, Leshem and Gaisler-Salomon 27 Behavioral effects differed by sex, such that female offspring were more fearful, anxious, cognitively impaired and less sociable; males were also less sociable, but were less fearful and more successfully avoidant.Reference Zaidan, Leshem and Gaisler-Salomon 27 Interestingly, pregestational stress increased the expression of corticotropin-releasing factor type 1 messenger RNA (mRNA) in the ova as well as in the brains of mothers and offspring, suggesting an epigenetic route of transgenerational transmission.Reference Zaidan, Leshem and Gaisler-Salomon 27 A second study found that pregestational stress to female rats 2 weeks before mating resulted in reduced anxiety, enhanced fear learning and improved adaptive learning for second-generation offspring.Reference Zaidan and Gaisler-Salomon 28 In addition, levels of the stress hormone corticosterone (CORT; an indicator of HPA functioning) was altered across the three generations in a sex-dependent manner.Reference Zaidan and Gaisler-Salomon 28 Structurally, pregestational maternal adversity has been found to affect offspring by altering medial prefrontal brain morphology and increasing the number and length of dendritic spines in certain brain regions; these regional effects were shown to vary depending on the temporal proximity between adversity and conception.Reference Bock, Poeschel and Schindler 29
Many researchers maintain that transgenerational transmission is likely mediated by stress-induced epigenetic programming of future gene activity in the oocyte, whereas others contend that continuity of psychosocial environment may mostly explain the effects. Another possibility is that the long-term consequences of pregestational adversity persist into pregnancy to affect fetal development and into the postpartum period to alter maternal, and thereby offspring behavior. Different maternal behavior toward male and female offspring might partially explain sex differences. Compromised maternal competence is detrimental, inter alia, to the developing stress physiology in rat and nonhuman primates,Reference Champagne, Francis and Mar 30 and its effects can persist across generations. Such effects are difficult to demonstrate in humans.
In sum, pregestational adversity to the mother may have effects on her offspring that persist into adulthood, differ by sex, differentially effect behaviors and depend on the temporal proximity of the stressful exposure to conception. Pregestational adversity to the mother can impart both resilience and impairment to offspring. Additional findings demonstrate behavioral effects on the second and third generations and elucidate physiological changes to support epigenetic routes of transmission, although further research is needed to tease apart confounding psychosocial factors. Future studies on gender differences in brain development and neural programming are also needed to better understand differential responses to maternal adversity.
Prenatal adversity
Importantly, prenatal maternal adversity (stress during pregnancy) impacts the fetal environment, and thus the fetus directly, whereas pregestational adversity does not. More research is available on maternal stress during rather than before pregnancy, and it is currently well understood that prenatal adversity can have long-term behavioral, neurobiological and psychological consequences for infants as well as mother–infant interactions postpartum.Reference Weinstock 31 , Reference Monk, Spicer and Champagne 32 Such effects of transgenerational transmission are demonstrated by experimental rat models.
To begin with, prenatal adversity has been shown to directly affect maternal behavior postpartum. In one study, female rats, who previously engaged in high pup-directed licking/grooming following an unstressed pregnancy, engaged in low levels of these maternal care behaviors following both a stressful pregnancy and a subsequent unstressed pregnancy.Reference Champagne and Meaney 33 The offspring showed anxious and low maternal care behaviors in adulthood, and along with their prenatally stressed mother, showed reduced oxytocin receptor binding.Reference Champagne and Meaney 33 Another study found that even brief and repeated separation of prenatally stressed pups from their dams could reverse deleterious effects on offspring avoidance learning,Reference Lehmann, Stöhr and Feldon 34 suggesting that the effects of adversity on maternal behavior might ameliorate with the passage of time. However, it is significant to note that cross-fostering by unstressed dams has been shown to attenuate rather than completely eliminate the effects of prenatal stress on offspring,Reference Fujioka, Fujioka and Tan 35 and altered maternal behavior may not be necessary for transgenerational transmission. Evidence suggests that the neuroendocrine system, and thus, epigenetic processes, may be the primary mediator of prenatal effects on behavior.Reference Monk, Spicer and Champagne 32 , Reference Bale 36 Indeed, prenatal stress can predict offspring’s behavior as well as their neuroendocrine stress reactivity;Reference Weinstock 31 the consequences are long-lasting, spanning from the juvenile period to adulthood.
The timing of the adverse event during pregnancy is important to consider. In one rat model, maternal stress during the 3rd, but not the 2nd, week of gestation was significantly associated with alterations in stress reactivity behaviors and prolonged elevations in glucocorticoid levels among adult male offspring.Reference Koenig, Elmer and Shepard 37 Elevated maternal and fetal exposure to glucocorticoids can permanently alter HPA function in a sex-specific manner.Reference Kapoor, Dunn and Kostaki 38 For rats, the 3rd week of gestation corresponds to a critical period during which time the fetal HPA axis begins to release its own ACTH and CORT.Reference Weinstock 39 However, the male offspring of mice exposed to stress earlier in gestation (1st week) have also been found to display maladaptive stress reactivity and depressive behaviors, accompanied by long-term differences in central corticotropin-releasing factor (CRF) and glucocorticoid receptor expression as well as increased HPA reactivity.Reference Mueller and Bale 40 In addition, early prenatal stress increased the expression of particular genes in male but not female placentas, suggesting that differences in placental epigenetic mechanisms may explain sex-specific transgenerational transmission.Reference Mueller and Bale 40
Many behavioral findings are sex specific, such that male offspring are often more vulnerable prenatally. In a study on rats, stress during pregnancy altered HPA regulation in the mother, her offspring and the second generation in a sex-dependent way; second-generation females demonstrated greater stress responses, whereas second-generation males demonstrated attenuated stress responses and heightened anxious behavior.Reference Grundwald and Brunton 41 Heightened anxiety was associated with greater CRH mRNA gene expression in the amygdala, and attenuated stress responses were associated with greater glucocorticoid mRNA expression in the hippocampus and impaired feedback to the HPA.Reference Grundwald and Brunton 41 Prenatal exposure to adverse events has been found to impact HPA and glucocorticoid receptor activation in the placenta and diverse regions of the brain.Reference Welberg, Seckl and Holmes 42 In one study, the offspring of rats exposed to either a daily injection of CORT or prenatal stress during the 3rd week of gestation all displayed decreased glucocorticoid receptor protein levels in the medial prefrontal cortex, hippocampus and hypothalamus, as compared with controls.Reference Bingham, Rani and Frazer 43
In humans, elevated levels of depression during the third trimester of pregnancy has been associated with increased DNA methylation of the glucocorticoid receptor gene (NR3C1) in newborns (but not mothers), which predicted cortisol and HPA stress reactivity at 3 months of age.Reference Oberlander, Weinberg and Papsdorf 44 Elevated levels of NR3C1 DNA methylation have also been found in the children of women who experienced intimate partner violence during pregnancy.Reference Radtke, Ruf and Gunter 45 In addition, prenatal adversity is associated with excess amounts of CRH and cortisol, impaired fetal habituation to stimuli, and temperamental difficulties in infants.Reference Austin, Leader and Reilly 16 Perceived maternal stress during pregnancy can predict infant cortisol reactivity during neonatal and postnatal assessments, and neonatal cortisol reactivity predicts behavioral reactivity at 10 months of age.Reference Leung, Tasker and Atkinson 46 Similarly, exposure to elevated concentrations of maternal cortisol during the second and third trimesters predicts larger infant cortisol responses to and prolonged behavioral recovery from a blood draw.Reference Davis, Glynn and Waffarn 47 In contrast, mothers with posttraumatic stress disorder (PTSD) who experienced trauma during pregnancy and their children had lower (rather than higher) cortisol levels.Reference Yehuda, Engel and Brand 48
In sum, prenatal adversity has been found to affect both maternal and offspring behavior. Transgenerational transmission may be mediated primarily through epigenetic processes, as there is a growing evidence base for an association between prenatal maternal stress and altered gene expression in offspring. Epigenetic pathways appear to operate via the neuroendocrine system, as glucocorticoid, cortisol and CRH levels have been found to be affected by prenatal stress. In turn, the HPA system plays a significant role in fetal programming and can lead to long-term consequences. Finally, models of prenatal stress have identified sex-specific and temporal-specific effects on offspring behavior, such as stress responses, that warrant further exploration.
Transgenerational enrichment
Enrichment refers to positive psychosocial and environmental exposures. In rodent modes, enrichment often involves the addition of a running wheel, tube or ladder to the environment or more opportunities for social interaction, which are believed to increase cognitive and/or motor stimulation. Maternal and/or offspring enrichment may confer positive benefits and protect against or ameliorate the negative effects of transgenerational transmission.
Early maternal enrichment has been found to have a positive impact on postpartum care and offspring behavior. In one study, female rats who were reared in socially enriched, communal conditions by foster mothers later showed improved maternal care when rearing their own offspring in standard conditions.Reference Curley, Davidson and Bateson 49 The offspring of rats raised in enrichment conditions exhibited reduced anxious behaviors, and female offspring displayed higher levels of maternal care when rearing their own pups, who also displayed less anxious behaviors.Reference Curley, Davidson and Bateson 49 Elevated oxytocin receptor binding and decreased vasopressin receptor binding was observed in both enriched mothers and their offspring.Reference Curley, Davidson and Bateson 49 Another study found similar effects, such that female rats raised in an enriched environment from weaning until breeding age demonstrated higher levels of maternal care behavior.Reference Cutuli, Caporali and Gelfo 50 This route of transgenerational transmission was linked to BDNF-induced neuroplasticity, such that the behavior of enriched females and their offspring was associated with profound rearrangements in neurotrophin pattern, and higher BDNF levels were expressed in the frontal cortex of enriched mothers and in the hippocampus of their offspring.Reference Cutuli, Caporali and Gelfo 50
In a previously discussed study by Leshem and Schulkin, female rats exposed to stress as weanlings were either raised in enriched or standard environment post-weaning until mating.Reference Leshem and Schulkin 12 Early maternal stress followed by subsequent pregestational enrichment had an anxiolytic effect on offspring, reducing anxious behaviors (elevated following early stress without enrichment).Reference Leshem and Schulkin 12 Pregestational enrichment prevented impairment of startle habituation in female offspring, and effects on social interaction were most marked in male offspring, suggesting that social behavior may be more vulnerable to transgenerational transmission among males.Reference Leshem and Schulkin 12 Offspring who were then raised in an enriched environment post-weaning exhibited reduced anxiety in the open field on all measures of explorative behavior.Reference Leshem and Schulkin 12 On other tests, the effects of offspring enrichment differed by sex: males (but not females) in the elevated maze demonstrated reduced anxiety as well as increased habituation to startle and improved avoidance learning.Reference Leshem and Schulkin 12
Similarly, when offspring of prenatally stressed rats were exposed to postnatal enrichment, negative behavioral effects were reversed in that offspring demonstrated greater play behavior and less emotionality.Reference Laviola, Rea and Morley‐Fletcher 51 Beyond environmental enrichment, there is evidence to suggest that postnatal drug treatment (pharmacological enrichment) can alleviate offspring impairment following prenatal stress.Reference Poltyrev, Gorodetsky and Bejar 52 Importantly, mice exposed to 2 weeks of pregestational maternal enrichment along with their offspring have both been found to have enhanced long-term potentiation (LTP), regardless of whether offspring were or were not exposed to postnatal enrichment,Reference Arai, Li and Hartley 53 supporting the existence of transgenerational transmission of maternal experience before pregnancy.
Prenatal enrichment has also demonstrated effects on maternal care and offspring behavior. Rats who were exposed to an enriched environment during pregnancy and until their offspring were weaned displayed higher levels of maternal care behaviors and their offspring exhibited altered reactivity to both chronic and acute stress.Reference Welberg, Thrivikraman and Plotsky 54 Female offspring exposed to chronic stress in adulthood had increased CORT levels and reduced ACTH responses to acute stressors, but only if they had not been exposed to enrichment (controls).Reference Welberg, Thrivikraman and Plotsky 54 Thus, the prenatal and postnatal enrichment selectively altered HPA programming. In another study with sex-specific results, prenatal maternal enrichment resulted in behavioral differences among female offspring and influenced cell proliferation in the hippocampus of female (but not male) fetuses.Reference Maruoka, Kodomari and Yamauchi 55
In sum, pregestational and prenatal maternal enrichment has been found to improve maternal care and ameliorate the transgenerational consequences of adversity on offspring in a sex-specific manner. Postnatal enrichment can also be remedial for offspring, annulling the effects of pregestational stress, depending on the test and sex. Although more research is needed, the effects of enrichment appear to be primarily anxiolytic, reducing anxiety and fear and promoting explorative and social behavior. If potential damage to progeny and its prevention can both occur in the parent generation, remedial pregestational, prenatal, and postnatal enrichment could be combined to protect the offspring generation from the transgenerational transmission of adversity. As animal models are limited in their ability to assess complex psychosocial enrichment, human studies are needed, as the consequences of adversity might be ameliorated through therapeutic interventions to promote resilience. The transgenerational transmission of enrichment could have broad implications that warrant further investigation.
Mechanisms of transgenerational transmission
Epigenetic and environmental mechanisms have been proposed to explain how experience is transferred across generations through transgenerational transmission. In the preceding sections, hormonal and neural changes associated with adversity and enrichment at different stages were discussed. These changes are thought to be epigenetic in nature. Epigenetic programming is a prominent explanatory mechanism of transgenerational transmission that will be discussed further in this section, along with explanatory environmental mechanisms that involve fetal development, nurturing and contextual stressors.
To begin with, transgenerational transmission cannot simply be attributed to evolution, culture and/or genetic transmission alone. From an evolutionary perspective, transgenerational transmission ensures that the next generation is better prepared for an adverse environment. However, it remains unclear how adverse outcomes, such as transgenerational transmission of PTSD or schizophrenia,Reference Yehuda and Bierer 56 , Reference Perrin, Brown and Malaspina 57 could represent adaptive effects. In light of evidence that environmental adversity (e.g. traumatic experiences) can become encoded with brain and behavior consequences for the next generation, recent human research links dysfunctional outcomes with epigenetics.Reference Youngson and Whitelaw 1 , Reference McGowan and Roth 58 Epigenetics refers to interactions between the genotype and the environment that alter the phenotype via heritable changes in gene expression without underlying change in DNA sequence. Key mechanisms involved in epigenetic gene regulation include DNA methylation (silencing or enhancing gene expression) and histone modifications, which alter the functional qualities of the DNA sequence. Evidence suggests that epigenetic processes can be affected by life experiences, prenatal environmental, postnatal mother–infant interactions and juvenile social rearing with long-term consequences that effect first and second-generation offspring in a differential, sex-specific manner.Reference Champagne 2
As previously discussed, maternal stress before or during pregnancy results in elevated cortisol levels in both the mother and the fetus, and the activation of cortisol or CORT impacts the expression of information molecules (e.g. CRH) as well as neurotransmitter and glucocorticoid receptor sites. Chronic and prolonged exposure to stress hormones may explain the effects of transgenerational transmission on pregnancy and offspring, and epigenetic changes in chromatin is proposed to underlie the transgenerational programming effects of maternal stress via alterations in glucocorticoid signaling.Reference Cottrell and Seckl 59 Experimentally increasing prenatal exposure to glucocorticoids results in reduced birth weight and increased likelihood of developing disorders related to cardiovascular function, glucose homeostasis, HPA activity and anxiety-related behaviors in adulthood.Reference Welberg, Seckl and Holmes 42 , Reference Cottrell and Seckl 59 In humans, it is also associated with lower birth weight and higher blood pressure in offspring.Reference Cottrell and Seckl 59 Interestingly, glucocorticoid facilitation of CRH gene expression can elicit avoidance behaviors through epigenetic mechanisms, such as histone modifications (e.g. deacetylation). Such long-term changes in CRH expression may explain transmission.
Epigenetic regulation of CRH and other information molecules has been detected in placental tissue.Reference Abou-Seif, Shipman and Allars 60 , Reference Di Stefano, Wang and Parobchak 61 Considering CRH and glucocorticoids are implicated in an arousal pathway involving exaggerated anticipation of negative events, it follows that the upward regulation of CRH in the placenta is linked with the pathogenesis of chronic anxiety, fear and heightened vigilance in offspring.Reference Abou-Seif, Shipman and Allars 60 In addition, CRH facilitates parturition and conditions of duress can cause glucocorticoids to prematurely elevate CRH levels in the placenta, increasing vulnerability to preterm delivery.Reference Erickson, Thorsen and Chrousos 62 , Reference Majzoub 63 Preterm birth may serve to ‘rescue’ the fetus from an adverse environment or reduce the allocation of resources to a pregnancy occurring during stressful circumstances.Reference McLean, Bisits and Davies 64 , Reference Power and Schulkin 65 It is important to note that higher levels of CRH during pregnancy also increases maternal vulnerability to a suppressed HPA axis after delivery and postpartum depression, for which the CRH-R1 receptor has been implicated.Reference Meltzer-Brody, Stuebe and Dole 66 – Reference Engineer, Darwin and Nishigandh 68 Changes in maternal and offspring HPA function, which can be altered via stress-induced changes to CRF (or CRH) expression,Reference Zaidan, Leshem and Gaisler-Salomon 27 are often accompanied by behavioral effects. One key feature impacting these outcomes is social contact. Early social experiences affect neural systems, including CRH and glucocorticoid receptors, as well as brain morphology,Reference Bock, Poeschel and Schindler 29 , Reference Curley, Jensen and Mashoodh 69 and cross-fostering experiments show that changes in demethylation are linked to the transmission of social behaviors as well as CRH expression.Reference Elliott, Ezra-Nevo and Regev 70 Together, CRH and social factors can impact behavior and brain function.
Parental responsiveness has profound effects on HPA as well as extra-hypothalamic CRH expression. Nurturing maternal behaviors (e.g. grooming) can buffer offspring from the over activation of HPA when they are later exposed to adversity in adulthood.Reference Francis, Champagne and Meaney 71 Furthermore, studies with monkeys show that rearing conditions have long-term implications for neuropeptides, steroids, neurotransmitter expression and behavior.Reference Erickson, Gabry and Schulkin 72 Maternal deprivation in macaques results in elevated levels of cortisol and CRH, whereas macaques in normal maternal-reared conditions develop elevated levels of oxytocin and lower levels of CRH.Reference Winslow, Noble and Lyons 73 , Reference Rosenblum, Smith and Altemus 74 In addition, macaques with the anxiety risk allele of the SLC6A4 gene, who were reared under conditions of maternal separation, were subsequently found to have increased DNA methylation of the gene.Reference Kinnally, Capitanio and Leibel 75 Evidence that maternal care can alter the expression of behavioral and endocrinal stress response genes (as well as hippocampal synaptic development) supports an epigenetic route for transgenerational transmission of stress reactivity.Reference Meaney 76 For instance, impaired maternal care in mice causes female offspring to exhibit increased fear of novel objects and decreased exploratory behavior.Reference Curley, Champagne and Bateson 77 Additional animal research has shown that maternal care influences the subsequent maternal behavior of female offspring, a self-perpetuating cycle of nurturing competence that may be partially explained by epigenetic effects (e.g. oxytocin receptor gene expression).Reference Champagne and Curley 78 , Reference Weaver, Cervoni and Champagne 79
Paternal care also plays an important role in the functional development of the brain. Father-deprived animals display dysfunctions in anxiety-related subcircuits, including CRH-expressing neurons in the medial bed nucleus of the stria terminalis (BNST), prefrontal and limbic regions.Reference Gos, Schulkin and Gos 80 – Reference Seidel, Poeggel and Holetschka 82 In light of such effects, the transgenerational transmission of paternal experience is receiving increasing attention.Reference Franklin, Russig and Weiss 13 It has been proposed that stress to the father affects biological and behavioral phenotypes, causing epigenetic modification of DNA that is expressed in germ cells and thus, transmitted to offspring.Reference Yehuda 83 , Reference Dietz, Laplant and Watts 84 In one study, the offspring of male mice exposed to social stress displayed more depressive and anxious behaviors as well as differences in levels of plasma CORT and vascular endothelial growth factor.Reference Dietz, Laplant and Watts 84 Furthermore, the father’s nutritional diet, toxicological exposures (e.g. alcohol, nicotine, cocaine), age and phenotypic variation can lead to alterations in offspring development that are suggestive of an epigenetic germline route of paternal transmission.Reference Malaspina, Reichenberg and Weiser 85 , Reference Curley, Mashoodh and Champagne 86 Alternatively, or in combination with paternal transmission, life experiences may lead to changes in mate quality and preference, which can subsequently influence maternal investment.Reference Curley, Mashoodh and Champagne 86
There is ample evidence to support the transmission of adaptations to environmental circumstances. In animal studies, for instance, experimentally improved LTP is transmitted to improve offspring LTP,Reference Arai, Li and Hartley 53 and mothers exposed to predation threat before reproduction transfer protective behaviors to their offspring.Reference Giesing, Suski and Warner 87 , Reference Mashoodh, Sinal and Perrot-Sinal 88 In addition, human observational studies suggest that phenotypic variation acquired from the parental generation’s environment can be transmitted.Reference Pembrey, Saffery and Bygren 89 Studies on the Dutch famine of 1944 indicate that poor maternal nutrition during pregnancy is associated with low birth weight as well as a greater risk of metabolic and cardiovascular disease in offspring.Reference Painter, Roseboom and Bleker 90 The grandchildren of women who were undernourished during pregnancy also displayed increased neonatal adiposity,Reference Painter, Osmond and Gluckman 91 a potentially protective adaptation to uncertain food supply. Furthermore, severe changes in the paternal grandmothers’ food availability (before puberty) is associated with their granddaughters’ cardiovascular mortality risk.Reference Bygren, Tinghög and Carstensen 92 While paternal grandmothers’ food supply during the slow growth period (SGP; mid-childhood) is associated with the mortality risk of granddaughters, paternal grandfathers’ food supply during the SGP is associated with the mortality risk of grandsons.Reference Pembrey, Bygren and Kaati 93 This time- and sex-specific influence of ancestral nutrition during the SGP has been replicatedReference Kaati, Bygren and Pembrey 94 and may be mediated by sex chromosomes.Reference Pembrey, Saffery and Bygren 89
On a related note, maternal stress can influence fetal programming of obesity and metabolic disease.Reference Entringer, Buss and Swanson 95 , Reference Wang, Walker and Hong 96 For instance, pregestational exposure to famine is associated with decreased DNA methylation of insulin-like growth factor 2 (IGF2), which affects growth and development,Reference Heijmans, Tobi and Stein 97 and increased methylation of interleukin 10 and leptin,Reference Tobi, Lumey and Talens 98 both of which are obesity-related candidate genes. Neonatal epigenetic gene promoter methylation has also been associated with adiposity during childhood,Reference Godfrey, Sheppard and Gluckman 99 maternal obesity increases proinflammatory cytokines that can cause peripheral and intrauterine inflammation as well as offspring brain inflammation, whereas maternal diabetes can lead to hyperglycemia, inducing chronic intrauterine fetal tissue hypoxia and oxidative stress.Reference Li, Fallin and Riley 100 The combination of maternal pregestational (and/or gestational) diabetes and obesity has been associated with an increased risk of autism spectrum disorder and co-occurring intellectual disability in offspring.Reference Li, Fallin and Riley 100 These findings support the metabolic imprinting theory that intrauterine exposures have ‘fetal programming’ effects that can permanently alter offspring metabolic patterns and long-term risk for disease.Reference Waterland and Garza 101
The intrauterine period has been implicated as the most sensitive time for the establishment of epigenetic variability, which in turn affects offspring development, cell- and tissue-specific gene expression and risk for a range of disorders.Reference Wang, Walker and Hong 96 For instance, epigenetic alterations may mediate the effects of prenatal and postnatal exposures on the development of food allergies and other allergic diseases, which develop through complex environmental and genetic interactions.Reference Hong and Wang 102 – Reference Hong, Hao and Ladd-Acosta 104 The in utero environment is regulated by the placenta, which is highly susceptible to maternal distress, and altered gene expression within the placenta has been associated with adverse birth outcomes.Reference Monk, Spicer and Champagne 32 Increased expression of IGF2 and decreased expression of IGF1 in the placenta, for example, is associated with greater risk of intrauterine growth retardation.Reference Lee, Jeon and Lee 105 In addition to epigenetic routes of transgenerational transmission, maternal–placental–fetal endocrine factors and maternal nutrition during pregnancy can exert programming effects via fetal telomere length.Reference Entringer, Buss and Wadhwa 106 A recent study also found that maternal stress during pregnancy altered vaginal microflora and bacterial exposure (e.g. swallowed) during birth, affecting neonatal gastrointestinal microbiota and brain amino acid composition.Reference Banks 107
In sum, there are many forms of fetal programming. In utero environment as well as fetal exposure to the physiological consequences of maternal stress can impact offspring neurotransmitter and neuropeptide expression, increasing the long-term risk for negative health and behavioral outcomes. Pregestational and prenatal conditions can also affect fetal brain development, impacting cognitive and affective functioning as well as the size of limbic and frontal brain regions.Reference Entringer, Buss and Wadhwa 106 Many effects of transgenerational transmission appear to occur independently from changes in the genomic DNA sequence, and there is evidence for the prominent role of epigenetic mechanisms. Of course, the other side of evolution is endless adaptation and expansion to limit adverse effects of transgenerational transmission.
Conclusion
Concern for the infant’s future underlies much of the research on transgenerational transmission. The developmental perspective that early experience is formative of long-term behavioral and cognitive patterns is reflected in the numerous studies that have demonstrated affects of adversity during the prenatal, perinatal and postnatal periods on offspring brain development, stress reactivity and behavior regulation. However, developmental logic has been applied to an even earlier time – before pregnancy – far less often. Currently, our empirical understanding of the link between stress before conception and its remote outcomes on progeny remains limited. This is especially relevant now, as nongenomic and epigenetic transmission offer a conceptualization of how experience may be transferred across generations without enculturation.
Going forward, a unifying view of stress vulnerability and resilience proposes connecting genetic predisposition and programming sensitivity to contexts of experience–expectancy and transgenerational epigenetic traits.Reference Bock, Poeschel and Schindler 29 Cross-fostering experiments in particular will be critical to determine whether stress before conception primarily exerts its effects on offspring through behavioral and/or epigenetic mechanisms of transmission. Future research should also address the adaptive and maladaptive nature of transmissions as well as the extent of individual, familial and societal consequences. A knowledge base for remedial interventions is also necessary to focus on educational and therapeutic strategies and stem the escalation of risk across generations.
Acknowledgments
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.




