Background
Chronic diseases including obesity are on the rise worldwide, many of which already start to manifest during childhood.Reference Hanson and Gluckman1 According to the World Health Organization (2018), the prevalence of overweight and obesity worldwide among children from 5 to 19 years of age was 18% in 2016, compared with just 4% in 1975.2 In the United States, 31.8% of all children from 2 to 19 years of age were estimated to be either overweight or obese in 2011–2012Reference Ogden, Carroll, Kit and Flegal3 and in the Netherlands, 13.4% of children between the ages of 4 and 20 were overweight or obese in 2016.4 Childhood overweight and obesity can persist into adulthood, increasing the risk of cardiovascular diseases, diabetes and various cancers later in life.Reference Llewellyn, Simmonds, Owen and Woolacott5 Overweight can affect every system in the body and is therefore associated with an increased risk of other health issues including joint problems, fractures, sleeping disorders, hypertension, nonalcoholic fatty liver disease, depression and anxiety.Reference Kumar and Kelly6
Modifiable early-life risk factors found in a systematic review by Woo Baidal et al.Reference Woo Baidal, Locks, Cheng, Blake-Lamb, Perkins and Taveras7 for childhood overweight include not only prenatal factors, such as maternal pre-pregnancy and excess gestational weight gain, smoking during pregnancy, high birth weight, but also postnatal factors, such as premature solid food introduction and infant antibiotics. The human gut microbiome may play a substantial underlying role in these early risk factors for overweight and obesity.Reference Cox and Blaser8
One of the factors that can disrupt the gut microbiome and may contribute to the development of childhood overweight and obesity is early-life antibiotic administration.Reference Nogacka, Salazar and Arboleya9 In the early 1950s, farmers had already started adding low doses of antibiotics to the food of various animals, including chickens and pigs, following the discovery that antibiotic administration could significantly increase their growth.Reference Jukes and Williams10 Later experimental studies in mice also demonstrate the likelihood of a critical window in which antibiotics could impose lasting effects; mice exposed to antibiotics in early life before weaning were more likely to become overweight than mice that were not exposed, or mice that were exposed to antibiotics after weaning.Reference Cho, Yamanishi and Cox11, Reference Cox, Yamanishi and Sohn12 Several observational studies have reported that alterations to the early developing microbiome may also be making children susceptible to overweight or obesity.Reference Azad, Moossavi, Owora and Sepehri13 Although the microbiome may eventually return to its original composition after the completion of antibiotic administration, the disruption caused by antibiotics may lead to permanent changes in metabolic and immune development.Reference Cox and Blaser8, Reference Korpela and de Vos14
Antibiotics are commonly administered to women during pregnancy for urinary and respiratory infections and just prior to or during delivery, to prevent neonatal Group B Streptococcus fever and maternal infections, including chorioamnionitis and infections arising from surgical wounds after caesarean sections.Reference Martinez de Tejada15 Stokholm et al.Reference Stokholm, Schjorring and Pedersen16 estimated antibiotic administration to be 37% for pregnancy and 33% during delivery in Denmark. In the Netherlands, 20.8% of women are estimated to have antibiotics during pregnancy,Reference de Jonge, Bos, van Langen, de Jong-van den Berg and Bakker17 but the proportion of intra-partum antibiotics is unknown. Antibiotics are given to over 50% of children in high-income countries, during their first years of life to treat mainly ear and respiratory tract infections.Reference Nogacka, Salazar and Arboleya9, Reference Dekker, Verheij and van der Velden18
As overweight and obesity rates are rising with such detrimental consequences and antibiotics are so commonly administered to women and children, it is important to ascertain whether and to what extent antibiotic exposure, a potentially modifiable factor, does increase the risk of developing childhood overweight. An initial review of the literature revealed that a substantial number of studies have been conducted on the relationship between infant antibiotic administration and childhood overweight, and far fewer studies have been conducted on prenatal antibiotic exposure and childhood overweight. This review therefore has two main aims: (1) to review the evidence reported by observational studies between 2008 and 2018 that have investigated prenatal antibiotic exposure and overweight or obesity in children up to 18 years of age and (2) to review the evidence that has been reported by systematic reviews between 2008 and 2018 that have investigated infant antibiotic exposure and overweight or obesity in children up to 18 years of age.
Methods
The databases Pubmed, Embase and Google Scholar were searched from the period 1st January 2008 till 1st December 2018. Search terms adjusted to the databases Pubmed and Embase (see Box S1) were used to identify the titles and abstracts of relevant English language publications. Various combinations of the same keywords were entered into Google Scholar to check for any additional publications. The titles and abstracts that were retrieved were screened by RB for potential inclusion in this review. The full texts of the selected titles/abstracts were then read and assessed independently by RB and MT to determine whether they were eligible for this review. The inclusion and exclusion criteria, quality assessments and data extraction methods differed somewhat for the two types of review conducted in this study and will therefore be described separately.
Prenatal antibiotic exposure
Inclusion and exclusion criteria
The inclusion criteria for study design were observational human studies, such as cohort, case-control or cross-sectional studies examining prenatal antibiotic exposure. We included only studies that were published, in order to have sufficient information to be able to conduct comparable assessments of the quality and findings of the studies. By including only studies that had been peer-reviewed, we aimed to increase the chance of our findings being accurate. Experimental animal studies and reviews were excluded.
The exposure for the review of prenatal antibiotic exposure was any type of antibiotic administered during pregnancy and during delivery; data could be obtained by means of medical databases or by maternal self-reports. All publications needed to report effect sizes, such as odds ratios, relative risks, body mass index (BMI) z-scores and their corresponding 95% confidence intervals. The outcome overweight or obesity could be measured at any time during childhood up to 18 years of age and determined by BMI (divided into weight categories, such as underweight/normal weight/overweight/obesity) or by BMI z-scores. These anthropometrics could be based on parental self-reports or measurements by trained personnel.
Quality assessment
To help determine how reliable the findings of the finally selected publications were on prenatal antibiotic exposure and childhood overweight, RB and MT independently assessed them for their risk of bias using the Newcastle–Ottawa Scale (NOS)Reference Wells, Shea and O’Connell19 (Table 1). This scale evaluates observational studies and gives points for three categories: ‘selection’ (assessing how representative the sample is, and how objective the measurements are), ‘comparability’ (assessing how well potential confounders are taken into account) and ‘outcome’ (assessing follow-up time and how missing data are handled). The maximum number of points that can be awarded is 9, with publications scoring 8–9 points considered ‘low risk’, 6–7 points ‘medium risk’ and publications with <6 points evaluated as ‘unclear’.
Table 1. Prenatal antibiotics exposure and childhood overweight/obesity

NOS, Newcastle-Ottawa Scale (low risk: 8–9 points, medium risk: 6–7 points, unclear risk: <6 points); CS, caesarean section; VB, vaginal birth; OR, odds ratio; RR, relative risk.
For the ‘comparability’ category, the main potential confounders we considered to be of importance due to their association with childhood overweight were caesarean section,Reference Kuhle, Tong and Woolcott25 postnatal antibiotics,Reference Azad, Moossavi, Owora and Sepehri13 maternal pre-pregnancy overweight,Reference Gillman26 childhood infectionsReference Li, Chen, Ferber and Odouli27 and maternal infection (due to its association with childhood infections).Reference Blomstrom, Karlsson, Gardner, Jorgensen, Magnusson and Dalman28
Data extraction
Data were also extracted independently from each publication by RB and MT. The data extracted by RB and MT were compared and any disagreement was discussed until consensus was reached. These data consisted of the study design, country, definitions of the exposure and outcome measurements, the main findings (i.e. effect sizes and confidence intervals), additional relevant factors that were adjusted for or examined and the study authors’ own conclusions. Information was collected on the potential confounding factors the studies had adjusted for, such as maternal infections and maternal BMI, and on whether authors had addressed multiple doses and types of antibiotics.
Infant antibiotic administration
Inclusion and exclusion criteria
The inclusion criteria were published systematic reviews that consisted of observational human studies examining the relationship between infant antibiotic exposure during the first 2 years of life and the development of childhood overweight or obesity. The systematic reviews needed to report statistical effect sizes and confidence intervals.
Quality assessment
The assessments of the systematic reviews were based on a tested criteria appraisal tool for umbrella reviewsReference Aromataris, Fernandez, Godfrey, Holly, Khalil and Tungpunkom29 and on the tool Risk of Bias in Systematic Reviews (ROBIS)Reference Whiting, Savovic and Higgins30 (Tables S1a and S1b). The first tool consists of 10 questions about the methodology used in the systematic review with the following answer options which we adjusted to ‘yes’, ‘no’, ‘unclear’ and ‘not applicable’. The ROBIS tool is used to help assess risk of bias in terms of four different domains: ‘study eligibility’ (i.e. the appropriateness of eligibility criteria), ‘identification and selection of studies’ (i.e. the appropriateness of methods used to identify publications), ‘data collection and study appraisal’ (i.e. efforts made to minimise error in data collection and risk of bias assessment) and ‘synthesis and findings’ (i.e. the appropriateness of the synthesis conducted).
Data extraction
Data were extracted from the systematic reviews on the authors, country, the number of studies included, whether a meta-analysis had been conducted, the main findings and pooled results, additional findings of influential factors, information about quality assessments conducted by the authors and the authors’ own summary conclusions regarding the evidence on infant antibiotic exposure and childhood overweight/obesity.
Results
Five studies were identified investigating prenatal antibiotic exposure and childhood overweight/obesity and were published during the period 2015–2018 (see Figure S1 for search process). Four systematic reviews were identified investigating infant antibiotic exposure and childhood overweight/obesity and were published from 2017 to 2018. Three of the systematic reviews had additionally conducted meta-analyses and one systematic review included had examined prenatal as well as infant antibiotic exposure. The authors that had not conducted a meta-analysis referred to their study as a narrative review in the title, but they had followed the same reproducible procedures in their review as other systematic reviews.
Prenatal antibiotic exposure
Study characteristics
Five cohort articles were identified examining the relationship between prenatal antibiotic exposure and childhood overweight or obesity (Table 1). The studies were conducted in the United States (n = 4) or in Denmark (n = 1) and the sample sizes ranged from 436 to 39,615 mother–child pairs. The prevalence of prenatal antibiotic exposure was 16% when intra-partum antibiotics were excluded,Reference Mueller, Whyatt and Hoepner23 57.5% in the study that included intra-partum antibioticsReference Cassidy-Bushrow, Sitarik and Levin21 and 25.2%–60.4% in the other three studies where it was unclear whether or not intra-partum antibiotics were included.Reference Wang, Liu and Zhang20, Reference Mor, Antonsen and Kahlert24, Reference Poulsen, Pollak, Bailey-Davis, Hirsch, Glass and Schwartz22 Childhood overweight/obesity ranged from 17.1% at 2 years of ageReference Cassidy-Bushrow, Sitarik and Levin21 to 24.7/8.9% (overweight/obesity) at 4 years of ageReference Wang, Liu and Zhang20 to ranges of 10.9%–25.2% at the ages of 7 and older.Reference Wang, Liu and Zhang20, Reference Mueller, Whyatt and Hoepner23, Reference Mor, Antonsen and Kahlert24
Study findings
There were generally positive trends found in the relationship between any prenatal antibiotic exposure and childhood overweight, although this relationship was significant in just two of the five studies: that is, overweight: prevalence rate (PR): 1.26 (1.10–1.45); obesity: PR: 1.29 (1.03–1.62) in a Danish sample of 7–16 year oldsReference Mor, Antonsen and Kahlert24 and relative risk (RR): 1.77 (1.25–2.51) in 7 year olds from a sample who had mothers identifying as African-American or DominicanReference Mueller, Whyatt and Hoepner23 (Table 1). Another study did not find an association with overweight or obesity categories, but with an increased BMI z-score at 3 years of age.Reference Poulsen, Pollak, Bailey-Davis, Hirsch, Glass and Schwartz22
The significance of the relationship found in the study conducted by Mor et al.Reference Mor, Antonsen and Kahlert24 was mainly driven by exposure to three or more antibiotic prescriptions. Although the other three studies found a general insignificant relationship between prenatal antibiotic exposure and childhood overweight/obesity, they all did reveal significant relationships under certain conditions, that is, the exposure consisted of multiple antibiotic prescriptions in a sample where mothers were overweightReference Poulsen, Pollak, Bailey-Davis, Hirsch, Glass and Schwartz22 and the exposure was multiple, mainly broad-spectrum antibiotic administration when overweight was measured at a later childhood age (7 versus 4 years of ageReference Wang, Liu and Zhang20). Other conditions were that antibiotic exposure had occurred only during certain trimesters [i.e. only first trimesterReference Cassidy-Bushrow, Sitarik and Levin21 or only second trimester for overweight at 7 years of age (but not at 4 years)Reference Wang, Liu and Zhang20].
Three of the four studies that had examined evidence for a dose–response relationship found a significant relationship only after multiple courses (i.e. 2+ courses when outcome was measured at age 7Reference Wang, Liu and Zhang20, 3+ coursesReference Mor, Antonsen and Kahlert24 and 3+ courses by overweight mothersReference Poulsen, Pollak, Bailey-Davis, Hirsch, Glass and Schwartz22). Two studies examined antibiotic types and found that sulfonamides, trimethoprim and mixed antibiotics had a stronger effect sizeReference Mor, Antonsen and Kahlert24 and that macrolides were associated with a higher BMI z-score.Reference Cassidy-Bushrow, Sitarik and Levin21
Influential factors
Common potential confounders that were usually adjusted for were maternal age, ethnicity, indicators of socio-economic status (such as maternal education or receipt of public assistance), parity, infant gender, maternal BMI and delivery mode. Birth weight, breastfeeding, maternal and child infections and early childhood antibiotic administration were less frequently considered.
Delivery mode: Studies conducting subgroup analyses on delivery mode revealed varying results. Wang et al.Reference Wang, Liu and Zhang20 found a stronger relationship between prenatal antibiotic exposure and overweight/obesity in a subgroup of children who had been born by caesarean section, Cassidy-Bushrow et al.Reference Cassidy-Bushrow, Sitarik and Levin21 found no difference in effect size in the two types of delivery mode and Mor et al.Reference Mor, Antonsen and Kahlert24 found similar effect sizes, but the relationship was only significant in children born vaginally. Caesarean section was found to be a significant independent predictor besides prenatal antibiotic exposure.Reference Mueller, Whyatt and Hoepner23
Pre-pregnancy maternal weight: This potential confounder was adjusted for in all studies but one; that study did not have information on maternal BMI, but did correct for estimates based on previous literature by Vidal et al.Reference Vidal, Murphy and Murtha31 and Whitaker et al.Reference Whitaker32 Only Poulsen et al.Reference Poulsen, Pollak, Bailey-Davis, Hirsch, Glass and Schwartz22 stratified by maternal weight and found that only mothers with overweight who had taken 3+ courses antibiotics were significantly likely to have a child with overweight or obesity.
Postnatal antibiotics: This factor was generally not examined as a possible confounder of prenatal antibiotic use and overweight. Poulsen et al.Reference Poulsen, Pollak, Bailey-Davis, Hirsch, Glass and Schwartz22 had observed that there was a significant trend of postnatal antibiotic use with prenatal antibiotic exposure. In their study, they examined postnatal antibiotic use correcting for prenatal antibiotics, but did not correct for postnatal antibiotics when examining prenatal antibiotics. They found that postnatal antibiotics were and prenatal antibiotic exposure was not associated with childhood overweight and obesity.
Maternal and childhood infections: Maternal infections were not examined in any publications, but Mor et al.,Reference Mor, Antonsen and Kahlert24 Wang et al.Reference Wang, Liu and Zhang20 and Cassidy-Bushrow et al.Reference Cassidy-Bushrow, Sitarik and Levin21 did mention in their discussions that a possible underlying role of maternal infection could not be ruled out in the relationships they had found between prenatal antibiotics and overweight/obesity. Mor et al.Reference Mor, Antonsen and Kahlert24 mentioned that there could be a genetic predisposition to infection and obesity. None of the studies adjusted for childhood infections in the relationship between prenatal antibiotic exposure and childhood overweight/obesity; however, Mueller et al.Reference Mueller, Whyatt and Hoepner23 did not find a significant relationship between prenatal and childhood infections.
Breastfeeding: Two of the five studies examining the relationship between prenatal antibiotic exposure and overweight/obesity adjusted for breastfeeding; in both studies, breastfeeding was defined as ever having breastfed. Other related child feeding practices with regard to weaning were never mentioned as possible confounders.
Gender: The gender of the infant was either adjusted for or examined in subgroup analyses. One study found that the relationship between prenatal antibiotic exposure and overweight in 7–16 year olds was only significant in boys,Reference Mor, Antonsen and Kahlert24 although there was a positive but insignificant effect in girls.
Birth weight: Mor et al.Reference Mor, Antonsen and Kahlert24 found a stronger relationship between prenatal antibiotic exposure and overweight within the lower birth weight sample than the higher birth weight sample.
Risk of bias in studies
Most studies had a medium risk of bias, usually scoring a 7 out 9, when assessed according to the NOS. Studies generally lost points by not taking important potential confounders into account (such as delivery mode, postnatal antibiotics or maternal and childhood infections) or by using self-reported as opposed to medically diagnosed measurements. There were other issues, however, that we believed could not be captured in the NOS scale, such as ambiguity at times about the types of analyses that had been conducted, and missing information, such as whether or not intra-partum antibiotics (administered just prior to or during delivery) had been included as part of the exposure prenatal antibiotics.
Heterogeneity between the studies on prenatal antibiotic exposure made them difficult to compare. There was heterogeneity with respect to the exposure (i.e. inclusion/exclusion of intra-partum antibiotics or inclusion not mentioned), various ages of outcome (ranging from age 2 in one study to 7–16 years in another study), the type of overweight measurements (i.e. BMI odd ratios versus z-scores) and the number and types of potential confounders taken into account.
Infant antibiotic administration
Study characteristics
The four systematic reviews that had been retrieved were conducted in Denmark, China, United Kingdom and Canada each covering between 12 and 17 publications on infant antibiotic administration. The latest date of the search period conducted by these four systematic reviews was October 2017. Nine publications were covered by all 4 reviews and 12 publications by at least 3 reviews (see Box S2).
Main findings
The four systematic reviews varied in their overall conclusions (see Table 2). One concluded there was substantial evidence for infant antibiotic exposure increasing the risk of childhood overweight/obesity,Reference Shao, Ding and Wang33 two concluded there was a small association between infant antibiotic exposure and childhood overweight/obesityReference Miller, Wu, Pedersen, de Klerk, Olsen and Burgner49, Reference Rasmussen, Shrestha and Bjerregaard34 and one concluded that there was no conclusive evidence of associations between infant antibiotic exposure and childhood overweight/obesity.Reference Partap, Allcock, Parker, Gurdasani, Young and Sandhu35 Reported pooled effect sizes found by the meta-analyses conducted in three of the systematic reviews were RR: 1.21 (1.09–1.33) for overweight and RR: 1.18 (1.12–1.25) for obesity (based on any exposure <24 months and any age of outcome from 2 to 12 years),Reference Shao, Ding and Wang33 odds ratio (OR): 1.11 (1.02–1.20) [based on any exposure (0–2 years) and any age of outcome from >2 to 12 years]Reference Rasmussen, Shrestha and Bjerregaard34 and OR: 1.05 (1.00–1.11) [based on earliest age of exposure (<24 months), latest age of outcome (2–18 years) and lowest number of prescriptions in each publication].Reference Miller, Wu, Pedersen, de Klerk, Olsen and Burgner49
Table 2. Summary of systematic reviews examining infant antibiotic exposure and childhood overweight or obesity
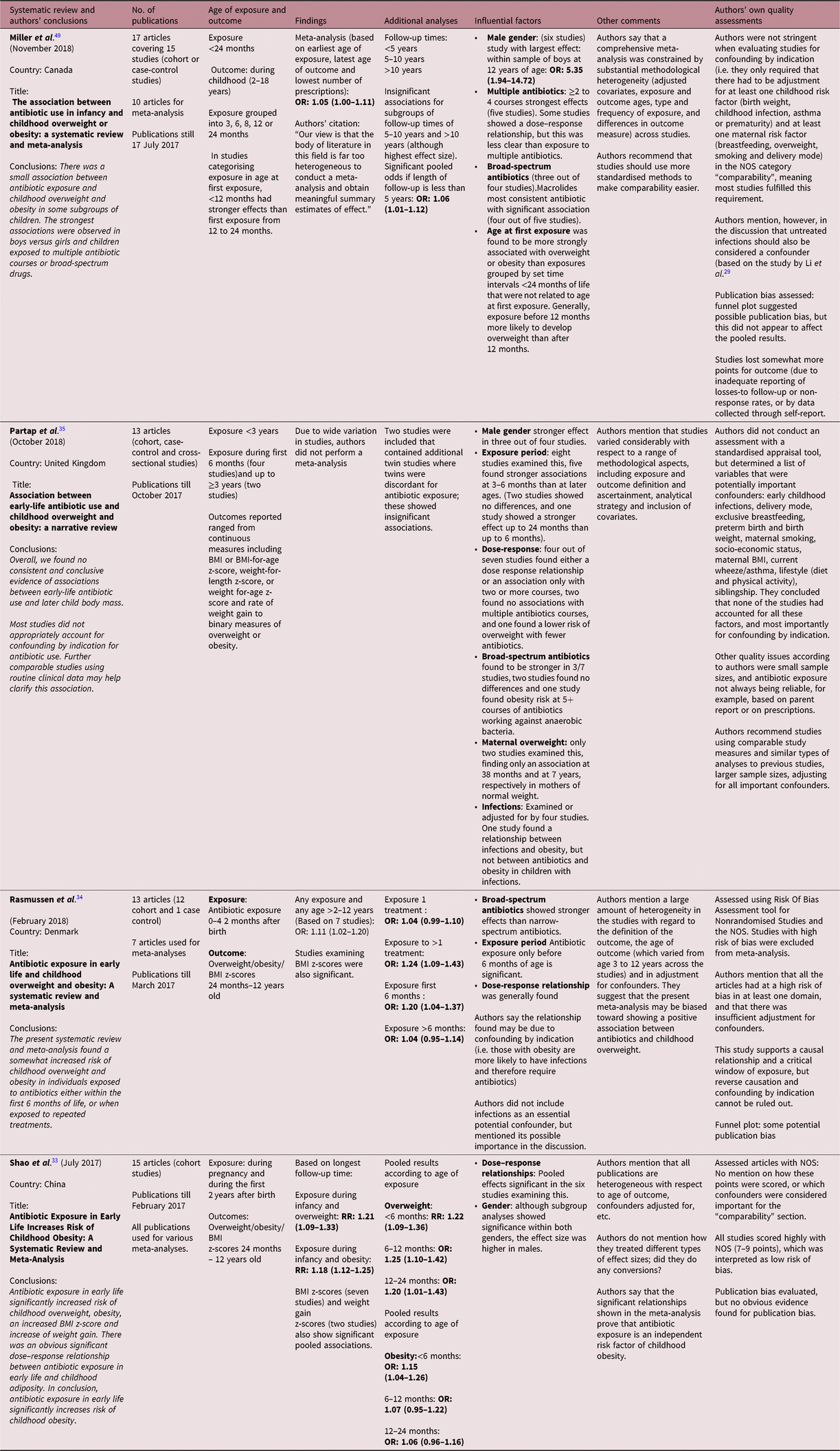
Notable influential factors with greater effects, as reported by these reviews, were multiple doses of antibiotics (a few studies within the reviews also reporting dose–response relationships), broad-spectrum antibiotics (macrolides being most consistently reportedReference Miller, Wu, Pedersen, de Klerk, Olsen and Burgner49) and male gender.
Other factors investigated were the follow-up times. Miller et al.Reference Miller, Wu, Pedersen, de Klerk, Olsen and Burgner49 found that shorter follow-up times (<5 years) were significant, whereas longer follow-up times up to 10 years and above 10 years had positive effects, but were insignificant.
The investigation of maternal overweight was only mentioned by Partap et al.Reference Partap, Allcock, Parker, Gurdasani, Young and Sandhu35 who found that in the two publications conducting sub-studies according to maternal weight, a significant association was only found in mothers of normal weight. Partap et al. also mentioned two twin studies that showed an insignificant relationship between infant antibiotics and childhood overweight.
The authors of the four systematic reviews all reported the potential confounders that had been adjusted for in each study; factors that were commonly adjusted for included gestational age, birth weight, maternal age, socio-economic status and breastfeeding. All authors of the systematic reviews acknowledged the likelihood of there being more potential confounders influencing the relationship between infant antibiotic administration and overweight/obesity, such as childhood infections.
Assessment of studies
Using the critical appraisal tool by Aromataris et al.Reference Aromataris, Fernandez, Godfrey, Holly, Khalil and Tungpunkom29 and the ROBIS to assess the methodology and synthesis, it was apparent the four systematic reviews varied in the criteria they adhered to (Tables S1a and S1b). Partap et al. were the most and Shao et al. the least stringent with regard to the methodology, study syntheses and evaluations. Three out of the four reviews assessed the studies using the NOS, with varying scores and evaluations. Shao et al. rated their included studies the most favourably, assigning scores from 7 to 9 to all the studies and evaluating scores of at least 7 as low risk of bias. More details on how the scores were obtained were not reported in their review. Their more relaxed criteria with regard to assessing articles, pooling findings from heterogeneous studies, interpreting publication bias and including them in their meta-analysis likely contributed to their more assertive conclusions that there is a causal relationship between early-life antibiotics and childhood overweight.
Miller et al. and Rasmussen et al. also assessed the included articles using the NOS and in general had similar proportions of scores [Miller: low risk (four studies), moderate risk (eight studies) and high risk (three studies) and Rasmussen: low risk (two studies), moderate risk (eight studies) and high risk (three studies)].
Miller assessed the ‘comparability’ category of the NOS (covering the adequacy of adjusting for confounders) in the studies they reviewed much more favourably, giving 12/15 of their studies the maximum of two points if relevant potential confounders had been adequately accounted for. Their criteria were adjusting for at least one childhood and at least one maternal risk factor to gain the full two points. They did mention in their discussion, however, that (untreated) infections should be treated as potential confounders in future studies. Rasmussen et al. only awarded 2 of 13 included studies the full 2 points for ‘comparability’, due to inadequate adjustment for confounding, although in their list of potential confounders, they did not include childhood infections. They did mention in their discussion, however, that infections were likely to be of importance and that most studies did not address this. The studies rated as high risk of bias were not included in their meta-analyses. Other points were often lost by the authors of these systematic reviews for inadequate reporting of loss-to-follow-up or non-response rates and self-reported data.
The most stringent assessment of publications was conducted by Partap et al.Reference Partap, Allcock, Parker, Gurdasani, Young and Sandhu35 who also had the most cautious conclusions regarding the association between infant antibiotic exposure and childhood overweight. The authors mentioned not conducting a meta-analysis, due to the heterogeneity of all the studies. Although they did not use a standardised method, such as the NOS to assess the studies, they did consider important potential confounders that studies should have adjusted for to obtain more reliable results. Important confounders mentioned in their review were early childhood infections, delivery mode, exclusive breastfeeding, preterm birth and birth weight, maternal smoking, BMI and socio-economic status, current wheeze/asthma, lifestyle and siblingship. None of the studies adjusted for all of these factors. They concluded that most studies did not sufficiently examine confounding by indication. Other critical points raised by this review were small sample sizes and parent-reported antibiotic use.
The three studies who conducted meta-analyses also created funnel plots to test for publication bias. One study concluded there was no indication of publication bias (Shao et al., 2017),Reference Shao, Ding and Wang33 one study mentioned there was possible publication bias, but this would unlikely affect the pooled resultsReference Miller, Wu, Pedersen, de Klerk, Olsen and Burgner49 and one study concluded there was some potential publication bias.Reference Rasmussen, Shrestha and Bjerregaard34
Three of the four reviews recommended further studies using more standardised and similar measures in order to make comparability across the publications more clear and reliable.
The studies that were assessed by the authors of the systematic reviews had similar quality issues to the assessments we had made of the observational studies on prenatal antibiotic exposure, the majority scoring a 7 when assessed by the NOS (medium quality). Similar issues were encountered, including much heterogeneity in the studies (e.g. in exposure/outcome measurements and potential confounders), and the use of self-reported data.
Discussion
In this review, our two main aims were to summarise and assess the evidence regarding prenatal antibiotics exposure and the development of childhood overweight/obesity and to summarise and assess the systematic reviews of publications regarding infant antibiotic exposure and overweight/obesity. A search for publications in the last 10 years resulted in the retrieval of five studies on prenatal antibiotic exposure published during the period 2015–2018 and four systematic reviews on infant antibiotic administration published from 2017 to 2018.
Main findings
The five studies that were identified generally showed a positive trend in the relationship between any prenatal antibiotic exposure and childhood overweight/obesity, which was significant in two of the studies. The other three studies showed significant relationships with overweight/obesity under certain conditions, such as multiple antibiotic courses or prescriptions, broad-spectrum antibiotics, older children, overweight mothers and during varying trimesters where exposure occurred.
The four systematic reviews similarly tended to conclude that there was either a clear relationship,Reference Shao, Ding and Wang33 there was some evidence of a relationshipReference Miller, Wu, Pedersen, de Klerk, Olsen and Burgner49, Reference Rasmussen, Shrestha and Bjerregaard34 and there was no clear evidence of a relationshipReference Partap, Allcock, Parker, Gurdasani, Young and Sandhu35 between infant antibiotic exposure and childhood overweight and obesity. All reviews mentioned there being much heterogeneity in the studies, and three reviews mentioned the lack of adjustment for confounding by infectious diseases in many studies as being a potential issue causing bias.
Overall, the studies examining both prenatal antibiotic exposure and infant antibiotic administration point to some evidence of a relationship with childhood overweight/obesity under certain circumstances, but these relationships cannot be concluded as definitive at this point.
The gut microbiome
Although we cannot conclude with certainty that early antibiotic exposure causes overweight or obesity in humans, there is some evidence that the gut microbiome may play a role in this relationship. The microbiome in the gut of overweight children is more likely to have a lower diversity and a higher ratio of firmicutes to bacteroidetes than normal weight children,Reference Slattery, MacFabe and Frye36 which in turn is associated with a higher BMI in later childhood.Reference Korpela, Zijlmans and Kuitunen37 A recent Norwegian cohort study showed that the composition of the gut microbiome during various times of the first 2 years of life predicted childhood BMI at 12 years old.Reference Stanislawski, Dabelea and Wagner38 Antibiotic use during pregnancy is also associated with increased staphylococcus species,Reference Stokholm, Schjorring and Eskildsen39 which in turn is associated with increased risk of childhood overweight.Reference Kalliomaki, Collado, Salminen and Isolauri40
Various factors are known to influence the colonisation of the gut microbiota of children early in life including maternal BMI and diet, smoking status and stress during pregnancy, preterm birth, hospitalisation, mode of birth (i.e. vaginal versus caesarean), type of milk feeding (i.e. breastfeeding versus formula), childhood infections and early antibiotic exposure.Reference Munyaka, Khafipour and Ghia41, Reference Fouhy, Ross, Fitzgerald, Stanton and Cotter42 Other drugs, such as acid-suppression medications, can also affect the microbiome and are associated with childhood diseases, such as obesity and allergies.Reference Mitre, Susi, Kropp, Schwartz, Gorman and Nylund43, Reference Stark, Susi, Emerick and Nylund44
Overweight and obesity are thought to occur through various bacterial mechanisms, including the bacterial role in increasing the efficiency of energy extraction from more food sources than normal weight children, influencing gastrohormonal changes in hunger and satiety, and increasing gut wall permeability leading to the higher plasma concentrations of lipopolysaccharides and liver fat, and increased production of proinflammatory cytokines. This subsequent low-grade inflammation is associated with a range of chronic diseases, including type 2 diabetes and obesity.Reference Munyaka, Khafipour and Ghia41, Reference Shreiner, Kao and Young45, Reference Federico, Dallio, DI Sarno, Giorgio and Miele46
Other influential factors
Some of the factors found by the studies in this review that may be influential in the relationship between prenatal antibiotic exposure and overweight/obesity fall in line with earlier studies. Caesarean section was found to have independent effects on the development of obesity, which corresponds with the findings of an earlier systematic review that CS was associated with childhood obesity.Reference Kuhle, Tong and Woolcott25 It is difficult, however, to ascertain what extent of this relationship may be due to caesarean section itself, or due to intra-partum antibiotics often given to prevent post-surgical wounds,Reference Nogacka, Salazar and Arboleya9 or whether their effects correlate when it comes to disrupting the microbiome. Subgroup analyses on delivery mode in the studies in this review showed mixed findings; sometimes there was a stronger effect of prenatal antibiotics in children who had had caesarean sections (for instance, Wang et al.Reference Wang, Liu and Zhang20) and sometimes there were only significant effects of antibiotics in children with vaginal births (for instance, Mor et al.Reference Mor, Antonsen and Kahlert24). These seemingly conflicting findings are plausible, however, if there are differences in intra-partum antibiotic policies in different medical settings with regard to caesarian sections,Reference Mackeen, Packard, Ota, Berghella and Baxter47 or when studies differ in whether or not intra-partum antibiotics are included in the exposure measurement. More studies are needed that can distinguish these effects on the microbiome, for instance, by comparing children who have had CS and intra-partum antibiotics before cord clampage with those who have had antibiotics following cord clampage.
In the studies examining infant antibiotic administration, male gender was also a factor found to be relatively more at risk of developing overweight or obesity when exposed to antibiotics. Kozyrskyj et al.Reference Kozyrskyj, Kalu, Koleva and Bridgman48 found that boys were more likely to develop overweight and obesity than girls, when their microbiome was disrupted. Gender differences were also found in mouse studies, with early penicillin exposure having a greater impact on male mice than female mice.Reference Cox, Yamanishi and Sohn12
In the studies on both prenatal antibiotic exposure and infant antibiotic administration, little consideration was generally given to maternal or infant infections being possible confounders. A recent Australian study found that mothers who had been exposed to antibiotics before and especially during pregnancy had an increased risk of children being hospitalised for infections.Reference Miller, Wu, Pedersen, de Klerk, Olsen and Burgner49 Infections in turn are also associated with childhood overweight.Reference Li, Chen, Ferber and Odouli27, Reference Block, Bailey and Gillman50 Li et al.Reference Li, Chen, Ferber and Odouli27 conducted a large birth cohort study in which they found that infections not treated with antibiotics were more strongly associated with childhood obesity in a dose–response manner, compared with those who had not had infections. Antibiotic use was not associated with childhood obesity when compared with those who had had infections not treated with antibiotics. Just as infections may play a greater role in the onset of obesity than antibiotics, it is possible that maternal infections may also play a role in the risk of childhood overweight and obesity. Maternal infections are associated with childhood infectionsReference Blomstrom, Karlsson, Gardner, Jorgensen, Magnusson and Dalman28 and with childhood eczema and asthma,Reference Zhu, Zhang, Qu and Mu51 but studies are still needed that examine the relationship between maternal infections and childhood overweight. Block et al.Reference Block, Bailey and Gillman50 found that infections appeared to cause some confounding in the relationship between antibiotics and overweight but that there was still a small significant effect of antibiotics on overweight. The authors concluded, however, that the clinical significance of antibiotics in contributing to the development of overweight was negligible at the individual level. Although it is important that antibiotic use is curbed in order to prevent the increasing morbidity and mortality caused by antibiotic resistance,Reference Nicolini, Sperotto and Esposito52 antibiotic reduction may not be the most effective intervention for the purpose of decreasing childhood overweight and obesity.Reference Block, Bailey and Gillman50, Reference Partap, Allcock, Parker, Gurdasani, Young and Sandhu35
Strengths of this review include the extensive search and assessments of available studies, enabling a complete overview of the existing evidence concerning the relationship between both prenatal and infant antibiotic exposure and childhood overweight/obesity. Our review contributes to increased insight into what is currently known about this relationship and calls for further well-designed studies. Some limitations include the scarcity of studies on prenatal antibiotic exposure, heterogeneity of the included studies, missing information on intra-partum antibiotics, the use of self-reported data and the lack of adjustment for confounding by infections. Differences in health care policies with regard to prescribing antibiotics and in patients with regard to treatment adherence are further issues to consider when comparing the findings across studies. Although many more studies have been conducted on infant antibiotic administration and overweight/obesity, the fact that within the last 10 years one systematic review was published in 2017 and the other three in 2018 shows that this topic is just beginning to gain more attention and this will likely increase in the coming years.
Conclusions
There is some evidence for a relationship between prenatal and infant antibiotic exposure and the onset of childhood overweight/obesity. There was a scarcity of studies on prenatal antibiotic exposure and potential confounders were often not accounted for. More well-designed studies are needed that include data on intra-partum antibiotic exposure that address important potential confounders (including maternal and childhood infections) and that use similar study measures as previous studies to increase comparability. The effects of antibiotic exposure, especially evident in certain children (i.e. exposed to multiple and broad-spectrum antibiotics, earlier postnatal exposure and male gender) merit further research.
Acknowledgements
None.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S2040174419000722
Financial Support
The study was funded by Winclove Probiotics.
Conflicts of Interest
One coauthor IB is employed by Winclove Probiotics, which is funding the study. The other authors declare that they have no competing interests.




