Introduction
Disturbed sleep is a known risk factor for metabolic dysregulation but in pregnancy, it remains under-recognized and poorly understood. Altered sleep patterns due to normal physiologic changes in pregnancy are common, but can be debilitating. They may pose significant risk to both the mother and developing fetus, particularly in obese women with commonly co-occurring risk factors. Disturbed sleep is implicated in a multitude of serious maternal/fetal complications that are exacerbated in obesity. These include gestational hypertension and preeclampsia, Reference Ayrim, Keskin, Ozol, Onaran, Yiidirim and Kafali1–Reference Chen, Kang, Lin, Wang, Keller and Lin9 gestational diabetes mellitus, Reference Bisson, Series and Giguere10,Reference Facco, Grobman, Kramer, Ho and Zee11 preterm birth, and unplanned cesarean delivery.Reference Louis, Auckley, Sokol and Mercer12,Reference O’Brien, Bullough and Owusu13 Sleep-disordered breathing (SDB) is one of the most common sleep disorders and impacts up to 32% of pregnancies. Reference Louis, Koch and Reddy14
By definition, SDB is a continuum that ranges from mild inspiratory flow limitation to obstructive sleep apnea (OSA) (see Dempsey et al. [2010]). Reference Dempsey, Veasey, Morgan and O’Donnell15 Mild SDB is characterized by snoring from inspiratory flow limitation. Reference Stoohs, Gold, Kryger, Roth and Dement16 OSA is the most severe form of SDB in which partial or complete collapse of the upper airway results in decreased airflow (hypopnea) or cessation of breathing (apnea) and frequent arousals. Reference Greenberg, Lakticòvá, Scharf, Kryger, Roth and Dement17 Loud snoring and somnolence (excessive daytime sleepiness) are common features of OSA. Apneas and hypopneas result in sleep fragmentation (SF) to restore airway patency. While the development of SDB is likely multifactorial, one of its strongest predictors is obesity Reference Epstein, Kristo and Strollo18 ; increasing fat in airway muscles and neck fat pads Reference Hernandez, Ballard and Weil19 make collapse of the upper airway more likely. Investigators have consistently reported that frequency of self-reported snoring increases through pregnancy. Reference O’Brien, Bullough and Owusu3,Reference Pien, Fife, Pack, Nkwuo and Schwab20 This is likely due to the anatomical and physiological changes associated with pregnancy, such as increased blood volume, gestational weight gain, and increasing abdominal pressure due to the enlarged uterus. Reference Izci Balserak21
Obesity is a state of insulin resistance (IR) in most individuals. Reference Defronzo22,Reference Ye23 Pregnancy itself produces an IR state as a normal adaptation to ensure an adequate supply of maternal fuels to the meet the metabolic demands of the placenta and growing fetus. IR in pregnancy is largely a result of placental hormones such as human placental growth hormone, human placental lactogen, increased levels of Tumor Necrosis Factor-alpha, and decrease in adiponectin. Reference Barbour, Farabi and Friedman24,Reference Barbour, McCurdy, Hernandez, Kirwan, Catalano and Friedman25 With increasing fetal growth, IR is maximal in the late 2nd and 3rd trimesters when there is an approximate 50% decrease in insulin-mediated glucose disposal and a 200%–300% increase in insulin secretion in response to glucose. Reference Catalano, Huston, Amini and Kalhan26,Reference Barbour, Friedman, Hernandez, Reece and Coustan27 It has been demonstrated that women with obesity enter pregnancy with heightened IR, upon which the effects of pregnancy are additive. Reference Catalano, Huston, Amini and Kalhan26–Reference Hernandez, Friedman, Barbour, Zeitler and Nadeau29
Pregnancy IR may be exacerbated by worsening SDB. Although the association between IR and SDB is likely bidirectional, Reference Aurora and Punjabi30 there is evidence suggesting that sleep disruption similar to that experienced in severe SDB precedes altered insulin sensitivity and glucose metabolism, suggesting causality. Reference Fung, Wilson and Lappas31–Reference Stamatakis and Punjabi34 The exact mechanisms underlying this relationship are not understood, but several hypotheses have been proposed. SF and intermittent hypoxia (IH) may increase IR through over-activation the sympathetic nervous system (SNS), Reference Collins and Surwit35–Reference Kunos, Ishac, Gao and Jiang37 activation of the hypothalamic-pituitary-adrenal axis with increased cortisol release, Reference Dunford and Riddell38 or increased inflammation and reactive oxidative stress. Reference Briancon-Marjollet, Weiszenstein, Henri, Thomas, Godin-Ribuot and Polak39,Reference Tasali and Ip40 Interestingly, IH, increased inflammation, and increased oxidative stress, particularly early in pregnancy, can also impair placental function and blood flow. Reference Challis, Lockwood, Myatt, Norman, Strauss and Petraglia41 Thus, it is possible that the duration and severity of SDB results in opposite growth outcomes to the fetus. Mild SDB that worsens over pregnancy may exacerbate pregnancy IR, resulting in nutrient over-exposure and offspring large-for-gestational age (LGA). In contrast, preexisting chronic OSA may increase risk of endothelial dysfunction and hypertensive disorders in pregnancy, result in abnormal placentation with reduced blood flow, and lead to earlier delivery and offspring small-for-gestational age (SGA). Both LGA and SGA are risk factors for childhood metabolic disease. Fig. 1 highlights the potential mechanisms by which SDB may exacerbate both IR and changes at the placenta.

Fig. 1. Sleep-disordered breathing in pregnancies affected by obesity: two offspring phenotypes. Pregnancy is a state of increased insulin resistance (IR) and increasing weight which exacerbates both SDB symptoms and preexisting IR of obesity. Preexisting IR is understood to be multifactorial and known mechanisms are listed. SDB involves intermittent hypoxia, sleep fragmentation, or a combination, which activate pathways that further increase IR. Severe SDB, manifested as OSA, may result in extreme sleep fragmentation and intermittent hypoxia that alters placental development. In extreme cases, altered placental functioning may lead to small-for-gestational age (SGA) infants. However, exacerbation of IR due to worsening SDB also may result in excessive fetal-placental nutrient exposure, leading to large-for-gestational age (LGA) infants. Alternatively, exposure to SDB and increased IR may result in newborns born with normal birthweight but increased adiposity or subclinical alterations in metabolism. Developmental Origins Theory posits that risk of future disease is along a continuum with both extremes (both SGA and LGA) leading to increased risk of future disease. AA, amino acids; BW, birthweight; FFA, free fatty acids; IR, insulin resistance; OSA, obstructive sleep apnea; ROS, reactive oxygen species; SDB, sleep disordered breathing; SNS, sympathetic nervous system; GLP-1, glucagon-like peptide-1; HGP, hepatic glucose production; AT, adipose tissue; SM, skeletal muscles; HPA, hypothalamic pituitary adrenal axis.
Both animal and human models support that in utero development is a time of tremendous plasticity during which the fetus is exquisitely sensitive to exposures within the intrauterine environment. In accordance with the Developmental Origins of Health and Disease (DOHaD) framework, in utero plasticity allows for the creation of environmentally matched phenotypes that are best suited to promote survival under altered conditions, such as under- or overnutrition, or IH. Reference Hanson and Gluckman42 These phenotypes, however, are vulnerable to dysregulation with reversal of those conditions postnatally (i.e., an environmental mismatch). After birth, a background of preset functional capacity and gradual loss of plasticity, in combination with an obesity-promoting postnatal environment, is thought to set the stage for offspring obesity and chronic disease risk. Reference Hanson and Gluckman42,Reference Barker and Bagby43 During pregnancy, maternal factors such as nutrient intake, metabolic disturbances, and hypoxia shape fetal environmental conditions; even normal variation in these exposures is now appreciated to have long-term offspring consequences. Sleep may influence these maternal factors, affecting fetal development and growth. Birth weight (BW) and body composition, specifically increased fat mass at birth, are strong predictors of future metabolic disease. Reference Catalano, Farrell and Thomas44 Emerging data continue to demonstrate that low and high BWs predict higher odds for overweight/obesity in school-aged children (OR 1.91–2.34), Reference Kapral, Miller, Scharf, Gurka and DeBoer45 and fat mass at birth is a stronger predictor of childhood obesity risk than BW. Reference Catalano, Farrell and Thomas44
Childhood obesity in the United States affects nearly one in five preschool children, suggesting a role for early environmental factors. Reference Ogden, Carroll and Lawman46 Because the risk for childhood obesity appears to be propagated by a number of in utero environmental conditions, and SDB is common, a better understanding of its potential impact on fetal outcomes might inform interventions that optimize the intrauterine environment, mitigating chronic disease risk beginning during fetal development. Accordingly, the aim of this paper is to systematically review and link data from both mechanistic rodent models and descriptive human studies to characterize the impact of maternal SDB on fetal development. While the data suggest that SDB in pregnancy may be an exposure to the developing fetus, we emphasize that controlled, appropriately designed, and prospective data are required to provide stronger support for this relationship. Significant confounders in human investigations, potential reasons for discrepant findings, and suggested areas for future research are also discussed.
Methods
PubMed, Embase, and CINAHL were searched using terms that included: “sleep disordered breathing” OR “obstructive sleep apnea” OR “snoring” OR “sleep fragmentation” OR “intermittent hypoxia” AND “pregnancy” OR “placenta” OR “fetus” OR “infant” OR “birthweight”. Original studies were included if: (1) full text was available; (2) written in English; (3) publication was between 01/2000 and 09/2019; and (4) infant outcomes were reported (e.g. BW, intrauterine growth restriction [IUGR], small or large for gestational age [SGA, LGA, respectively]) in relation to SDB. Animal studies were included if the disruptions common to SDB (SF or IH during the sleep period) were used in pregnant animals and metabolic outcomes of the offspring were assessed. Notably, only studies where IH, not chronic hypoxia, were included because in the human condition, SDB is limited to IH during the sleep period. PRISMA guidelines were followed for the review. Quality of studies was reviewed using the NIH tool for observational studies and NIH tool for case–control studies 47 in humans and the SYRCLE tool Reference Hooijmans, Rovers, de Vries, Leenaars, Ritskes-Hoitinga and Langendam48 for animal studies. For human studies, control for the major confounders of maternal age, gestational age, BMI, gestational hypertension, diabetes, and smoking was assessed in each study.
Results
Fig. 2 shows the flow of study selection. Initially, a total of 1374 abstracts were retrieved from the 3 databases. After careful review, 57 articles (48 in humans and 9 in rodents) in which assessment of maternal SDB during pregnancy with fetal, placental or infant birth-weight outcomes were included. Additionally, five meta analyses met criteria for inclusion and were reviewed. All studies in humans were descriptive or case–control studies. Table 1 provides information on studies reviewed in rodents, and Table 2 provides information on studies reviewed in humans. The quality review of human studies is summarized in Table 3. Supplementary Table S1 provides quality ratings of the animal studies.
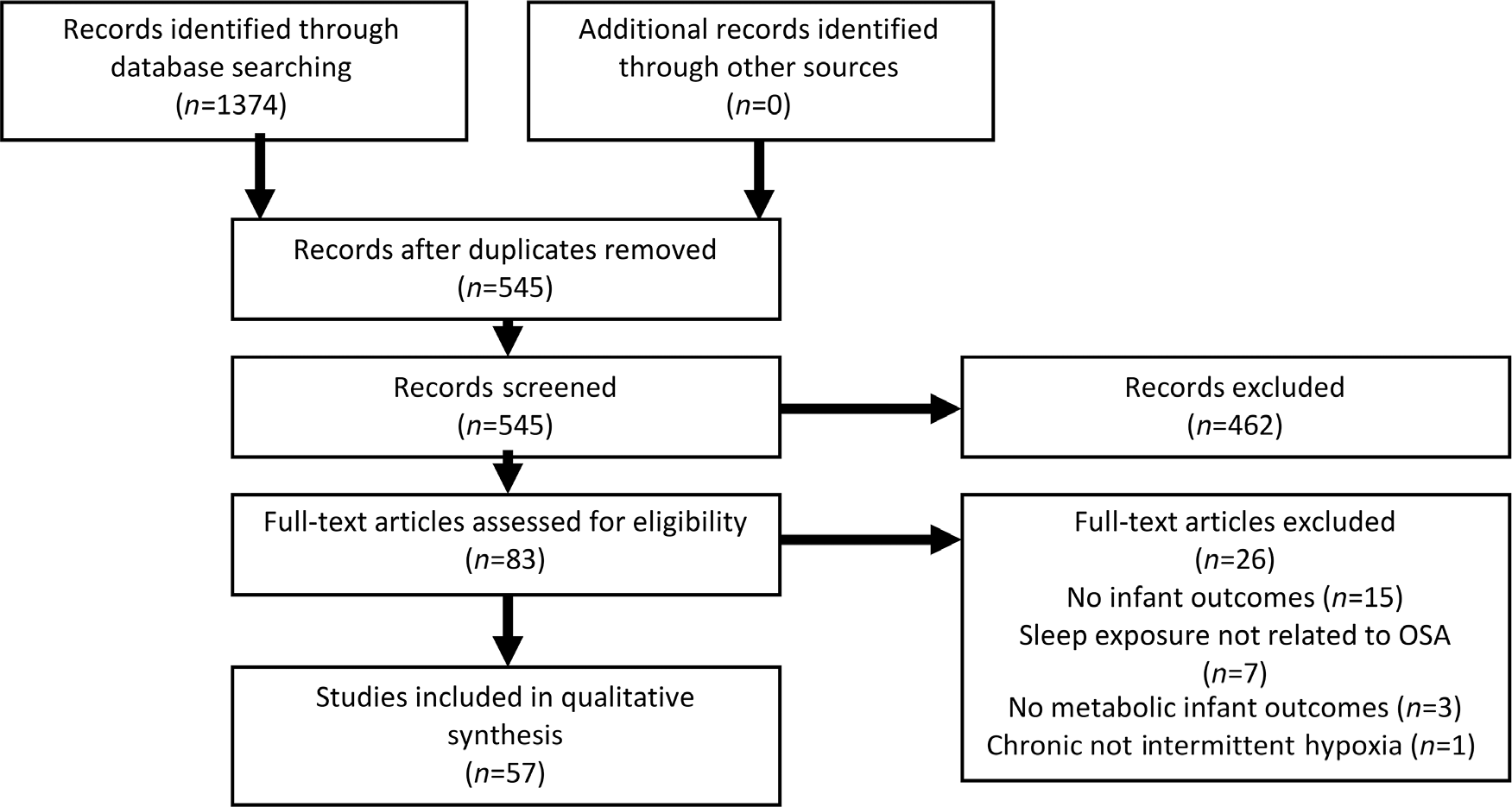
Fig. 2. Prisma flow diagram for selection of articles.
Table 1. Overview of studies conducted in rodents

Table 2. Overview of studies conducted in humans in which the association between SDB and infant growth outcomes was examined

Negative finding (−); Positive finding (+); Trend (±); IUGR, intrauterine growth restriction; LBW, low birth weight; LGA, large for gestational age; SGA, small for gestational age.
Table 3. Quality review of human studies included in systematic review

Studies were reviewed using the NIH tools for observational or case–control studies.
Rodent models of gestational SDB show metabolic aberrancies in offspring growth and development
Rodent models (mice and rats) of the effects of gestational SDB allow for mechanistic investigation of the independent effects of SF and IH as separate exposures to the developing offspring. In the human SDB condition, it is not possible to isolate the effects of IH vs SF due to co-occurrence and interacting effects. While all of the studies in rodents were randomized control trials, most of the reports lack description of the key bias indicators such as similarity of groups at baseline, sequence generation and random housing (Table S1).
Intermittent hypoxia: Investigators reported either no difference in BW, Reference Khalyfa, Cortese and Qiao49,Reference McDonald, Dempsey and O’Halloran50 significantly lower BW Reference Gozal, Reeves, Row, Neville, Guo and Lipton51 or growth restriction Reference Iqbal and Ciriello52 in offspring exposed to gestational IH during pregnancy compared to control animals. In two studies, IH-exposed offspring had lower BW but rapid catch-up growth, Reference Gozal, Reeves, Row, Neville, Guo and Lipton51,Reference Iqbal and Ciriello52 which has been shown to be related to increased risk of childhood obesity in humans. Reference Ong, Ahmed, Emmett, Preece and Dunger53 At 6–12 weeks of life, male offspring exposed to gestational IH had greater fat mass compared to controls. Reference Iqbal and Ciriello52 Khalyfa et al. found that male, but not female, offspring exposed late gestational IH (days 13–18 of gestation) had higher body weight and visceral adipose tissue at 24 weeks compared to controls. Reference Khalyfa, Cortese and Qiao49 HOMA-IR and plasma leptin were higher, and plasma adiponectin was lower in these male offspring exposed to maternal IH compared to controls at 24 weeks, Reference Khalyfa, Cortese and Qiao49 suggesting that there may be sex differences in exposure outcomes. The investigators further reported upregulation of gene methylation pathways associated with energy production in adipose tissue of IH-exposed offspring, suggesting a potential mechanism by which IH-exposure may result in increased risk of obesity. Reference Khalyfa, Cortese and Qiao49
Sleep fragmentation. Exposure to SF in utero may have deleterious effects on offspring metabolism which do not manifest until later in life. In mouse offspring exposed to gestational SF, BW was not different than controls. Reference Cortese, Khalyfa, Bao, Andrade and Gozal54–Reference Khalyfa, Mutskov, Carreras, Khalyfa, Hakim and Gozal56 However, as the mice entered adulthood (16–18 weeks of life), offspring of SF-exposed mothers had significantly higher mean body weight, fat mass, blood glucose, and higher fasting total triglyceride and cholesterol concentrations than controls. Reference Mutskov, Khalyfa, Wang, Carreras, Nobrega and Gozal55,Reference Khalyfa, Mutskov, Carreras, Khalyfa, Hakim and Gozal56 The negative metabolic effects of gestational SF on offspring were more pronounced in males, once again, supporting sex differences. Reference Khalyfa, Carreras, Almendros, Hakim and Gozal57 Similar to IH, changes in gene methylation patterns for PPARα activation (critical for fatty acid oxidation, ketogenesis, Reference Sander58 and hepatic gluconeogenesis) were seen in male offspring exposed to SF compared to controls. Reference Khalyfa, Carreras, Almendros, Hakim and Gozal57 In a separate study, offspring exposed to gestational SF had epigenetic changes resulting in overexpression of the FoxO1 gene, a transcription factor that plays an important role in gluconeogenesis and glycogenolysis. Reference Mutskov, Khalyfa, Wang, Carreras, Nobrega and Gozal55 Taken together, these findings suggest that SF exposure, especially in males, changes the offspring’s ability to regulate glucose and lipid metabolism, increasing risk of future metabolic disease. Alterations in offspring metabolism may, in part, be due to an integrated stress response involving cortisol and sympathetic activation. Reference Trzepizur, Khalyfa, Qiao, Popko and Gozal59 Offspring of double mutant mice of integrated stress response genes CHP and GADD34 (unable to mount a stress response) exposed to SF did not have metabolic perturbations as seen in normal mice offspring exposed to SF. Reference Trzepizur, Khalyfa, Qiao, Popko and Gozal59 Importantly, postnatal physical activity implemented early in SF-exposed offspring reduced FoxO1 acetylation and methylation to levels similar to controls. Reference Mutskov, Khalyfa, Wang, Carreras, Nobrega and Gozal55
The evidence from rodent models is compelling; however, it must be underscored that unlike humans, mice and rodents are born with very little fat and size at birth is in part due to the size of the litter and driven more by lean body mass. In summary, evidence from these rodent models suggests distinct mechanisms whereby maternal IH results in normal or low BW and subsequent accelerated postnatal growth, and maternal SF results in normal BW followed by later metabolic derangement.
Human studies: strong link between SDB and birthweight outcomes
Over 30 published reports described a strong association between maternal SDB and birth outcomes in humans, supporting that mechanisms and outcomes in rodent models may have translational relevance. In accordance with the DOHaD model, infants born with IUGR/SGA or LGA are both at risk for future metabolic diseases such as obesity and T2D. Reference Hales and Barker60 Notably, while the purpose and study population were clearly defined in most reports, most lacked power for infant outcomes, made a single assessment of SDB during pregnancy (instead of multiple measures over gestation), did not measure severity of SDB, and body composition at birth was rarely measured (Table 3). In 32 of the 48 studies, investigators attempted to at least partially control for important confounding variables: maternal age, gestational age, BMI, smoking, and preexisting hypertension, which could highly influence growth outcomes (Table 3). It is also notable that all humans studies are descriptive in nature, so cause and effect cannot be determined. Further, even after attempting to control for known confounders, there may still be unmeasureable or unknown variables which can influence study outcomes.
IUGR/SGA. 27 studies were reviewed in which SGA or IUGR were reported as outcomes, three of which were meta-analyses. Ding et al. reported a significant association between SDB and IUGR across 8923 women in 11 studies (OR = 1.44 [95% CI: 1.22–1.71]). Reference Ding, Wu and Xu61 Similarly, Warland et al. reported by meta-analysis that SGA was significantly associated with SDB across 9478 women (13 studies) in which SDB was measured subjectively (adjusted OR = 1.6 [1.1–2.2]) and across 56,423,715 women (7 studies) which included objective measures (adjusted OR = 1.4 [1.1–1.9]). Reference Warland, Dorrian, Morrison and O’Brien62 In contrast, Brown et al. found no significant association between SDB and SGA by meta-analysis of 21 studies (adjusted OR 1.19, [0.94–1.51]). Reference Brown, Turner and Kumar63 Findings were mixed across the 24 original (non-meta-analysis) studies reviewed for this manuscript. While investigators reported an association between SDB and growth restriction in nine studies, Reference Franklin, Holmgren, Jonsson, Poromaa, Stenlund and Svanborg7,Reference Chen, Kang, Lin, Wang, Keller and Lin9,Reference O’Brien, Bullough and Owusu13,Reference Fung, Wilson and Lappas31,Reference Micheli, Komninos and Bagkeris64–Reference Miyagawa, Emori, Kawano, Sakurai and Tanigawa68 no significant association was detected across 15 studies. Reference Bourjeily, Raker, Chalhoub and Miller4,Reference Louis, Auckley, Sokol and Mercer12,Reference Facco, Ouyang and Zee69–Reference Okun and O’Brien81 Mixed findings may be due to lack of consistent assessment of SDB, the severity of the SDB and provocation of IH, and a lack of adequate power (Table 3). Timing of maternal SDB assessment varied widely across studies with the majority of subjective SDB assessment done at time of delivery. Type of SDB assessment was also highly varied; the majority of studies included self-reported symptoms of snoring, which does not reflect severity of SDB. Of the 19 studies, only five Reference O’Brien, Bullough and Owusu13,Reference Fung, Wilson and Lappas31,Reference Pamidi, Marc and Simoneau65,Reference Olivarez, Ferres and Antony71,Reference Yin, Williams and Burton78 were powered to detect an association between SDB and SGA as the primary outcome; a positive association was reported in three of the studies and no association was reported in two (Table 2). In the majority of the studies (n = 20), investigators controlled (at least in part) for important maternal variables (BMI, hypertension, diabetes, smoking) that could influence infant growth outcomes (Table 3). Studies in which severity of OSA is measured objectively throughout gestation (pre-pregnancy through 3rd trimester) in relation to SGA are lacking. In summary, the results from descriptive studies in humans are mixed in support of an association between SDB and SGA which may be due to a lack of consistency of measurement of SDB, a lack of power or other confounding variables that were not measured.
BW: Results from three meta-analyses supported a relationship between SDB and low BW with similar ORs (OR = 1.75 [95% CI: 1.33–2.32], Reference Ding, Wu and Xu61 1.67 [1.0–2.78], Reference Brown, Turner and Kumar63 1.39 [1.14–1.65] Reference Pamidi, Pinto, Marc, Benedetti, Schwartzman and Kimoff82 ), while Li et al. concluded that there was no association between SDB and BW in a meta-analysis across 8749 women (15 studies). Reference Li, Zhao, Hua and Li83 OSA diagnosis (documented by ICD-9) was associated with an increased risk of low BW (<2500 g; OR = 1.76 [1.28–2.40], n = 4746). Reference Chen, Kang, Lin, Wang, Keller and Lin9 Further, self-reported frequent snoring in the 3rd trimester was associated with low BW (RR = 2.6 [1.2–5.4]); however, after adjustment for pre-maturity, snoring was no longer associated with low BW. Reference Micheli, Komninos and Bagkeris64 SDB was not associated with increased risk of low BW in three smaller studies, Reference Owusu, Anderson and Coleman84–Reference Sharma, Nehra and Sinha86 none of which were powered for BW as the outcome. Louis et al. reported that compared to women with obesity alone, women with OSA had infants with significantly lower BW (3288 ± 590 vs. 3013 ± 968 g). Reference Louis, Auckley, Sokol and Mercer12 Diagnosis of OSA was made either before or during pregnancy and continuous positive airway pressure use/adherence was not reported, important because treatment of OSA could have affected BW in the OSA group. In three studies where BW was compared between women with OSA and those without, one group of investigators reported higher BW in women with OSA Reference Olivarez, Ferres and Antony71 and two reported no significant difference. Reference Sahin, Koken and Cosar74,Reference Louis, Auckley and Miladinovic87 Higgins et al. reported significantly higher BW in infants of women with a positive Berlin Score (high risk of OSA), Reference Higgins, Leong, Park, Facco, McCarthy and Wong88 but Ugur et al. reported slightly lower BW in infants of women with a positive Berlin score. Reference Ugur, Boynukalin, Atak, Ustuner, Atakan and Baykal89 Discrepant findings may be due to multiple factors. There was inconsistent timing of SDB measurement throughout the studies from pre-pregnancy to delivery, severity was not consistently assessed, and the degree of IH was usually unreported. BW was used differently across studies; five of the studies used low BW (<2500 g) as a categorical variable Reference Chen, Kang, Lin, Wang, Keller and Lin9,Reference Micheli, Komninos and Bagkeris64,Reference Owusu, Anderson and Coleman84–Reference Sharma, Nehra and Sinha86 (requiring a larger sample to detect an association), while the other 10 studies used BW as a continuous variable. Reference Ayrim, Keskin, Ozol, Onaran, Yiidirim and Kafali1,Reference Olivarez, Ferres and Antony71,Reference Sahin, Koken and Cosar74,Reference Tauman, Sivan, Katsav, Greenfeld and Many77,Reference Louis, Auckley and Miladinovic87,Reference Bassan, Uliel-Sibony, Katsav, Farber and Tauman90–Reference Leung, Hui, Leung, Yuen and Lau92 Further, none of the studies were powered on the BW outcome and body composition was not assessed. Importantly, low BW is different from IUGR as it is not corrected for gestational age (i.e., a preterm infant is expected to have a lower BW but may or may not have growth restriction). In seven of the studies found, investigators made an effort to at least partially adjust for confounding factors, while in nine of the studies, there was no control for confounding factors (Table 3). In summary, overall results from studies support an association between SDB and low BW, but, in over half of the studies, potential confounding variables were not controlled thus limiting the strength of evidence to support this association.
LGA: One meta-analysis supported an association between SDB and LGA across 678,310 women (7 studies) (OR = 1.6 [95% CI: 1.3–1.9]), Reference Warland, Dorrian, Morrison and O’Brien62 while the additional four meta-analyses did not include LGA as an outcome. In eight studies, investigators reported an association between SDB and LGA. Reference O’Brien, Bullough and Owusu13,Reference Facco, Ouyang and Zee69,Reference Telerant, Dunietz, Many and Tauman79–Reference Okun and O’Brien81,Reference Bin, Cistulli and Ford93,Reference Howe, Signal and Paine94 Seven groups found a significant association between SDB symptoms and LGA (BW > 90th percentile) and one group reported a significant association between SDB and macrosomia (defined as BW > 97th percentile). Maternal diagnosis of OSA in the year before or during pregnancy (across 636,227 women) was significantly associated with increased risk of LGA in a large, retrospective cohort (adjusted OR = 1.27 [1.04–1.55], p < 0.05), after adjustment for obesity. Reference Bin, Cistulli and Ford93 Similarly, women who self-reported apnea had increased frequency of LGA compared to women who denied apnea (22.4% vs. 12.8%, p = 0.049). Reference O’Brien, Bullough and Owusu13 Maternal report of pregnancy-onset breathing pauses during sleep was associated with LGA (OR = 3.5 [95% CI: 1.3–9.6], p = 0.01). Reference Howe, Signal and Paine94 OSA+ women (identified via ICD-9 codes) had higher rates of macrosomia (BW > 97th percentile), but this group had a significantly higher rate of maternal diabetes and higher body mass index (BMI) which could also explain the macrosomia rate. Reference Ravishankar, Bourjeily, Lambert-Messerlian, He, De Paepe and Gundogan73 Telerent et al. reported that the occurrence of LGA was significantly more frequent in infants born to mothers who had mild sleep apnea compared to those who did not. Reference Telerant, Dunietz, Many and Tauman79 The investigators studied women in their 3rd trimester and excluded women with more severe sleep apnea (apnea hypopnea index [AHI] > 15), which might be expected to result in placental insufficiency and attenuated growth. Reference Telerant, Dunietz, Many and Tauman79 Facco and colleagues, on the other hand, did not find an association between OSA (diagnosed using in-home objective measurement) and LGA (19.4% LGA in no SDB group vs. 12.5% in Moderate/Severe SDB group). Reference Facco, Ouyang and Zee69 The lack of significant finding for this study may be due to the fact that it was designed to look at maternal and not fetal outcomes and a higher cutoff for definition of LGA (>95th percentile). Across studies, there are limited data on the classes of obesity, and no measures of newborn body composition. We recently completed a study in which we examined the relationship between severity of maternal OSA and infant % body fat in maternal obesity (BMI 30–40). There was no correlation between severity of OSA (by AHI) and infant % body fat (r = 0.34, p > 0.05) Reference Farabi, Barbour, Heiss, Hirsch, Dunn and Hernandez95 ; however, overnight minimum oxygen saturation was correlated with infant % body fat, suggesting that increasing severity of maternal OSA (with greater reduction oxygen saturation) was related to higher newborn fat (r = −0.63, p = 0.02). Reference Farabi, Barbour, Heiss, Hirsch, Dunn and Hernandez95 Importantly, none of the studies were powered on LGA, the assessment of SDB symptoms were variable and often did not include the degree of hypoxia, and the timing of assessment across studies was inconsistent. However, in seven of the eight studies, investigators attempted to control for confounding factors (Table 3). The available evidence supports an association between more severe maternal SDB and fetal overgrowth even after controlling for maternal BMI (in most studies).
Clinical studies investigating potential mechanisms
Some investigators studied the relationship between SDB in pregnancy and biomarkers that may indicate potential mechanisms for the link between SDB in pregnancy and fetal outcomes.
SDB and fetal stress. Maternal apneas were accompanied by fetal heart rate decelerations in three of four women with OSA, Reference Sahin, Koken and Cosar74 suggesting increased fetal stress with maternal apnea. In contrast, Olivarez and colleagues found no association between degree of oxygen saturation and fetal heart rate in 20 women with OSA (severity of OSA not reported). Reference Olivarez, Maheshwari and McCarthy96
SDB, inflammation and oxidative stress. Koken and colleagues Reference Koken, Sahin and Cosar97 reported that markers of oxidative stress (maldondialdehyde [MDA], Myeloperoxidase [MPO]) were higher and an anti-oxidant marker (Glutathione peroxidase [GSH-Px]) was lower in plasma of women who reported snoring compared to non-snorers. Of note, women who snored were older with higher BMI, which may confound the relationships reported, and the gestational week of blood collection was not controlled. In another study, women who reported snoring during pregnancy (n = 48) had higher levels of the inflammatory cytokine IL-6 in cord blood compared to women who denied snoring (n = 75). Reference Tauman, Many and Deutsch98 Erythropoietin and nucleated (immature) red blood cell counts were higher in the cord blood of women who reported snoring, Reference Tauman, Many and Deutsch98 supporting that exposure to SDB in utero may result in hypoxic changes in the fetus. In contrast, Khan and colleagues reported in a retrospective study that women with diagnosed OSA (vs. self-reported non-snorers) had lower oxidative and carbonyl stress markers (Advanced glycation end products [AGE] and advanced oxidation protein products [AOPP]), but higher anti-oxidative stress markers (total antioxidant capacity [TAC]). Reference Khan, Lambert-Messerlian and Monteiro99 Taken together, these findings support that SDB, and especially OSA, may increase fetal exposure to oxidative stress, inflammation, and hypoxia-induced compensatory enhanced erythropoiesis, however further study is warranted as the findings are inconsistent. This is interesting as inflammation and oxidative stress may exacerbate IR. Reference Chen, Chen, Wang and Liang100,Reference Khodabandehloo, Gorgani-Firuzjaee, Panahi and Meshkani101
SDB and placental changes. Placental expression of carbonic anhydrase IX (CAIX), a marker of hypoxia, was higher in women who snored or with OSA compared to non-snoring women (n = 47). Reference Ravishankar, Bourjeily, Lambert-Messerlian, He, De Paepe and Gundogan73 Fetal normoblastemia (immature red blood cells), a sign of exposure to hypoxia, was significantly higher in placentas of women who snored/had OSA. However, women who snored and women with OSA had significantly higher BMI and higher rates of chronic hypertension and diabetes (vs. non-snorers), which likely contributed to differences in biomarkers (preeclampsia rates were similar between groups). Reference Ravishankar, Bourjeily, Lambert-Messerlian, He, De Paepe and Gundogan73 Women with OSA had lower levels of placenta-associated plasma protein-A (PAPP-A) Reference Bourjeily, Curran, Butterfield, Maredia, Carpenter and Lambert-Messerlian102 and lower estriol levels Reference Bourjeily, Butterfield, Curran and Lambert-Messerlian103 compared to women without OSA, suggesting poorer placental and fetal well-being, which could affect growth. Salameh et al., however, showed no differences in markers of fetal well being (PAPP-A, AFP, uE3, hCG, inhibin-A) between snorers and non-snorers. Reference Salameh, Lee and Palomaki104 Women with mild OSA (mean AHI 7.8 ± 2.7), objectively measured in the 3rd trimester, had significantly higher placental weight and higher placental leptin mRNA expression compared to women without OSA, even after controlling for maternal BMI. Reference Kidron, Bar-Lev, Tsarfaty, Ariel and Tauman105 Placental weight was positively correlated with infant adiposity (measured by skinfolds). Reference Kidron, Bar-Lev, Tsarfaty, Ariel and Tauman105 This was a small study and a power analysis was not reported; however, the findings support that mild OSA may result in placental changes that contribute to increased infant adiposity. The effect of OSA on maternal IR was not explored in any of these studies as a possible mechanism for the increased in adiposity.
SDB and chronic disease risk. Salihu and colleagues reported that women at high risk of OSA (measured by Berlin Questionnaire) had neonates with shorter telomeres than women at low risk of OSA (n = 64 women). Reference Salihu, King and Patel106 Telomere length is indicative of chromosomal aging and has been proposed as a mechanism contributing to chronic disease development, Reference D’Mello, Ross, Briel, Anand, Gerstein and Pare107 suggesting that exposure to OSA could have long-term implications in accordance with DoHad theory. Reference Hanson and Gluckman42
Long-term implications of SDB exposure in humans. In a single longitudinal study, BW between infants born to mothers with SDB were similar to controls. Reference Tauman, Zuk and Uliel-Sibony108 However, despite similar neurological development, at 1 year, mothers with SDB vs. control were more likely to report infant snoring (41.7% vs. 7.5%, p = 0.004). Reference Tauman, Zuk and Uliel-Sibony108 Human studies in which body composition of the infant, and longer term metabolic phenotyping of offspring into childhood and young adulthood are needed to elucidate if SDB exposure that increases risk for future offspring metabolic disease as studies in rodents suggest.
Discussion
SDB is a common problem that worsens throughout pregnancy. Outside of pregnancy, SDB is often associated with obesity, a metabolic syndrome characterized by IR, glucose, and triglyceride elevations that are expected to enhance fetal growth. However, obese women are also at risk of endothelial dysfunction, hypertensive disorders, placental insufficiency and the development of preeclampsia, which could attenuate fetal growth independent of the effect of SDB. Although mechanistic studies using rodent models of SF and IH during pregnancy suggest that these exposures negatively impact long-term offspring metabolic outcomes, investigations in humans have been discrepant, focused mainly on BW, and lacked a focus linking underlying mechanisms to long-term outcomes of gestational SDB exposure. Further, all studies in humans were descriptive. While most investigators made an effort to control for known confounding factors (n = 32; Table 3), there is significant possibility that unmeasured and unknown variables could influence the study outcomes. Despite these limitations, the overall evidence suggests a link between SDB, characterized by IH, SF or both, and offspring SGA and LGA, both of which are related to increased future obesity and metabolic disease risk.
Rodent models suggested that pathophysiological changes accompanying SDB during pregnancy may have lasting effects on metabolic regulation in offspring, and BW in offspring may not demonstrate the true deleterious effects of exposure to gestational SDB. Indeed, mice born to mothers exposed to SF had similar BW to control mice; however, as the mice reached adulthood, the exposed mice had significantly more metabolic abnormalities. Reference Cortese, Khalyfa, Bao, Andrade and Gozal54–Reference Khalyfa, Mutskov, Carreras, Khalyfa, Hakim and Gozal56,Reference Trzepizur, Khalyfa, Qiao, Popko and Gozal59 Further, animal models suggested that SDB exposure may have a sex-dependent effect. Reference Khalyfa, Cortese and Qiao49,Reference Mutskov, Khalyfa, Wang, Carreras, Nobrega and Gozal55,Reference Trzepizur, Khalyfa, Qiao, Popko and Gozal59 This supports previous findings that males may be more vulnerable to short-term changes or exposures in the womb, while females may not manifest differences until later, especially in the context of postnatal longer term exposures. Reference Eriksson, Kajantie, Osmond, Thornburg and Barker109 There are many limitations to rodent models. Rodent gestation demonstrates marked differences in placental development which result in no spontaneous preeclampsia. The further birth of multiple pups with minimal fat mass at birth, and the strong influence of postnatal development(including fat accretion) make translation to human pregnancies difficult. Importantly, rodents do not spontaneously develop SDB. Instead, investigators mimicked a condition that would not normally exert influence on rodent physiology. Thus, growth and body composition in rodents at birth will not closely reflect what could be expected in humans. Despite these limitations, rodent models support that exposure to gestational SDB may have negative long-term outcomes for the metabolic health of the offspring.
Studies in humans suggested a link between maternal SDB and both SGA and LGA, but a number of considerations impact interpretation of the data. A salient problem across human studies was inconsistency in reporting of infant outcomes. Measurement of SDB also varied widely. While some studies used objective measurement of SDB (such as laboratory or in home polysomnography), Reference Pamidi, Marc and Simoneau65,Reference Facco, Ouyang and Zee69,Reference Sahin, Koken and Cosar74,Reference Telerant, Dunietz, Many and Tauman79,Reference Pien, Pack, Jackson, Maislin, Macones and Schwab85,Reference Louis, Auckley and Miladinovic87 others used a single question about presence of snoring. Reference Ayrim, Keskin, Ozol, Onaran, Yiidirim and Kafali1,Reference Bourjeily, Raker, Chalhoub and Miller4,Reference Franklin, Holmgren, Jonsson, Poromaa, Stenlund and Svanborg7,Reference Perez-Chada, Videla and O’Flaherty72,Reference Tauman, Sivan, Katsav, Greenfeld and Many77,Reference Owusu, Anderson and Coleman84 Importantly, the majority of studies were not powered to detect a relationship between SDB and infant outcomes. Only five were powered on an infant outcome, Reference O’Brien, Bullough and Owusu13,Reference Fung, Wilson and Lappas31,Reference Pamidi, Marc and Simoneau65,Reference Olivarez, Ferres and Antony71,Reference Yin, Williams and Burton78 and none on infant body composition. Investigators found an association in three of the studies and no association in two (Table 2). Thus, many studies may not have detected an association due to inadequate power. Timing of SDB assessment varied widely across studies in humans. Symptoms of SDB (i.e. snoring) were assessed at delivery in many studies, while others assessed SDB earlier in pregnancy. Severity of SDB was not typically reported. It is speculated that women who enter pregnancy with chronic OSA have higher risk for altered placental function that would result in compromised nutrient supply and placental perfusion as well as fetal under-development and growth restriction. Alternatively, we hypothesize that mild SDB without significant hypoxemia and a deleterious effect on placental development may exacerbate pregnancy IR, promoting nutrient excess and fetal overgrowth (Fig. 1). It is possible, and highly likely, that mild SDB either develops or worsens over pregnancy in those affected by obesity, but in many, never becomes severe enough to compromise placental development and perfusion. Thus, while mild IH and SF are speculated to increase oxidative stress and inflammation to some degree, the placenta may still develop normally. If maternal IH and/or SF worsen IR, excess fetal nutrient exposure may result in LGA. Alternatively, with more severe IH, placental insufficiency may attenuate fetal overgrowth in spite of an effect on worsening maternal IR and nutrient excess in late gestation. Assessment of SDB symptoms (i.e. presence/absence of snoring) only at the end of pregnancy provides incomplete information about the timing and severity of an exposure that evolved over gestation. It is also possible that SDB serves as a marker for other underlying conditions, such as obesity, gestational hypertension, IR, inflammation, or elevated glucose levels, which mediate many of the alterations in birth outcomes. Future, more highly controlled, studies in which SDB is objectively categorized into mild/moderate or severe OSA, its occurrence measured in in the 1st, 2nd, and 3rd trimesters, and its associated complications including IR, inflammation, hypoxia, oxidative stress, altered glucose and lipid metabolism, elevated blood pressure, and endothelial dysfunction are better quantified will help to delineate if there truly are opposing effects of the SDB spectrum on infant outcomes, allowing for better understanding of SDB as an exposure.
Based on rodent models, it is also possible that BW may be normal in infants exposed to SDB in utero, but body composition (percentage of body fat) may differ. Reference Barbour, Hernandez and Reynolds110 Higher neonatal adiposity is associated with increased risk for childhood obesity. Results from our lab indicate a potential relationship between severity of maternal OSA and infant adiposity. In a recent study, we found that infant % body fat, measured at 2 weeks postpartum, was significantly correlated with overnight oxygen desaturation in a group of women with obesity who had previously undiagnosed mild OSA, possibly mediated by higher 24-h glycemic patterns as measured by continuous glucose monitoring. Reference Farabi, Barbour, Heiss, Hirsch, Dunn and Hernandez95 We also found a positive correlation between OSA severity and fasting free fatty acids as well as hepatic IR, Reference Farabi, Barbour, Heiss, Hirsch, Dunn and Hernandez95 suggesting potential mechanisms by which SDB exacerbates IR in the mother and contributes to excessive infant adiposity. Future studies should include measurement of infant body composition as infant adiposity to provide a stronger indicator of future disease risk rather than BW alone. Reference Catalano, Farrell and Thomas44
Mechanistic studies in humans looking at the effects of SDB during pregnancy showed evidence of hypoxia at the level of the placenta, increased inflammation and oxidative stress, and suggested increased risk of disease development in the offspring (telomere length). However, these studies are limited by very small sample sizes, lack of objective measurement of SDB, and were not carefully controlled. Controlled studies in which participants are carefully enrolled based on like phenotypes are needed to elucidate mechanisms behind the relationship between SDB, inflammation and IR, such as the role of HIF1-α, a transcription factor induced by hypoxia, linked to both inflammation and IR. In Fig. 1, we highlight other potential mechanisms by which SDB could exacerbate IR including at the level of smooth muscle, adipose tissue, or the liver, as well as effects on hormones, such as GLP-1 and cortisol, and sympathetic stimulation.
In summary, prevailing evidence across human and rodent models implicates in utero exposure to SDB as a developmental origin of future metabolic health. Since OSA is tightly linked to obesity, prenatal screening of obese pregnant women for risk of SDB may help to identify women who have undiagnosed OSA for treatment prior to pregnancy. Prenatal counseling that directly promotes weight loss prior to pregnancy may help to further prevent worsening SDB with pregnancy. Treatment of SDB may be important to eliminate the exposure of hypoxia and alleviate exacerbation of the IR of pregnancy resulting in altered glucose and lipid metabolism and inflammation. While there remain no large prospective studies in humans investigating the impact of SDB treatment on offspring outcomes, the evidence suggests that treatment targeting factors such as IH and SF may reduce future offspring disease risk. Prospective human trials over the course of pregnancy designed to discern the mechanistic pathways by which SDB influences placental development, fetal growth, and future risk of offspring metabolic disease are of paramount importance to inform timing and type of interventional therapy. With the ever-rising prevalence of obesity both in pregnancy and in childhood, understanding if SDB during pregnancy, still largely unrecognized in clinical practice, has important long-term implications for offspring is vital as it may be one modifiable contributor of significant morbidity from metabolic disorders in adulthood.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174420000355
Financial support
This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK101659), and by the CU Center for Women’s Health Research.
Conflicts of interest
None.








