Introduction
Depression is characterized by persistent low mood and associated cognitive, emotional and physical symptoms, such as low self-esteem, and disturbed sleep and appetite. Depression is the 4th leading cause of disability worldwide; each year 6% of adults experience an episode of depression, and over the course of a lifetime, 15–20% of the population will have at least one depressive episode.Reference Wang, Dwyer-Lindgren and Lofgren1 At a biological level, depression involves changes in monoamine neurotransmitters, hypothalamic–pituitary–adrenal (HPA) axis function, and immune system alterations; however the exact pathophysiology and etiology remains unclear.
A number of risk factors are associated with depression onset. These include: geneticReference Kendler, Gardner and Neale2 and personality risk factors;Reference Boyce, Parker and Barnett3 psychosocial factors such as unemployment and poverty;Reference Bruce4 and comorbidities with other diseases such as diabetes and cardiovascular disease.Reference Kilzieh, Rastam and Maziak5 Over the past decade it has become increasingly clear that environmental stressors during early life, such as childhood maltreatment, also increase risk of depression. Consistent findings from humans and animals support an association between early life stress and childhood and adult psychopathology, particularly depressive symptoms.Reference Kessler and Magee6–Reference Schilling, Aseltine and Gore11
A relevant new concept in this field, which has come to the forefront of the literature recently, is that risk factors for depression may present even earlier than previously thought: risk for depression may extend into the prenatal period. That is, a fetus that is exposed to a compromised intrauterine environment may be predisposed to develop depression in adulthood. For example, there have been consistent findings of associations between high levels of maternal prenatal stress, and adverse offspring outcomes,Reference O’Connor, Heron and Glover12–Reference Talge, Neal and Glover14 including depression.Reference Van den Bergh, Van Calster and Smits15 This raises the intriguing possibility that risk for depression may, in some cases, be determined before birth.
However, given this notion is in its infancy, there is currently little information about the nature of prenatal environmental adversity, timing of exposure, and mechanism by which prenatal environmental factors may increase risk for offspring depression. This review is a focused overview of the current state of the literature that provides evidence for associations between prenatal environmental exposures and offspring depression. We begin with a discussion of the role of low birth weight (LBW) in the prediction of adult health and disease, in line with the Barker hypothesis, before considering a number of key fetal exposures for depression in adulthood, including: maternal undernutrition, psychological distress and toxin exposure. We then discuss potential mechanisms by which the intrauterine environment may influence offspring depression risk, and the challenges of unravelling such mechanisms.
Developmental origins of health hypothesis
The recent impetus to study antenatal factors as markers of later health risk originated with a consideration of LBW. In 1989, Barker reported an association between LBW and cardiovascular disease and diabetes, such that infants born with LBW were more likely to develop cardiovascular disease and diabetes in adulthood.Reference Barker, Winter and Osmond16, Reference Barker17 Although the underlying cause of the LBW was unclear, it was evident that the infant’s growth within the womb had been compromised. This research highlighted the possibility that environmental factors within the womb may have implications for adult health and disease; until this time it was widely assumed that the intrauterine environment was protected from outside influences. From this research the ‘Barker hypothesis’, or ‘fetal programming hypothesis’, was formed, which proposes that the developing fetus adapts to maternal cues within the womb, and that this adaptation has the aim of ultimately increasing the chance of offspring survival. Recently, the fetal programming hypothesis has been expanded to explain in evolutionary terms how other environmental factors, such as maternal prenatal stress, may result in adverse offspring outcomes.Reference Talge, Neal and Glover14, Reference Glover18 It has been suggested that high levels of maternal stress during pregnancy may be as a result of environmental pressures such as competition for food or conflict with rival groups. As such, the fetus responds to maternal cues that the postnatal environment will be one of adversity, and adapts in a way that would be beneficial for survival in such a stressful environment. For example, the child may be more likely to be aggressive and have a rapidly distracted attention,Reference Talge, Neal and Glover14, Reference Glover18 which could benefit survival in such a high-stress environment.
Here, we consider the evidence from human studies in relation to the outcome of depression in adult life; in particular those studies assessing the hypothesis that LBW, maternal undernutrition, psychological distress and toxin exposure during pregnancy may predispose offspring to increased risk for depression.
Low birth weight and offspring depression
LBW is a crude indicator of intrauterine growth restriction, and is commonly associated with undernutrition in pregnancy. However, there are a number of problems with using LBW as a proxy for prenatal adversity, as the exact cause of the LBW cannot be determined. For example, LBW is not only associated with maternal undernutrition, but is also related to smokingReference Jaddoe, Troe and Hofman19–Reference Lieberman, Gremy and Lang21 and psychosocial stressReference Newton and Hunt22–Reference Bhagwanani, Seagraves and Dierker24 during pregnancy. Further, LBW is often not dissociated from small for gestational age (SGA), and it is likely that the two have different origins. Nonetheless, numerous early studies of development used LBW as a measure of sub-optimal intrauterine growth, and the results still offer useful insights into the effects of the fetal environment on later health outcomes.
Since the initial findings from Barker, a still-growing body of research has linked LBW to a variety of adult health outcomes, including: cardiovascular disease,Reference Barker, Winter and Osmond16, Reference Barker25, Reference Huxley, Owen and Whincup26 hypertension,Reference Barker, Bagby and Hanson27 and type 2 diabetes.Reference Whincup, Kaye and Owen28 The literature regarding the association between low LBW and adult depression however is conflicting, with evidence both forReference Thompson, Syddall and Rodin29–Reference Gudmundsson, Andersson and Gustafson35 and againstReference Osler, Nordentoft and Andersen36–Reference Vasiliadis, Gilman and Buka39 an association. A recent meta-analysis of 18 studies found a weak association between LBW and later depression/psychological stress (OR=1.15, 95% CI=1–1.32).Reference Wojcik, Lee and Colman40 However, this association attenuated after correcting for potential publication bias (OR=1.08, 95% CI=0.92–1.27), and also when the analysis was restricted to examining depression alone. Therefore, there is currently insufficient evidence to support the hypothesis that LBW is associated with later depression. Just one study has considered SGA, independently of LBW, as a risk for adult depression. Haavind et al.Reference Haavind, Bergin and Brubakk41 assessed 8519 adolescents, and similarly found that those who were SGA were no more likely to have depression or anxiety than controls. Considering that inconsistencies in the LBW literature could potentially be due to individual variation in underlying causes of LBW, it is possible that more accurate and concise measures of intrauterine growth restriction may yield more reliable findings.
Maternal undernutrition during pregnancy and offspring depression
The 1944 Dutch famine provided a unique opportunity to directly examine the impact of maternal undernutrition. This famine took place as a result of a transport embargo on food supplies imposed by the German occupying forces in the Netherlands during WWII. Official rations fell below 900 kcal/day by November 1944, and were as low as 500 kcal/day by April 1945. The dramatic effects of the famine on the course and outcome of pregnancy have been recorded,Reference Smith42, Reference Stein, Ravelli and Lumey43 and follow-up studies have documented long-term consequences among the offspring.Reference Stein, Kahn and Rundle44–Reference Roseboom, de Rooij and Painter47 In 2000, Brown et al. identified that risk of developing an affective disorder was significantly increased for those exposed to famine during the second and third trimesters, compared to an unexposed control group (OR=1.5, P<0.05). These effects were present in both men and women, and for unipolar and bipolar affective disorders.Reference Brown, van Os and Driessens48 Further findings from this cohort have reported increases specifically in depressive symptoms of prenatally exposed adults, compared with sibling and hospital controls (OR=1.27, P<0.05).Reference Stein, Pierik and Verrips49 However, it is important to consider that these adults were also exposed to high levels of prenatal stress, and it has been suggested that prenatal malnutrition and stress may exert effects on fetal neurodevelopment via similar mechanisms.Reference Monk, Georgieff and Osterholm50
It is also important to consider that famine during wartime is extreme, and perhaps not reflective of the undernutrition that many women around the world may experience during pregnancy today, which may be indexed by low body mass index or by a current eating disorder. Findings from the Avon longitudinal Study of Parents and Children (ALSPAC) cohort have linked eating disorders during pregnancy with an increased rate of emotional, conduct and hyperactivity disorders in childhoodReference Micali, Stahl and Treasure51 and with adolescent emotional disorders.Reference Micali, De Stavola and Ploubidis52 However, there is a distinct lack of data linking prenatal low BMI or eating disorders with offspring depression and the findings from the Dutch famine cohort require replication.
Maternal psychological distress during pregnancy and offspring depression
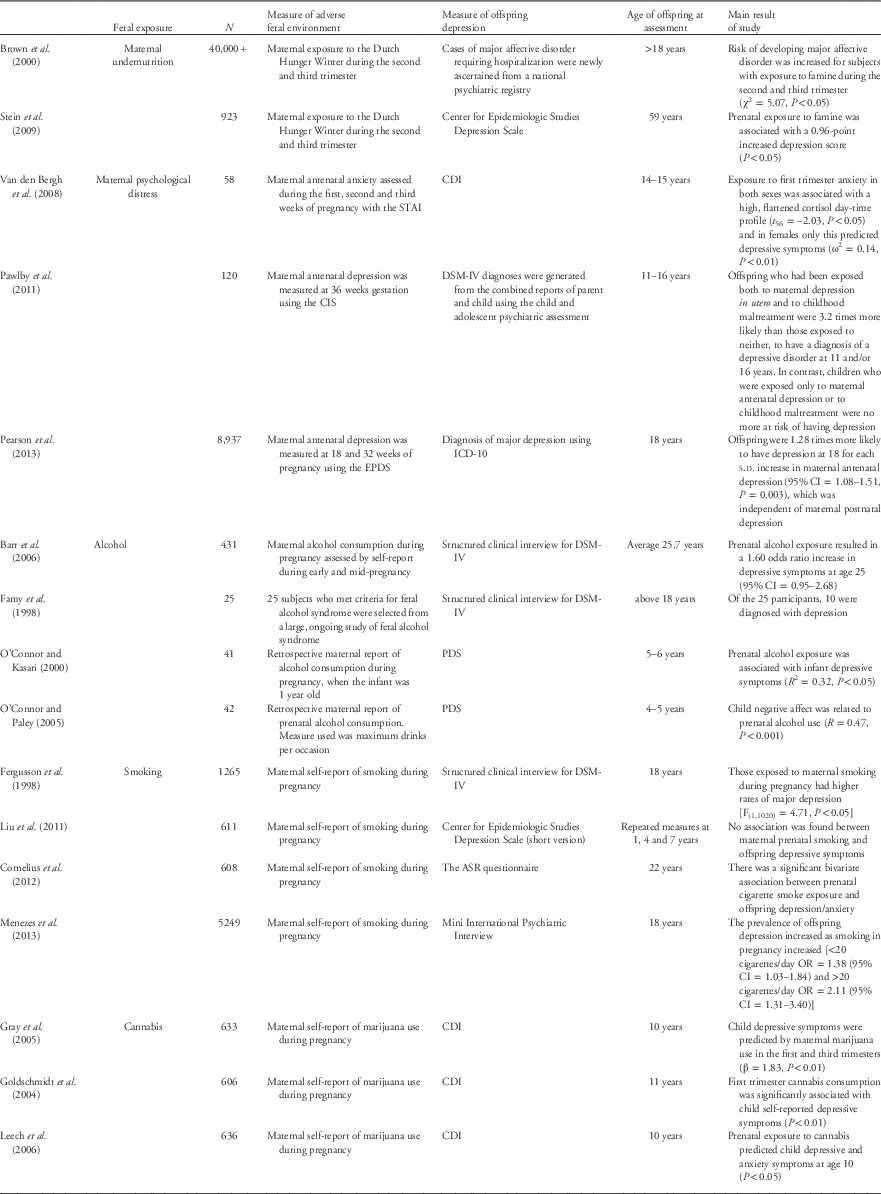
Many studies have shown that prenatal maternal psychological stress increases risk of child emotional and behavioral problems.Reference O’Connor, Heron and Glover12, Reference Talge, Neal and Glover14, Reference O’Connor, Heron and Golding53, Reference Field54 However, to date relatively few have followed offspring into adulthood, which is when the incidence of depression increases significantly. Three studies have reported offspring depression in the context of prenatal stress (see Table 1). A prospective observational study of 120 mother-offspring dyads found that adolescents aged 11–16 years were 4.4 times more likely to have a diagnosis of depressive disorder when exposed to antenatal depression and childhood maltreatment.Reference Pawlby, Hay and Sharp55 However, those offspring exposed only to antenatal depression or childhood maltreatment were no more at risk of having depression. Van den Bergh et al. investigated the effects of prenatal mood disturbance on adolescent depression (n=58). They found that 14 and 15-year-old adolescents exposed to prenatal anxiety had a high, flattened cortisol daytime profile (P<0.05) and in females only, this was associated with increased depressive symptoms (P<0.01).Reference Van den Bergh, Van Calster and Smits15 Similarly, in a larger study of over 4500 mother–infant dyads using data from the ALSPAC cohort, Pearson et al. also found that offspring were 1.28 times more likely to have depression at 18 years of age for each standard deviation increase in maternal antenatal depression (95% CI=1.08–1.51, P<0.01),Reference Pearson, Evans and Koundali56 and this remained significant when controlling for confounding variables. Thus, although there is some discrepancy, two of these three initial studies are suggestive of a significant and positive association between maternal psychological distress during pregnancy and offspring depression.
Table 1 Summary of studies which have investigated specific prenatal environmental exposures and offspring depressive symptoms

STAI, state trait anxiety inventory; CDI, children’s depression symptoms inventory; CIS, clinical interview schedule; EPDS, Edinburgh Postnatal Depression Scale; PDS, Pictoral Depression Scale; ASR, adult self-report.
Maternal exposure to environmental toxins during pregnancy
Alcohol
Fetal alcohol spectrum disorder (FASD) is an over-arching term used to describe the range of adverse effects that can occur in children of women who consume alcohol during pregnancy. Fetal alcohol syndrome is the most severe end of the spectrum, and can occur as a result of chronic consumption of alcohol during pregnancy.Reference Hellemans, Sliwowska and Verma57 FASD presents a number of central nervous system defects, including: diminished intellectual capacity, deficits in executive function, and a wide range of maladaptive and clinically significant behavioral characteristics.Reference Mattson, Crocker and Nguyen58 A number of studies have also found an association between FASD and depressive symptoms during both childhood and adulthood.Reference Famy, Streissguth and Unis59–Reference Barr, Bookstein and O’Malley62 However, these studies have a number of limitations, which should be considered. Famy et al. reported that adults with FASD suffer from mental illness, including depression, but this descriptive study is limited by a small sample (n=25) with no control group. Two studies by O’Connor et al. reported an association between maternal alcohol consumption during pregnancy and child depressive symptoms at age 4–6 years. However, these two studies again had very small sample sizes (n=41 and 42), and the statistical analysis of these studies was limited, for example no multivariate analyses were used. Just one larger cohort study with 400 mother–infant dyads has investigated this association; Barr et al. found no significant increase in depression risk following prenatal alcohol exposure. Therefore, there is currently insufficient evidence to support a causal relationship between prenatal alcohol exposure and offspring depression.
Smoking
There is more convincing evidence from the human literature to suggest that maternal smoking during pregnancy influences depressive symptoms. In a study of 611 children at age 7, those prenatally exposed to smoking had higher anger temperament scores than non-exposed controls, but no association was found between prenatal smoking exposure and other negative emotions, including depression and anxiety.Reference Liu, Gatsonis and Baylin63 However, this may be attributable to the young age of the children, and an inability to detect significant levels of psychopathology. Two large birth cohort studies and a large observational study have assessed older adolescents and adults, and have all reported findings of a positive association. Cornelius et al. found a significant association between prenatal nicotine exposure and self-reported depression/anxiety at age 22 years (n=608).Reference Cornelius, De Genna and Leech64 Similarly, in a sample of 1265 participants at age 18, Fergusson et al.Reference Fergusson, Woodward and Horwood65 reported that those exposed to prenatal nicotine had a higher incidence of major depressive disorder (P<0.05). Further, Menezes et al. analyzed data from a Brazilian cohort (n=5249, aged 18), and found that prevalence of offspring depression increased as smoking in pregnancy increased [<20 cigarettes/day OR=1.38 (95% CI=1.03–1.84) and >20 cigarettes/day OR=2.11 (95% CI=1.31–3.40)].Reference Menezes, Murray and Laszlo66 Thus, initial findings suggest a positive and significant association between prenatal nicotine exposure and depression in late adolescence/early adulthood.
Cannabis
Research into the consequences of prenatal cannabis exposure has focused primarily on infant cognitive and behavioral development, and evidence regarding mental health outcomes is limited. However, one longitudinal study has documented this at the age of 10 years (see Table 1). A regression analysis found that prenatal cannabis use significantly predicted child depressive symptoms (β=1.83, P<0.01).Reference Goldschmidt, Richardson and Cornelius67–Reference Leech, Larkby and Day69 Interestingly, Grey et al. expanded their analysis to assess the effect of trimester, and found that while first trimester cannabis exposure predicted offspring depressive symptoms at 10 years, effects in the second and third trimester appeared to be accounted for by first trimester exposure. Therefore, it is possible that the first trimester may be a ‘sensitive period’ for prenatal cannabis exposure to influence offspring depressive symptoms.
Fetal risk exposure and offspring depression: a summary
Few human studies have investigated the relationship between prenatal exposures and offspring depression, a summary is available in Table 1. These preliminary findings suggest that prenatal exposure to undernutrition, psychological distress and cannabis increase risk for offspring depression. However, it is important to note that the majority of measures of fetal exposures are based on retrospective maternal reports, which may be both unreliable due to reduced recall accuracy, and are also subject to reporter bias. It is also important to note that observational studies, such as those presented above, have a number of methodological limitations. For example, they are subject to residual confounding, which is the distortion that remains after controlling for cofounders in the study design, and some also use repeated measures, which results in autocorrelation of variables. The ideal design to understand whether these risk factors are causal, and to model the mechanisms through which they may affect risk for depression, would be to use randomized intervention trials. Clearly for most of these risk exposures, such as drug use or undernutrition, it is not possible to do this ethically, so we have to rely on human evidence from the best alternative study designs, such as longitudinal studies and natural experiments. However, our knowledge is also supplemented by studies in animals, where a wider range of study designs can be undertaken.
Many of the risk factors addressed in this review have been modeled in animals, and in the main, the findings have been congruent. For example, prenatal exposure to acute stress, alcohol and cannabis has been associated with offspring depression and anxiety-like behaviors.Reference Jaddoe, Troe and Hofman19, Reference Weinstock70–Reference O’Shea, McGregor and Mallet73 However, modeling prenatal risk factors in animals presents a number of challenges. Particularly, the methods used to induce stress, such as open field or elevated maze paradigms, have limited translational relevance to human antenatal mood disorder.
Outstanding questions remain regarding fetal risk exposure. For example, findings from Gray et al. suggest that the first trimester is a sensitive period for exposure to cannabis and the development of depressive symptoms. It is currently unclear, however, whether other fetal exposures also have a sensitive period of impact, and if so when that may be. It is also unclear whether the strength of exposure may impose its effect in a ‘dose-dependent’ manner. For example, it may be that chronic exposure to an adverse fetal exposure results in more severe offspring depressive symptoms than an acute exposure. It is not possible to currently address these questions directly in the human literature, as experimental study designs where the timing and intensity of prenatal risk are controlled are clearly unethical and unfeasible.
Pathways of risk transmission from the prenatal environment to offspring depression
There are a number of real challenges to understanding the pathways of risk from the prenatal environment to offspring depression. First, it is extremely difficult to disentangle the individual effects of shared genes between mother and infant. Second, the continuation of some prenatal environmental exposures into the postnatal period makes separation of pre and post-natal exposures difficult. Finally, research disaggregating the remaining biological mechanisms occurring in utero that mediate these effects is in its infancy.
In this section, we briefly consider the roles of genetic and ongoing postnatal environmental exposure. However, there is evidence that there is an effect of prenatal environmental risk exposure over and above the effect of shared genes and postnatal environment. Therefore, the main focus of this section is on potential intrauterine biological mechanisms that might mediate prenatal risk exposure. The majority of the research here has focused on programming of the fetal HPA axis; however we also consider the roles of the maternal immune and sympathetic nervous systems. It is also important to note that, in the main, this field of research has been conducted in the context of exposure to prenatal psychological distress.
Shared genetics
Depression has a genetic component;Reference Levinson74 from twin studies it is estimated that heritability of Major Depressive Disorder is 0.33 (95% CI=0.26–0.39).Reference Sullivan, Neale and Kendler75 A number of genotypes have been reported to increase risk or susceptibility to depression, such as the serotonin transporter polymorphism (5-HTTLPR)Reference Caspi, Sugden and Moffitt76 and a single nucleotide polymorphism in the brain-derived neurotrophic factor gene.Reference Ribeiro, Busnello and Cantor77 However, a number of replication attempts have failed to identify susceptibility genes convincingly. A more recent approach to identifying genetic variants associated with depression is via genome-wide association studies,Reference Hek, Demirkan and Lahti78–Reference Ripke and Wray81 however it has been estimated that a very large sample size upwards of 50,000 cases would be required to detect specific genes for depression.Reference Hek, Demirkan and Lahti78
Nonetheless, as depression has a genetic component it is logical to presume that shared risk genes between the mother and offspring may underlie the relationship between prenatal environmental exposure and offspring depression. Consequently, teasing apart the independent influences of genetic and environmental factors poses a significant challenge.
In a very elegant study, Rice et al. capitalized on the use of in vitro fertilization, to design a ‘prenatal cross-fostering’ study in which pregnant mothers were genetically related or unrelated to their child, in order to disentangle maternally inherited and environmental influences on offspring.Reference Rice, Harold and Boivin82 They examined 574 mother-related dyads and 205 mother-unrelated dyads, and found that associations between prenatal stress and offspring birth weight, gestational age and antisocial behavior were evident in both the mother-related (β=0.207, P<0.001) and mother-unrelated pairs (β=0.211, P<0.01), which is consistent with the idea that prenatal stress is an important environmental influence. However, different patterns emerged for associations between prenatal stress and other outcomes such as anxiety. Although this study did not examine offspring depression specifically, it does offer useful insights into the independent roles of genes and environment in moderating the effects of prenatal stress on offspring outcomes. This study highlights that, although shared genes are important in this relationship, there is more to the association than genetics alone can account for, and therefore the environment must play a significant role.
Continuation of environmental exposure
A further challenge is to dissociate the independent effects of the prenatal and postnatal environment. Often, adverse environmental influences are present during both the prenatal and postnatal period. For example, prenatal depression is the main risk factor for postnatal depression,Reference Leigh and Milgrom83 and women who smoke while pregnant are also likely to smoke postnatally. Further, there is also evidence from the animal literature to suggest that prenatal stress has implications for postnatal maternal behavior, which potentially increases risk for offspring depression.Reference Champagne and Meaney84 Thus, it is difficult to discern whether it is the prenatal or postnatal exposure, or both, which poses risk to offspring development. Another challenge is to understand whether prenatal and postnatal exposure may exert different influences on offspring development. To some extent this was addressed by Rice et al., who found that maternal postnatal stress increased risk for offspring anxiety, whereas prenatal stress presented risk for antisocial behavior.Reference Rice, Harold and Boivin82 One approach, which has been used to infer causality from prenatal risk factors, is Mendelian randomization; genetic variants known to be reliably associated with a modifiable exposure are used to make inferences about those exposures and disease risk.Reference Lewis, Relton and Zammit85 An alternative approach is via the use of large cohort studies, where it is possible to statistically partial out the effects of the postnatal environment in order to study independent prenatal effects. O’Connor et al. used this method in 2002. They found that maternal prenatal anxiety predicted infant emotional and behavioral problems in boys (OR=2.14, 95% CI=1.48–3.10) and girls (OR=1.88, 95% CI=1.30–2.69), which remained significant after controlling for postnatal anxiety.Reference O’Connor, Heron and Glover12 Pearson et al. took the same approach when analyzing data from the ALSPAC cohort, as discussed previously. They found that antenatal depression was a significant risk factor for offspring depression, independently of postnatal depression.Reference Pearson, Evans and Koundali56 Thus, the evidence points to an independent effect of the prenatal environment on offspring depression.
Intrauterine biological mechanisms
It is clear that prenatal maternal psychological distress may increase risk for offspring depression, independently of the postnatal environment and shared genetics. This indicates that biological mechanisms in utero mediate at least part of the association between prenatal distress and offspring depression. A number of potential mechanisms have been proposed to explain this association, including: increased maternal noradrenaline, which may cause vasoconstriction and reduce fetal blood flow, and immunological mechanisms, which increase maternal inflammation. Of course, it is likely that a combination of mechanisms play a role in this association; however there is a paucity of research here. The majority of the research to date has focused on the programming of maternal and infant HPA axis as the biological mechanism linking prenatal stress with adverse offspring outcomes, arguably because it is the most accessible system to measure.
HPA axis
The HPA axis forms a major part of the neuroendocrine system and has many biological roles, including regulation of stress responses. Acute stress initiates the release of corticotrophin-releasing hormone (CRH) from the hypothalalmus, which stimulates the anterior pituitary gland to release adrenocorticotrophic hormone (ACTH). This in turn initiates the release of glucocorticoid hormones (mainly cortisol) from the adrenal glands, which act to suppress the release of CRH and ACTH from the hypothalamus and anterior pituitary, via the activation of glucocorticoid receptors (GRs) in a negative feedback loop (see Fig. 1). Cortisol is the main stress hormone and has a number of biological effects in the body.

Fig. 1 Action of the HPA axis in response to a stressful stimulus. CRH, corticotrophin-releasing hormone; ACTH, adrenocorticotrophic hormone.
The HPA axis has been implicated in the etiology of depression: currently depressed individuals have over-active cortisol reactivity,Reference Owens, Herbert and Jones86, Reference Bhagwagar, Hafizi and Cowen87 as do individuals with high genetic risk for depression,Reference Mannie, Harmer and Cowen88, Reference Portella, Harmer and Flint89 and also those recovered from depression.Reference Bhagwagar, Hafizi and Cowen90 However, the evidence for dysregulation of the HPA axis as a marker of depression risk has not been entirely consistent.Reference Carnegie, Araya and Ben-Shlomo91–Reference Doane, Mineka and Zinbarg94 The effects appear to be small and may be restricted to more severe symptoms of depression, and it unclear whether this relationship is causal.
Van den Burgh et al. were the first to demonstrate the involvement of the HPA axis in the link between prenatal mood disturbance and offspring depression in humans. 14–15-year-old adolescents exposed to prenatal anxiety had a high, flattened cortisol daytime profile and, in females only, this was associated with increased depressive symptoms.Reference Van den Bergh, Van Calster and Smits15 In support, there is further evidence that increased maternal glucocorticoids during pregnancy alter infant HPA function. Davis et al.Reference Davis, Glynn and Waffarn95 found that elevated glucocorticoids during the second and third trimester predicted larger infant cortisol responses to the heel-stick procedure 24 h after birth. Similarly, Brennan et al. Reference Brennan, Pargas and Walker96 showed that prenatal anxiety and depression predicted baseline and mean cortisol levels at 6 months, and O’Connor et al. found that prenatal anxiety was associated with individual differences in awakening and afternoon cortisol levels in 10-year-old children.Reference O’Connor, Ben-Shlomo and Heron97 The animal literature also provides evidence that prenatal stress is associated with altered offspring HPA function. Rodents exposed to prenatal restraint stress demonstrated enhanced activity of the HPA system,Reference Abe, Hidaka and Kawagoe71 corticosterone release was prolonged following stress exposure, and central GR expression was reduced.Reference Maccari, Darnaudery and Morley-Fletcher98
Some preliminary findings suggest that the process by which prenatal stress may influence offspring HPA function is via epigenetic changes. Epigenetics describes changes in gene activity that is not caused by a change in the DNA sequence. Examples of epigenetic changes are DNA methylation and histone modification. These mechanisms can alter gene expression, and are processes by which the environment can influence phenotype.
As mentioned above, prenatal restraint stress is associated with reduced GR expression, and evidence suggests that downregulation of the GR is mediated by epigenetic regulation of the GR gene, NR3C1. Chronic stress has been shown to increase methylation of NR3C1, which subsequently downregulates the receptors’ expression. This results in an over-active stress response, as usually activation of the GR receptor initiates the negative feedback loop of the HPA axis, and ‘turns off’ cortisol release.Reference Witzmann, Turner and Meriaux99 Exposure to early postnatal stress also results in increased methylation of the NR3C1 gene and subsequent downregulation of its expression in the hippocampus of both rodentsReference Liu, Diorio and Tannenbaum100, Reference Weaver, Cervoni and Champagne101 and humans.Reference McGowan, Sasaki and D'Alessio102 Interestingly, Oberlander et al. found that newborns exposed prenatal depression also had increased methylation of the promoter region of the NR3C1 gene, and at 3 months this was associated with increased salivary cortisol response to an acute stressor.Reference Oberlander, Weinberg and Papsdorf103 Thus, the results of these initial studies suggest that exposure to maternal prenatal psychological distress causes increased methylation of the offspring NR3C1 gene, resulting in decreased hippocampal GR expression and reduced feed-back efficiency of the HPA system, leading to over-active and sustained stress responses.
The biological process linking raised maternal glucocorticoids with epigenetic alterations remain unclear, however recent research has begun to delineate this. Usually, maternal cortisol is metabolized at the placental barrier by the enzyme 11β-hydroxysteroid dehydrogenase-2 (11β-HSD-2). However, when maternal cortisol levels are high, such as in the case of prenatal psychological distress, 11β-HSD-2 is downregulated, and more active cortisol enters fetal circulation.Reference Mairesse, Lesage and Breton104–Reference O’Donnell, Bugge Jensen and Freeman106 Animal studies suggest that downregulation of 11β-HSD-2 is mediated by increased methylation of the 11β-HSD-2 gene promoter.Reference Jensen Pena, Monk and Champagne107 Thus, it appears that prenatal psychological distress is associated with offspring epigenetic alterations, which affect gene and protein expression in both the offspring brain and placenta. However, intracellular mechanisms linking fetal cortisol with epigenetic modifications remain unclear.
Sympathetic nervous system
Although this review has focused on the HPA axis mediating the link between prenatal psychological distress and offspring depression, it is likely that other mechanisms are also involved. Indeed, there appear to be rapid mechanisms which link maternal emotional state with fetal heart rate responses,Reference Monk, Myers and Sloan108 which cannot be explained by the relatively slow activation of the HPA axis.
As well as the HPA axis, psychological distress also activates the sympathetic nervous system, which results in the release of noradrenaline. Noradrenaline does not directly cross the placenta;Reference Giannakoulopoulos, Teixeira and Fisk109 however it is possible that its release could indirectly affect the fetus by initiating vasoconstriction and disrupting uterine blood flow. Human research in this field is limited, but a significant association between high anxiety during the third trimester and increased uterine artery resistance index has been demonstrated.Reference Teixeira, Fisk and Glover110 Further, animal research has shown that both acute stress exposure and intravenous infusions of noradrenaline induce decreased uterine blood flow.Reference Stevens and Lumbers111, Reference Shnider, Wright and Levinson112 Thus, sympathetic nervous system alterations could in part mediate the association between prenatal psychological distress and offspring depression.
Immune system
Antenatal stress has also been linked to offspring immune function in a number of animal studies.Reference Coe, Lubach and Karaszewski113–Reference Diz-Chaves, Astiz and Bellini118 Although these investigations have used a wide range of prenatal stress exposures and measures of offspring immune function, there is a consensus that prenatal stress is linked with compromised offspring immunity. Human studies have generally used indirect measures of infant immune function. For example, associations have been found between prenatal mood disturbance and infant asthmaReference Lefevre, Moreau and Semon119, Reference Khashan, Wicks and Dalman120 and infectious disease.Reference Nielsen, Hansen and Simonsen121 Further, an association has been found between the method of delivery, as an index of maternal stress, and lymphocyte subset cell counts,Reference Duijts, Bakker-Jonges and Labout122 and prenatal stress has also been linked to altered innate and adaptive immune responses in cord blood mononuclear cells.Reference Wright, Visness and Calatroni123
One study has directly examined prenatal anxiety and adaptive immune responses of infants at 2 and 6 months of age to a hepatitis B vaccine. O’Connor et al.Reference O’Connor, Winter and Hunn124 reported that prenatal anxiety predicted lower hepatitis B titers at 6 months of age, and altered responder cell frequencies to antigen application. Thus suggesting that prenatal anxiety changes the adaptive immunity of the infant. Further, it highlights the possibility that immune system alterations may be one mechanism by which prenatal psychological distress exerts influence on offspring development.
Pathways to transmission of risk: summary
It is difficult to dissociate the pathways that mediate the relationship between prenatal environmental exposures and offspring depression, and it is likely that both shared genetic factors between mother and infant and continuation of exposure from the pre- to postnatal period contribute. Nonetheless, it is also clear that intrauterine biological mechanisms account, at least in part, for this association. The majority of the research has focused on alterations of the infant HPA axis. However, it is likely that a number of systems are affected in a complex manner as a result of a compromised intrauterine environment.
Conclusions and future directions
In conclusion, a developing body of evidence suggests that depression may, in some cases, have a very early developmental origin. Although research to date is limited, it is suggestive of an association between fetal undernutrition, maternal psychological distress, cannabis exposure, and an increased risk of offspring depression. However, a number of key questions regarding this association remain unanswered. For example, it is currently unclear whether the intensity and timing of the exposure are significant outcome severity. Further, the full extent of biological mechanisms which may mediate this association are yet to be elucidated. More thorough and extensive longitudinal cohort studies, and intervention studies tackling depression and anxiety, are required to further characterize these associations, as well as detailed neurodevelopmental studies to fully understand the underlying mechanisms. Understanding these mechanisms is an extremely important step in targeting public health prenatal and postnatal interventions to best help ‘at risk’ mothers, and to potentially limit depression risk before birth.
Acknowledgements
None.
Financial Support
This research was funded by a UK Medical Research Council Studentship awarded to Miss Elizabeth Braithwaite (grant number MR/J500501/1). Dr Susannah E. Murphy is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme.
Conflicts of Interest
Dr Susannah E. Murphy has received consultancy payments from p1Vital and has participated in paid speaking engagements for Lilly UK.