Introduction
Early stressful life events (SLEs) predict a myriad of poor health outcomes over the life course.Reference Cohen, Murphy and Prather1 Compromised reproductive function is generally not considered to be among these wide-ranging health outcomes, though stress has been linked to infertility.Reference Lynch, Sundaram, Maisog, Sweeney and Buck Louis2–Reference Chrousos and Gold4 It is hypothesized that stress may influence infertility via disruption of the hypothalamic–pituitary–adrenal (HPA) axis, which can dysregulate reproductive hormones and adversely impact normal reproductive function.Reference Magiakou, Mastorakos, Webster and Chrousos5 However, the link between stress and infertility has not been explored from a life course perspective, as most longitudinal studies of stress as an antecedent to infertility are limited to general periconceptual stress among couples trying to conceive.Reference Lynch, Sundaram, Maisog, Sweeney and Buck Louis2,Reference Buck Louis, Lum and Sundaram3,Reference Lynch, Sundaram, Buck Louis, Lum and Pyper6,Reference Park, Stanford, Porucznik, Christensen and Schliep7 Because stress is also implicated as a mechanism linking infertility to current health indicators and chronic health conditions later in life,Reference Hanson, Johnstone, Dorais, Silver, Peterson and Hotaling8–Reference Cedars, Taymans, DePaolo, Warner, Moss and Eisenberg10 it is critical to investigate exposure to stress over the life course to better understand the links between stress, reproductive health, and overall health.
Only three studies have considered the experience of SLEs prior to the occurrence of infertility, and only one has evaluated the effect of early life stressors.Reference Santos, Sobral and Martins11–Reference Jacobs, Boynton-Jarrett and Harville13 The link between early SLEs and infertility later during the life course is biologically plausible and consistent with life course theory, which proposes that an accumulation of stressors over time contributes to biologic wear and tear and later poor health outcomes,Reference Seeman, McEwen, Rowe and Singer14 with emphasis on the relevance of stressful events that occur during critical developmental periods.Reference Elder, Johnson, Crosnoe, Mortimer and Shanahan15 In terms of reproductive function, events that occur prior to or during puberty may permanently alter the HPA axis and other systems that maintain normative reproductive function.Reference Flinn, Nepomnaschy, Muehlenbein and Ponzi16 Specifically, chronic experiences of stress during childhood and adolescence can alter baseline functioning of the HPA axis, including the amount of cortisol released during the stress response, creating a stress-response system that is over-responsive to stimuli throughout the life course.Reference Flinn, Nepomnaschy, Muehlenbein and Ponzi16 Perturbation of the HPA axis can inhibit the release of gonadotropin-releasing hormone, which normally promotes reproductive hormones critical to ovulation and other normal reproductive function.Reference Magiakou, Mastorakos, Webster and Chrousos5,Reference Nakamura, Sheps and Arck17 In animal models, this early life disruption of normal endocrine function has been linked to early puberty, irregular estrus cycles, hormonal disruption, and impaired oocyte development, all of which impair normal reproduction.Reference Li, Hu and Li18,Reference Wu, Yuan and Li19 In humans, early life stressors have been associated with early reproductive function, including alterations in pubertal timing and earlier age at menarche.Reference Ellis and Essex20,Reference Clutterbuck, Adams and Nettle21 It has also been proposed that HPA functioning may be altered by early life stressors via epigenetic programming, which influences gene expression. Such programming has been proposed as a causal mechanism linking early life stress to poor health outcomes in adulthood, and emerging research in animal models supports the notion that epigenetic changes to HPA may be similarly linked to adult fertility.Reference Wu, Yuan and Li19,Reference Geraghty, Muroy, Zhao, Bentley, Kriegsfeld and Kaufer22–Reference Heim and Binder26
Maternal responsiveness, which promotes cognitive and social–emotional development,Reference Bornstein and Tamis-LeMonda27 has been shown to moderate the association between stressful events in childhood and both physiologic indicators of stress and long-term morbidity.Reference Evans, Kim, Ting, Tesher and Shannis28–Reference Gunnar and Fisher30 High maternal responsiveness has been shown to reduce the longitudinal accumulation of allostatic load, an index of upregulated biologic systems, in response to chronic stressors.Reference Evans, Kim, Ting, Tesher and Shannis28 Among children with high allostatic load, those with responsive mothers have a better working memory, which may suggest a mitigating effect against accumulated stress.Reference Doan and Evans31 Furthermore, recent investigations have demonstrated that children with non-nurturing mothers have shorter telomeres, a sign of biologic wear and tear, than children with nurturing mothers.Reference Knutsen, Filippov and Knutsen32–Reference Asok, Bernard, Rosen, Dozier and Roth34 Among children exposed to adversity, those with high parental responsiveness have longer telomeres than those with low parental responsiveness, again indicating a protective effect against stress.Reference Asok, Bernard, Roth, Rosen and Dozier29 Thus, we believe that the buffering effect of maternal responsiveness may positively program the same biologic pathway as that linking SLEs to infertilityReference Rodriguez-Caro and Williams35 and consider whether early life exposure to maternal responsiveness moderates the association between early life stress and infertility.
The objective of this investigation is to explore whether experiences of SLEs in childhood, adolescence, and early adulthood are associated with self-reported infertility in adulthood and whether maternal responsiveness moderates these associations. Below, we present the first longitudinal study exploring the association between SLEs and infertility among a nationally representative population-based sample from the United States.
Method
This study utilized data from the National Longitudinal Survey of Youth’s 1997 cohort (NLSY-97). The United States Bureau of Labor Statistics employed complex stratified sampling methods to recruit a nationally representative sample of men and women in the same birth cohort.Reference Moore, Pedlow, Krishnamurty and Wolter36 The NLSY-97 cohort was born between 1980 and 1984 and first surveyed in 1997, then interviewed annually or biannually thereafter. In 2011, the last year for which data was used for this current study, 3680 women were interviewed (retention rate = 84%).37,38 The analytic sample included all female participants who had ever tried to get pregnant with complete data on SLEs and reported infertility (n = 1652). The study procedures were considered exempt by the Institutional Review Board at the University of Maryland.
Outcome: infertility
Infertility information was collected during two surveys between 2009 and 2011. The few participants who were unavailable in 2009 were interviewed in 2010 or 2011.Reference Moore, Pedlow, Krishnamurty and Wolter36 First, participants indicated whether they had “ever been part of a couple that had problems getting pregnant or having a baby.” Those who responded they had never tried to have a child were not analyzed further, as they may not be aware of their fertility status.
Infertility was coded dichotomously (1 = yes, 0 = no) based on a definition consistent with the clinical criteria for infertility (regular sexual intercourse over at least 12 months without the use of contraception and without conceiving a child).Reference Zegers-Hochschild, Adamson and Mouzon39 This definition is used to identify infertility internationally by the World Health Organization, as well as in the US by the Centers for Disease Control and Prevention and American Society for Reproductive Medicine.Reference Zegers-Hochschild, Adamson and Mouzon39–41
Exposure: SLEs
In the first survey (1997), participants were asked if, prior to the age of 12, they: ever had their house/apartment broken into; were ever the victim of repeated bullying; and ever saw someone get shot or shot at with a gun. After respondents reached the age of 18, they were asked if any of these same events had occurred between the ages of 12 and 18. Because some respondents had this set of questions repeated in multiple survey years, only one response for each question was counted toward the total number of SLEs (e.g., limiting to one report of house/apartment broken into between the ages of 12 and 18) to prevent counting duplicates. In 2002, respondents were asked about the following additional events, and the age at which they happened: death of a close relative, victim of a violent crime (“for example, physical or sexual assault, robbery, or arson”), homelessness, household member stayed in the hospital for at least a week for treatment of illness or injury, adult member of household incarcerated, household member unemployment, and parents’ divorced. These questions were repeated in 2007, and again in subsequent rounds if the question was not answered in 2007 (i.e., participant was unavailable for interview). Thus, it was possible to ascertain all surveyed SLEs that occurred prior to the age of 23, since respondents would answer these questions for the prior 5 years through the age of 28. In total, respondents could have reported up to three possible SLEs prior to age 12, 10 between the ages of 12 and 18, and seven between the ages of 19 and 23. A total score for the number of SLEs across age groups was initially calculated, and then scores were grouped into categories, based on the distribution (0–1, 2, 3, 4+). Questions used to count SLEs in NLSY-97 were selected from Brugha’s List of Threatening Experiences, a validated inventory of SLEs that has also been used to assess pregnancy outcomes in response to stress.Reference Brugha, Bebbington and Tennanp42–Reference Witt, Cheng and Wisk44
To assess the impact of timing of exposure to SLEs on infertility, three separate SLE dichotomous variables were created based on the age at which the event occurred: any event prior to the age of 12, any event between the ages of 12 and 18, and any event between 19 and 23 (any event = 1, no event = 0). These were coded differently from the main analysis (counts of SLEs described in the preceding paragraph) because the number of SLEs that could have been included was not consistent across age categories.
Potential confounders
We selected confounders using a directed acyclic graph (DAG) which defined true confounders as covariates which were associated with both the potential for experiencing a stressful life event and infertility, but did not lie on the causal pathway between these variables (Supplementary Figure 1).Reference Textor, van der Zander, Gilthorpe, Liśkiewicz and Ellison45 Demographic confounders included race/ethnicity and household income-to-poverty ratio in adolescence. In NLSY-97, participants could identify their race/ethnicity as: Hispanic, non-Hispanic/non-Black, non-Hispanic Black, or multiracial. Household income-to-poverty ratio was calculated by dividing family income, as reported in Round 1 (1997), by the federal poverty level, accounting for household size.
Household-level confounders included biological siblings living in the household and physical environmental risk factors in adolescence. Detailed information about siblings in the home appears in the household roster, and the number of biological siblings was analyzed as a continuous variable.38 The physical environment has been linked to both reproductive health and child development, which may facilitate or contribute to SLEs.Reference Evans46–Reference Collins, Wambach, David and Rankin48 We used a validated index developed in the first survey round (1997),49 based on whether the household usually had electricity or heat when needed in the past month, the condition of buildings on the youth’s street, the condition of the interior of the home (reported by interviewer), whether interviewer felt concerned for their safety in the youth’s neighborhood (reported by interviewer), and the number of days the youth hears gunshots in a typical week. Based on the distribution of scores on the index, physical environment risk was coded into three categories representing the physical conditions and safety of the home and neighborhood (0, 1–2, 3+).
Finally, health and health behavior confounders included an index variable reflecting respondents’ cigarette, alcohol, and marijuana use (i.e., substance use) during rounds 1–4 (1997–2000) and BMI.49 Participants received one point for each substance used each year (0–3), and the score was summed over this 4-year period to produce a total possible score of 0–12. Scores were averaged over the analytic sample, and substance use was coded dichotomously as above or below the mean (>4.4 and ≤4.4). These survey cycles were chosen for assessment of substance use because usage may have occurred early enough in development to affect later reproduction. Use may then have occurred simultaneously with SLEs, but not after, when substance use could then be considered a mediator. BMI was calculated using CDC growth charts.Reference Ogden, Carroll, Kit and Flegal50 Given the U-shaped relationship between BMI and fertility, respondents were classified as underweight, normal weight, overweight, or obese, based on their BMI percentile for age at the time of survey. We also considered inclusion of age at menarche, given its association with stress and reproductive potential, but determined it would likely be a consequence of SLEs and thus would lie on the causal pathway between stress and infertility.
While we selected confounders from time periods that preceded or were concurrent with our exposure, we could not fully differentiate the timing of each confounding variable relative to SLEs within these periods. For example, substance abuse or weight loss or gain are well-recognized sequelae of stressors experienced in early childhood and adolescenceReference Andersen51,Reference Miller and Lumeng52 and thus are likely to mediate the causal pathway between stress and infertility (Supplementary Figure 1). Moreover, BMI measured at any time point would not influence the experience of the SLEs included in this analysis. Therefore, we constructed several models to assess the robustness of our findings after controlling for different sets of potential confounders.
Effect modifier: maternal responsiveness
Maternal responsiveness can be defined as prompt, appropriate responses to everyday exchanges with children.Reference Bornstein and Tamis-LeMonda27 If early life stressors impact infertility, then factors that buffer stress could act as moderators. We examined maternal responsiveness as a potential moderator using an adaptation of the Conger and Elder (1994) Parent–Youth Relationship Scale, which has acceptable psychometric properties and has been used to assess maternal responsiveness in other NLSY-97 studies.Reference Moore, McGroder and Mariner53,Reference Lanza, Huang, Murphy and Hser54 Respondents, assessed during adolescence (1997), provided responses ranging from never (0) to always (4) to the following questions about the mother figure with whom the respondent resided and how often she: praises you for doing well, criticizes you or your ideas, helps you do things that are important to you, blames you for her problems, and makes plans with you and cancels for no good reason. Negative items were reverse coded to allow items to be summed into a composite score ranging from 0 to 20. The final maternal responsiveness variable was dichotomized based on the recommended cut point of 15 (high = 15+, low = less than 15).Reference Moore, McGroder and Mariner53
Analysis
First, the prevalence of infertility among those who reported trying for pregnancy was calculated based on respondents reporting infertility (i.e., trying for > 12 months). Ever infertile and never infertile women were then compared across all variables included in the study using t-tests for continuous variables and Rao-Scott modified chi-squared analyses for categorical variables. All continuous variables were normally distributed allowing for parametric testing. Because NLSY uses complex, stratified sampling, survey weights were custom-made for each dataset generated through NLSY Investigator. Survey commands were used across analyses to account for the complex survey design, though survey weights were only used when generating frequency tables and descriptive statistics, consistent with the analytic guidelines.Reference Moore, Pedlow, Krishnamurty and Wolter36
Next, logistic regression models were fit to examine the association between SLE categories for reported infertility, adjusted for covariates in a stepwise manner: (1) unadjusted; (2) demographic variables only (race/ethnicity and household income-to-poverty ratio); (3) demographic and household/environmental factors (added number of biologic siblings and physical environment risk index); and (4) demographic, environmental, and health/health behavior variables (added substance use and BMI). To test whether maternal responsiveness moderated these associations, separate regression models were fit with interaction terms for each combination of maternal responsiveness multiplied by the number of SLEs. Stratified models were then fit for high vs. low maternal responsiveness. Model 3 was considered the main model for all additional analyses given that Model 4 included potential mediators related to behavioral risk factors, as described previously.
Additionally, to examine differences in infertility based on the timing of SLEs, separate models were fit predicting the risk of reported infertility for SLEs according to their age of occurrence: (1) prior to the age of 12; (2) between ages 12 and 18; and (3) between ages 19 and 23. SLEs were analyzed as a dichotomous variable comparing none vs. any event in each group.
Results
In the analytic sample (n = 1652), the prevalence of clinical infertility was 24.1%. The demographic characteristics of the sample can be viewed by infertility outcome in Table 1. Because the sample is weighted to be nationally representative, unweighted frequency counts may not match weighted percentages, and weighted frequency counts are typically in the millions. Thus, Table 1 provides weighted percentages to maximize interpretability of sample characteristics. Women who reported infertility had significantly higher mean SLEs than other women. When compared to reportedly fertile women, a significantly higher proportion of respondents reported being Hispanic or non-Hispanic Black, having a lower income-to-poverty ratio, fewer biological siblings, a higher number of physical environmental risk factors, higher mean substance use, and higher mean BMI. There was also a higher proportion of women reporting low maternal responsiveness among those reporting infertility.
Table 1. Demographic characteristics of women included in analysis from the National Longitudinal Survey of Youth 1997 cohort, weighted (n = 1652)
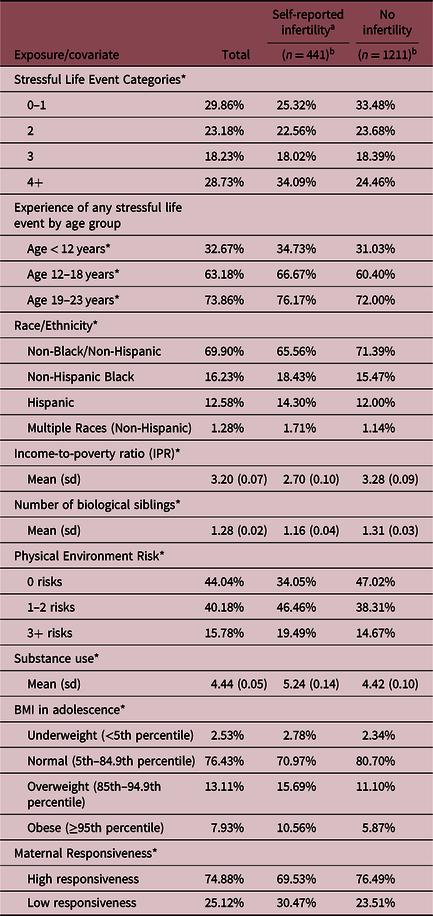
* Indicates significant difference at p < 0.05 in t test or Rao-Scott chi square for infertility vs. not.
a Self-reported infertility defined as failure to achieve pregnancy after 12 months or more of sexual intercourse without contraceptive use.
b Refers to unweighted sample size.
In comparison to respondents reporting 0–1 SLEs, those who experienced 3 or 4+ SLEs had higher odds of reporting infertility across all models (Table 2). For the main model (Model 3), the odds of infertility increased with each SLE, with odds of 1.21 (0.90, 1.64) for 2 events, 1.68 (1.16, 2.42) for 3 events, and 1.88 (1.38, 2.57) for 4+ events. Across each of the models, these elevated odds ranged from 1.30 (CI 1.09, 2.29) for two events in the environmental model (Model 2) to 1.78 (CI 1.19, 2.67) for 4+ events in the fully adjusted model (Model 4). The results were only marginally diluted for Model 4, which adjusted for BMI and substance use, both of which may lie on the causal pathway, though findings remained significant for four or more events.
Table 2. Adjusted odds ratios for the association between stressful life events and infertility, National Longitudinal Survey of Youth 1997 cohort, weighted (n = 1652)

a Model 2 adjusted for race/ethnicity and income to poverty ratio.
b Model 3 is considered the main model and is adjusted for race/ethnicity, income to poverty ratio, number of biological siblings in the household, and physical environment risk.
c Model 4 included only 1525 respondents with complete information on additional covariates, which adjusted for substance use and BMI in addition to covariates in Model 3; Results in bold significant at p < 0.05.
The experience of any stressful life event increased the odds of infertility across all age categories, though the magnitude of the associations did not differ substantially between age groups (Table 3).
Table 3. Adjusted odds ratios for timing of stressful life events (SLEs) and clinical infertility National Longitudinal Survey of Youth 1997 cohort, weighted (n = 1652)

Note: Model adjusted for race/ethnicity, income-to-poverty ratio, number of biological siblings, and physical environment risk.
Results in bold significant at p < 0.05.
After stratification, respondents reporting low maternal responsiveness in adolescence had higher odds of infertility across all categories of SLEs, and odds ratios increased with increasing number of SLEs (Figure 1). Among those reporting high maternal responsiveness, this association was only observed among those reporting four or more SLEs (aOR = 1.53; CI 1.05, 2.25). Results of Model 4, which additionally adjusted for substance abuse and BMI (Supplementary Figure 2), are similar to the results of Model 3, with SLEs predicting infertility only among women reporting low maternal responsiveness. However, given the smaller sample size with more strata across SLEs and responsiveness, the interaction terms are not significant for either Model 3 or Model 4. Nevertheless, the magnitudes of association represent distinct differences in patterns of SLEs and infertility in low compared to high maternal responsiveness groups.

Fig. 1. Adjusted odds ratios with 95% confidence intervals for the association between stressful life events (SLEs) and infertility (clinical), moderated by high or low maternal responsiveness National Longitudinal Survey of Youth 1997 cohort (n = 1652); *Indicates significance at p < 0.05.
Discussion
In this first investigation to include a nationally representative sample, we demonstrated a temporal association between the experience of SLEs in childhood, adolescence, and early adulthood and reports of infertility during adulthood. We found a dose–response association between SLEs and the risk of self-reported infertility, as well as elevated risk that persisted across age categories of SLEs. While this association did not vary across age categories to suggest an effect of event timing, in total, our results highlight the deleterious effect of chronic stressors that persist across age categories and may alter normal reproductive function. We also found evidence that the association between SLEs and infertility is moderated by maternal responsiveness in adolescence. Our finding is in line with evidence that the quality of parental interactions can mitigate or exacerbate the biologic response to stress across the life course,Reference Evans, Kim, Ting, Tesher and Shannis28–Reference Sosnowski, Kliewer, York, Amstadter, Jackson-Cook and Winter33 thus suggesting another window for positive programing to mitigate harms of stressors that could have later life influences, as demonstrated by other studies.Reference Rodriguez-Caro and Williams35
The association between number of SLEs and risk of infertility is supported by evidence that SLEs increase allostatic load, an indicator of biologic “wear and tear” on the body.Reference Seeman, McEwen, Rowe and Singer14 Chronic wear and tear can affect regulation of the stress response and increase inflammation and dysregulation of the immune system to negatively impact health.Reference Chrousos and Gold4 As mentioned previously, stress in childhood and adolescence can alter baseline functioning of the HPA axis, making it over-responsive to stimuli throughout the life course.Reference Elder, Johnson, Crosnoe, Mortimer and Shanahan15,Reference Flinn, Nepomnaschy, Muehlenbein and Ponzi16 Thus, in the face of repeated stressors in childhood, adolescence, and early adulthood, there may be a cumulative effect of HPA perturbation and dysfunction that negatively impacts reproduction. As a result, while most women will have no difficulty conceiving, given the same environmental and genetic circumstances, some women may experience infertility as a result of chronic stressors and an overactive stress response.
It has been suggested that, because the HPA system does not discriminate against types of stressors, all stress, at any point in the life span could be detrimental for fertility.Reference Boivin, Sanders and Schmidt55 Generally, we found that only the experience of multiple stressful events influenced infertility, but our sample was relatively young (27 years at final survey), so it is possible that the effect of stressors could amplify or otherwise change as the individual ages. In terms of timing, we did not observe a differential impact of SLEs across age categories, though it was difficult to isolate a critical period because 97% of respondents who reported an SLE prior to the age of 12 also reported an SLE between ages 13 and 22 (72% concordance at age 12–18; 70% at age 19–22; data not shown). This is a challenge consistent with exposure assessment in life course studies, where early life adversity is often highly correlated with later adversity.Reference Shenassa56
Our findings are consistent with two of only three other studies conducted to date to empirically test the link between lifetime stressors and indicators of infertility.Reference Santos, Sobral and Martins11–Reference Jacobs, Boynton-Jarrett and Harville13 Santos and colleagues found no difference in the experience of lifetime stressors between cases of infertility and controls among a clinic-based sample (n = 376).Reference Santos, Sobral and Martins11 However, the sample included both men and women, and cases were identified from medical records, which limits generalizability given issues related to access and infertility diagnosis and treatment (i.e., one may not seek a diagnosis if they do not have the means to access treatment, which may also be related to a higher likelihood of experiencing early life stress). For example, the National Survey of Family Growth indicates that women who access fertility services are more likely to be non-Hispanic White than other ethnicities and have high education and income levels.Reference Chandra, Copen and Stephen57 In addition, controls had unknown fertility status, as they self-reported fertility, but had no children, suggesting they may have never tried to get pregnant. Another study of women recruited from a fertility clinic (n = 89) reported a family history of abuse was associated with diminished ovarian reserve.Reference Pal, Bevilacqua and Santoro12 Finally, among a cross-sectional sample of women recruited from OB/GYN and WIC clinics (n = 742), respondent reports of four or more adverse childhood experiences were associated with longer time to pregnancy and other fertility difficulties.Reference Jacobs, Boynton-Jarrett and Harville13 This is the only study of lifetime experiences of stress and infertility among a sample recruited outside of a fertility or gynecologic clinic and would be most comparable to ours.
One strength of the current study is that, unlike previous studies, data were collected longitudinally. Second, respondents were surveyed about SLEs within 5 years of their occurrence, which reduces the likelihood of recall bias associated with differential recall of SLEs by infertile compared to fertile women. Third, the NLSY sample is nationally representative of the US, which makes our findings more generalizable than prior studies examining similar relationships. Fourth, we used a broad definition of infertility to capture women who may not have sought such a diagnosis or treatment, which is estimated to be as high as 50% among women with fertility difficulties.Reference Chandra, Copen and Stephen57 Moreover, this definition is better aligned with the definition of infertility used in prospective time-to-pregnancy studies, which are the gold standard for assessing risk factors associated with conception delay as they do not exclude couples who may never become pregnant or never seek treatment.Reference Joffe, Key, Best, Keiding, Scheike and Jensen58,Reference Slama, Ducot, Keiding and Bouyer59
When considering all reproductive-aged women in our sample, i.e., including women who reported never trying to get pregnant, the prevalence of infertility is 10.9% (data not shown). The higher prevalence of infertility observed in our eligible sample of women reporting they had ever tried to get pregnant may be reflective of our inclusive definition, which would encompass the “hidden infertile” who are not typically included in infertility research.Reference Greil, Slauson-Blevins and McQuillan60 Though higher than some US estimates (24% vs. 16%), the prevalence of infertility in our eligible sample was similar to estimates from population-based studies conducted in France and the US that did not limit respondents to those trying to conceive, with prevalence estimates ranging from 24% to 35% over 12 months.Reference Slama, Hansen and Ducot61–Reference Jacobson, Chin, Mertens, Spencer, Fothergill and Howards63 Thus, the prevalence in our study could indicate some lack of precision in assessing pregnancy intent but includes women who may still experience an inability to conceive despite being at risk of pregnancy, which may indicate underlying pathology.
Our study should also be viewed in light of its limitations. First, our list of SLEs was not exhaustive and may be missing experiences that might influence later health. This limited number of SLEs may have attenuated our results. Second, as a survey reliant on respondent reports, it is likely that there are inaccuracies in recalling length of time trying to conceive, though the window of recall is shorter given that the questions were asked when respondents were in their 20s. Recall has been shown to be relatively reliable, with higher reliability for interviews and shorter recall windows,Reference Cooney, Louis, Sundaram, McGuiness and Lynch64 and inaccuracies in recall were likely random and not differential by fertility status, biasing our results toward the null. Additionally, we do not have information on the male partner’s contribution to infertility among women in this sample, but population-based data generally lack cause-specific information, which relies on individuals seeking and receiving a diagnosis.40 Focusing on a subpopulation of individuals with this information would limit the generalizability of findings to those who can regularly interact with the health care system to receive a diagnosis. Consistent with other time-to-pregnancy studies and population-based infertility studies,Reference Lynch, Sundaram, Maisog, Sweeney and Buck Louis2,Reference Buck Louis, Lum and Sundaram3,Reference Lynch, Sundaram, Buck Louis, Lum and Pyper6,Reference Slama, Hansen and Ducot61 we lacked information on male risk factors and diagnosed causes of infertility. More precise information on the cause of infertility (female factor or unexplained) may have further strengthened the magnitude of our associations; however, it would exclude over half of all infertile couples. Thus, our findings would likely be an underestimate of the true effect. Additionally, the wording of the question associated with infertility may not account for time spent away from the partner or other events that may have limited intimacy in any given month during the 12 or more months respondents reported trying to conceive. Although we only considered the moderating effect of maternal responsiveness, we expect other familial interactions could also modify the association between stress and infertility. Paternal factors may moderate our observed associations, either independently or in conjunction with maternal responsiveness. Future studies should assess the validity of cut points for assessing paternal responsiveness to further explore these associations.
Our findings suggest that stressful events that accumulate in early life may influence reproductive health. Given that adverse reproductive function may act as an early marker for intervention to improve later life health outcomes, this study highlights a potential shared pathway between infertility and other health outcomes and indicates that certain types of interventions to prevent infertility may have more of an impact if delivered prior to adulthood. More longitudinal research should be conducted to further elucidate these pathways across the life course to better understand the holistic impact of early life stressors on reproductive health. Future research should explore whether different types of SLEs have a differential impact on infertility. Considering that maternal responsiveness may mitigate some of the physiologic effects of stress,Reference Asok, Bernard, Roth, Rosen and Dozier29,Reference Doan and Evans31 interventions that support families and provide resources to mitigate stress and stressors, while also teaching skills to adolescents and young adults to cope with stress, may mitigate later life health implications of SLEs.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174420000690
Acknowledgments
The authors would like to thank Drs. Sandra Quinn and Robert Gold for their careful review of the manuscript.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.







