Introduction
Endocrine-disrupting chemicals (EDCs) are exogenous compounds that can disrupt the morphology and function of endocrine and reproductive systems of animals and humans.Reference Fowler, Bellingham and Sinclair1 Many EDCs have the potential to mimic, antagonize, or change the levels of endogenous hormones, such as androgens and estrogens.Reference Frye, Bo and Calamandrei2 17α-Ethinylestradiol (EE2), an example of endocrine disruptor, is a synthetic estrogen that makes up most contraceptive drugs. Moreover, this compound can be found in the environment, especially in water, in the ground, and in food.Reference Nozawa, Nagaoka and Zhang3
The prenatal, neonatal, and pubertal periods can be severely altered by EDCs because during these phases, changes in the serum levels of steroid hormones may impact the development of female reproductive organs.Reference Ryan, Hotchkiss, Crofton and Gray4 In rodents, the oocyte development occurs in the last half of fetal life and in the beginning of postnatal life by the rupture of oocyte nests and the formation of primordial follicles. During these phases, estrogens play an important role in the regulation of proliferation and apoptosis of follicle nests and stroma cells.Reference Kezele and Skinner5 Furthermore, during the reproductive life, these hormones are essential for ovary hormonal response and folliculogenesis by promoting proliferation of theca and granulosa cells and maturation of follicle.Reference Drummond and Fuller6
On the other hand, exposure to EE2 and other EDCs during developmental periods may impair the reproductive function of rodents by promoting changes in expression of the luteinizing hormone receptor gene, irregularities in the estrous cycle,Reference Nozawa, Nagaoka and Zhang3 changes in ovarian follicle development,Reference Zhang, Nagaoka and Usuda7 and the hormonal profile of rodents.Reference Zhang, Taya, Nagaoka, Yoshida and Watanabe8
Organ senescence is associated with several diseases, including cancer, diabetes, obesity, and cardiovascular disease. In the case of ovaries, aging is characterized by gradual declines in ovarian follicle number and function, which leads to infertility. The aging process is affected by numerous factors, including lifestyle and drugs, as well as environmental and genetic factors.Reference Zhang, Chen and Du9 Thus, it is important to understand how EDCs, like EE2, may alter ovarian development and the consequences of these changes during aging.
Mongolian gerbils (Meriones unguiculatus) are rodents used as experimental models to study changes in aging, including in reproductive organs, such as the ovary.Reference Vincent, Rodrick and Sodeman10 In these animals, exposure to low doses of EE2 during prenatal or pubertal periods promotes morphological and functional changes in the senile male and female prostate.Reference Perez, Biancardi and Caires11,Reference Perez, Biancardi and Caires12 Other EDCs, such as quinestrol and levonorgestrel, promote changes in hormonal levels and receptor and protein expression in those animals.Reference Lv and Shi13,Reference Su, Chen, Qin, Wang, Wang and Liu14
However, the effects of exposure to EE2, during prenatal and pubertal periods, in reproductive function and ovary morphology of old gerbils remain unclear. On these bases, this study was developed to evaluate the effects of exposure to low doses of 17α-ethinylestradiol in prenatal and/or pubertal periods in ovary morphology, follicle availability, and hormonal levels in 12-month-old female gerbils.
Materials and methods
Animals and experimental design
The female gerbils (Meriones unguiculatus) used in this study were maintained in a vivarium in São Paulo State University (UNESP) (São José do Rio Preto) inside polyethylene cages under controlled conditions of temperature (23°C) and light (12 hours dark/12 hours light). They were provided filtered water in glass bottles, and rodent food ad libitum composed by 23% of protein, 12% of minerals, 5% of fiber, and 4% of total lipids (Nestle Purina PetCare, St. Louis, MO). Animal handling and experiments were in accordance with ethical principles of animal research and were approved by the Committee for Animal Research (protocol nº 020/09 CEEA) of UNESP.
Four nulliparous adult female gerbils (90–120 days) were maintained with a male of the same age for the formation of different families and separated into four different groups. Mating was confirmed by the presence of spermatozoids in the vaginal smear (the day 0 of pregnancy).
As shown in Fig. 1, in the EE/PRE group, four pregnant females received 15 µg/kg/day of 17α-ethinylestradiol (Sigma, St. Louis, MO, USA) diluted in the total volume of 100 µl Nujol® mineral oil (CAS 8020-83-5; Sigma-Aldrich) by gavage. The dose of EE2 used in the experiment is similar to the dose found in the contraceptive pills.Reference Thayer, Ruhlen and Howdeshell15 Pregnant females in the EE/PRE groups received EE2 from the 18th to the 22nd day, taking into account that the gestational time of the gerbil is 26 days. The treatment was performed in a window of susceptibility of follicle development, considering that primordial follicle formation occurs during late fetal and early postnatal periods in rodents.Reference Zhang, Nagaoka and Usuda7 Then, four pups of the females treated during the gestational period formed the EE/PRE group.

Fig. 1. Schematic representation of the experimental design. Pregnant females received 15 µg/kg/day of 17α-ethinylestradiol (EE2) from the 18th to the 22nd gestational day. The pups of these females composed the prenatal group (EE/PRE). The pubertal group (EE/PUB) was composed of gerbils that received 15 µg/kg/day of EE2 between the 42nd and 49th days of postnatal life. In the prenatal and pubertal group (EE/PRE-PUB), the pups of treated pregnant females received the same dose of EE2 in the pubertal period. Gerbils in the control group were not exposed to EE. In all the groups, the gerbils (n = 4) were killed at 12 months (360 days) of age.
In the EE/PUB group, 42-days-old female gerbils received by gavage the same dose of EE2 for 1 week during the puberty period.Reference Pinto-Fochi, Negrin, Scarano, Taboga and Góes16,Reference Siegford, Hadi Mansouri and Ulibarri17 The EE/PRE-PUB group was formed by four pups of pregnant female treated with EE2 during the gestation and received the same dose of EE2 during the pubertal period. In the control group, the females did not receive any treatment. During the experimental procedure, we did not utilize vehicle controls because previous studies by our group had shown no significance between the vehicle group and intact animals.Reference Santos, Falleiros-Júnior, Corradi, Vilamaior and Taboga18–Reference Scarano, Vilamaior and Taboga20
The 12-month-old animals were euthanized by anesthesia in CO2 followed by decapitation. After death, the females were weighed and ovaries were removed and also weighed. Before euthanasia, all females were cycled, and at the time of euthanasia all were in the proestrus phase of the estrous cycle, as described in previous studies.Reference Nishino and Totsukawa21
The normal estrous cycle of Mongolian gerbils was 4–6 days long. However, several irregularities could be found in the late phases of the cycle, such as pseudopregnancy. The proestrus phase, the beginning of the estrous cycle, has a more constant duration and few variations in serum levels of estrogen and progesterone when compared with the other phases.Reference Nishino and Totsukawa21,Reference Fochi, Perez and Bianchi22 Thus, the cycling of animals in this phase facilitates the standardization of the evaluation of tissues highly dependent on the levels of sex hormones, such as the ovary.
Serum hormone levels
The blood of 12-month-old female gerbils from experimental groups was collected at decapitation. The blood was centrifuged (3000 rpm for 20 min) and stored at −80°C. The serum hormonal levels were determined in duplicate by Enzyme-Linked Immunosorbent Assay (ELISA). In this study, high sensitivity kits (Testosterone ELISA kit No. 582701 and 17β-estradiol ELISA kit No. 501890, Cayman Chemical Company, MI, USA) following the instructions of manufacturers were used. The sensitivity was 20 pg/ml for estradiol and 6 pg/ml for testosterone. The limit of detection for estradiol was 6 pg/ml. The readings were performed using SpectraMax Plus 384, at 405 nm (Molecular Devices, Sunnyvale, CA, USA).
Morphological and morphometric analysis
Both ovaries of the experimental groups were fixed in methacarn in a 1:3:6 proportion of acetic acid, chloroform, and methanol and subjected to paraffin (Histosec, Merk) inclusion. Both gonads of each animal (n = 4) were sectioned (5µm) using a semiautomatic rotating microtome (RM2245, Leica Wetzlar, Germany) and were subjected to Hematoxylin-Eosin (HE),Reference Feldman and Wolfe23 Periodic Acid Schiff (PAS),Reference Kligman and Mescon24 and Gomori’s trichrome (GT)Reference Gomori25 for morphological, morphometrical, and histopathological analysis.
The images for this analysis were scanned using ScanScope® AT Turbo (Leica Wetzlar, Germany) and analyzed using Aperio ImageScope® Software version 12.4.05.043 (Leica Wetzlar, Germany). To aid in histological analysis, an optical microscope (Leica Byosystems DM750 Wetzlar, Germany) was also used. The morphometric analysis of the thickness of epithelium and the tunica albuginea was performed using Software Image-Pro Plus version 6.0 for Windows (Media Cybernetics, Inc., Silver Spring, MA).
The number of ovarian follicles was quantified, in HE staining, according to maturation stage, and classified as primordial, initial primary, late primary, secondary, and mature.Reference Luo, Huang, Fu, Xu and Qian26,Reference Takagi, Yamada, Miki, Umegaki, Nishimura and Sasaki27 Only follicles with evident oocytes were considered upon visualization of the nucleus or cytoplasmic delimitation.
The number of atretic follicles in PAS staining was also performed. The atretic follicle was identified by overall shrinkage, oocyte degeneration, and thickening of the basement membrane.Reference Saidapur and Kamath28,Reference Wang, Liu and Tian29 Atretic follicles were easily identified in PAS staining considering the thickening of the basement membrane.
The quantification of the number of corpus luteum, in HE staining, was also performed.Reference Luo, Huang, Fu, Xu and Qian26 The quantification of the area occupied by lutein cells in ovarian tissue was also analyzed by stereological multipurpose graticulate with 130 points and 10 test lines.Reference Weibel30 Quantification was performed in 400× magnification, with all ovarian tissue evaluated in the process. Both ovaries of all the animals (n = 4) were included in the counting and analyzed in duplicate.
The interstitial gland is a poorly characterized structure in ovarian morphology, presenting cells with clear cytoplasm and intracytoplasmic granules in HE staining.Reference Díaz-Hernández, Caldelas, Montaño and Merchant-Larios31 The morphological analysis of the interstitial gland included the comparison between HE, PAS, and GT stains to better clarify the components of the structure. The morphometrical analysis was done using the Weibel’s method. As in the morphometry of the interstitial gland, lipofuscin was quantified by Weibel’s method after the morphological identification in HE of cells with lipofuscin granules.Reference Angelousi, Szarek, Shram, Kebebew, Quezado and Stratakis32
The thickness of the ovarian epithelium was measured between the interface of the apical region of the epithelium and the basal membrane. The thickness of the tunica albuginea was measured between the end of the ovarian epithelium and the end of the dense irregular connective tissue. The analysis was carried out using methods previously described,Reference Perez, Biancardi and Caires12 being used 10 microscopic 400× magnification fields per animal and 10 measurements per field, with a total of 400 measurements per experimental group. The measurement fields of the ovarian epithelium and the tunica albuginea were randomly selected. Different representative spots were considered, excluding areas with cystic degenerationReference Lunde, Hoel and Sandvik33 or histological artifacts.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism software version 5.00 (Inc., La Jolla, CA, USA). The morphological and morphometric data obtained in this study were subjected to parametrical tests, including analysis of variance (ANOVA), followed by multiple comparison between groups by the Tukey test. The hormonal data were subjected to nonparametric Kruskal-Wallis and Dunn comparative tests. The level of significance adopted was P < 0.05, expressed as mean and standard deviation.
Results
Exposure to EE2 did not change biometric parameters
The body and ovary weight and relative weight of the ovary are presented in Table 1. EE/PUB and EE/PRE groups present heavier ovaries than the control and EE/PRE-PUB groups; however, no significant statistical difference was found between the biometric data in experimental groups.
Table 1. Biometric data of 12-month-old female gerbils from experimental groups

The data are expressed as mean ± standard deviation (n = 4).
Higher levels of serum hormones were found in treated groups, especially in EE/PUB
The serum levels of estradiol and testosterone are shown in Table 2. The 12-month-old females of the EE/PRE and EE/PUB groups showed a significant increase in estradiol levels when compared to the control group. As for testosterone levels, only females in EE/PUB group showed a significant increase when compared to the control group. No significant difference was found when comparing the treated groups with each other.
Table 2. Serological data of 12-month-old female gerbils from experimental groups

The data are expressed as mean ± standard deviation. n = 4.
Significant difference between the groups was highlighted by superscript letters (a,b).
*P < 0.05, **P < 0.01.
Exposure to EE2 alters folliculogenesis, especially during pubertal exposure
No evident morphological changes were observed in the follicles of the experimental groups. The ovarian tissue of most animals showed a low density of viable follicles, with the presence of ovarian stroma being predominant. The morphology of viable and atretic follicles was shown in Fig. 2.

Fig. 2. Histological sections of follicles stained with Hematoxylin and Eosin (HE) (A, B, C) and Period Acid Schiff (PAS) (D). The images highlight the morphology of the primordial and primary unilaminar follicles (A), primary multilaminar follicles (B), secondary follicle (C), and atretic follicles (D). Yellow circles: Primordial follicles. White arrow: Primary unilaminar follicle. Black arrows: Atretic follicles. Scale bar of A, B, and D: 50μm. Scale bar of C: 100 μm.
The data of the follicle count can be seen in Table 3. A significant increase in primordial follicle count was found in the EE/PUB and EE/PRE-PUB groups when compared to the control and EE/PRE groups. There was no statistical difference between primordial follicle count in EE/PUB and EE/PRE-PUB groups.
Table 3. Morphometric data of 12-month-old female gerbils from experimental groups

The data are expressed as mean ± standard deviation. n = 4.
Significant difference between the groups was highlighted by superscript letters (a,b,c,d). *P < 0.05, **P < 0.01, ***P < 0.001.
Considering the count of primary unilaminar follicles, a significant reduction was showed in EE/PRE group when compared to EE/PRE-PUB, control, and EE/PUB. In the analysis of primary multilaminar follicles, all treated groups showed a significant reduction in count when compared to the control group. The greatest reduction was observed in the EE/PRE group. There was no statistical difference between the treated groups.
As for secondary follicles, most animals did not have a large amount of this group of follicles. A significant reduction of secondary follicle count was showed in EE/PUB when compared to control, EE-PRE and EE/PRE-PUB groups. Finally, only the EE/PUB and EE/PRE-PUB groups had mature follicles in some of the animals. There was no statistically significant difference between groups.
Exposure to EE2 in the prenatal period increases the number of corpus luteum in 12-month-old gerbils
There were no changes in the morphology of the cells that make up the corpus luteum. However, as seen in Table 3, 12-month-old females exposed to EE2 during the prenatal period had a greater amount of corpus luteum compared to the control, EE/PUB group, and EE/PRE-PUB group. EE/PRE was the group with the largest number of corpus luteum, followed by control, EE/PRE-PUB, and EE/PUB. In addition, considering the morphometry of this structure, the EE/PRE group also had the largest relative proportion lutein cells in the ovary, with a significant increase when compared to other experimental groups. Finally, the proportion of lutein cells in ovaries in the EE/PUB group was significantly reduced when compared to the control group and also to the EE/PRE group.
Exposure to EE2 did not change the number of atretic follicles
Table 3 shows the data related to quantification of atretic follicles. The atretic follicles were easily identified by strong reactivity to PAS as shown in Fig. 2. No significant changes were observed in the morphology and quantification of atretic follicles in the treated groups when compared to the control group.
Exposure to EE2 during puberty increases the frequency of interstitial gland cells in ovary of 12-month-old gerbils
The cells of the interstitial gland presented a clear cytoplasm with eosinophilic granules in HE staining (Fig. 3a, 3d, 3g, 3j). Poor reactivity was observed in PAS staining (Fig. 3b, 3e, 3h, 3k), with a predominance of the reactivity in cytoplasmic granules and cell membrane. Some of the cells have more than one evident nucleus. Lipid droplets were also evident in interstitial gland cells and could be identified by circular empty spaces (Fig. 3).

Fig. 3. Histological sections of interstitial gland and lipofuscin stained with Hematoxylin and Eosin (HE) (A, D, G, J), Period Acid Schiff (PAS) (B, E, H, K), and Gomori’s Trichome (GT) (C, F, I, L). Lines 1, 2, 3 and 4 represent, respectively, the Control, EE/PRE, EE/PUB, and EE/PRE-PUB groups. The histological sections highlight the interstitial gland composed by cells with clear cytoplasm with intracytoplasmic granules and their arrangements in strings (more evident in Control group) or nests (more evident in EE/PUB group), permeated by fibrous septa. Lipid droplets (red arrows) can be seen in interstitial gland cells. Lipofuscin (yellow dashed) can be identified by brownish material in HE stain, PAS reactivity and dark green to brown in GT stain. Scale bar: 50 μm.
In all groups, the cells of the interstitial gland were arranged in strings (Fig. 3a–3c) or nests (Fig. 3g–3i). In the control and EE/PRE groups, the cells of the interstitial gland were located predominantly below the ovarian epithelium (Fig. 3a–3f). On the other hand, in the EE/PUB and EE/PRE-PUB groups, a higher number of interstitial gland cells were noticed, occupying more extensive areas in the ovarian cortex. In these groups, in addition to the increasing number of cells, hypertrophy of interstitial cells and more evident cytoplasmic granules were also observed (Fig. 3g–3l). The glandular formations are surrounded by fibrous tissue and permeated by septa of connective tissue, which are better evidenced in the GT staining.
The morphometry data of the interstitial gland are shown in Table 3. All treated groups had a higher percentage of interstitial cells, with statistical difference in the EE/PUB and EE/PRE-PUB groups when compared to the control group. The highest percentage was observed in the EE/PUB group, followed by EE/PRE-PUB, EE/PRE, and control groups. The groups treated during puberty also showed a statistical difference when compared to the EE/PRE group, and the highest percentage in the EE/PUB group was also significant when compared to the EE/PRE-PUB group.
Exposure to EE2 during puberty increases the deposits of lipofuscin in ovaries of 12-month-old gerbils
All experimental groups showed deposits of lipofuscin along the ovarian tissue. The pigment was identified by HE staining through deposits of brownish material in the cytoplasm (Fig. 3a, 3d, 3g, 3j). Lipofuscin also showed PAS reactivity with variable intensity along the section (Fig. 3b, 3e, 3h, 3k). In GT staining, the identification of the deposited material is difficult, but it can be seen in shades that varied from dark green to brown (Fig. 3c, 3f, 3i, 3l). The deposits of this pigment occurred mainly in interstitial gland cells.
As seen in Table 3, a greater area occupied by cells with lipofuscin deposit was observed in the EE/PUB and EE/PRE-PUB groups when compared to the control and EE/PRE groups. There was no difference between the EE/PRE group and the control group or between the EE/PUB group and the EE/PRE-PUB group.
Exposure to EE2 in EE/PRE-PUB group increases the thickness of ovarian epithelium of 12-month-old gerbils
As seen in Fig. 4, the ovarian epithelium of 12-month-old female gerbils often varied from simple squamous epithelium (Fig. 4Aa–4d) to simple cuboidal (Fig. 4e, 4f). In the case of the EE/PRE-PUB group, areas of simple columnar epithelium were also observed (Fig. 4g, 4h).
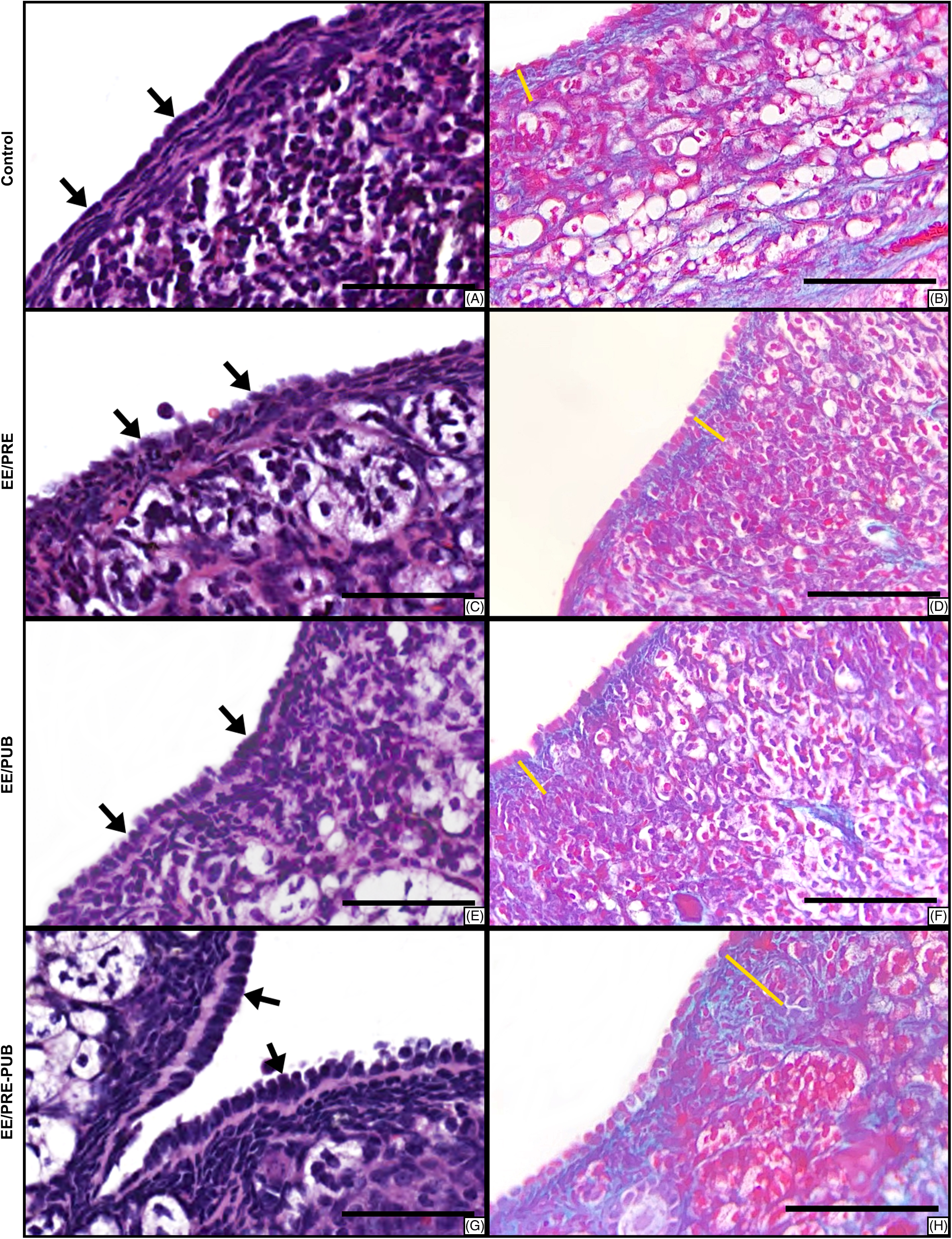
Fig. 4. Histological sections of ovarian superficial epithelium and tunica albuginea stained with Hematoxylin and Eosin (HE) (A, C, E, G) and Gomori’s Trichrome (GT) (B, D, F, H). Ovarian epithelium (black arrows) varied between simple squamous epithelium (Control and EE/PRE groups), simple cuboidal (EE/PUB group), and simple columnar (EE/PRE-PUB). The thickness of tunica albuginea (yellow line) was significant higher in treated groups. Scale bar: 50 μm.
The data regarding the effect of exposure to 17α-ethinylestradiol on the ovarian epithelium are shown in Table 3. The higher epithelial thickness was found in the EE/PRE-PUB group, followed by the EE/PRE, EE/PUB, and control groups. The ovaries of females in the EE/PRE-PUB group showed a statistically significant increase in epithelial thickness when compared to the control, EE/PRE, and EE/PUB groups. There was no difference between EE/PRE, EE/PUB, and control groups.
Exposure to EE2 increases the thickness of tunica albuginea of the ovary of 12-month-old gerbils
In all experimental groups, the tunica albuginea was formed by dense irregular connective tissue, which showed individual nonsystematic variation in cellularity and deposit of collagen fibers. The Gomori’s trichrome stain highlights the collagen composition of tunica albuginea with a green color (Fig. 4b, 4d, 4f, 4h).
In the statistical analysis, there was a significant increase in the thickness of tunica albuginea between the ovaries of all females in the experimental groups. As seen in Table 3, the thickest tunica albuginea was found in the EE/PRE-PUB group, followed by the EE/PUB, EE/PRE, and control groups.
Discussion
The senescence period is marked by a substantial reduction in ovarian follicles, especially mature follicles.Reference Atkins, Willson and Silverstein34,Reference Nichols, Bavister, Brenner, Didier, Harrison and Kubisch35 Considering the importance of folliculogenesis and the maintenance of follicle levels for female fertility, studies have been developed to identify protective and aggressive factors in this process.Reference Zhang, Nagaoka and Usuda7,Reference Zhang, Taya, Nagaoka, Yoshida and Watanabe8
Exposure to EDCs in critical periods of development alters the hypothalamic-pituitary-gonadal (HPG) axis and changes gonadal hormonal production.Reference Xi, Lee and Yeung36 We found that exposure to EE2 during puberty increases the levels of testosterone and estradiol. These changes may occur due to an imbalance in regulatory feedback mechanism of HPG axis.Reference Zhang, Taya, Nagaoka, Yoshida and Watanabe8,Reference Perez, Biancardi and Caires12 Furthermore, exposure during prenatal period also increases the levels of estradiol. It is possible that the exposure during developmental periods promotes epigenetic changes in critical signaling pathways, such as kisspeptin expression.Reference Zhang, Taya, Nagaoka, Yoshida and Watanabe8
In addition, previous studies showed that early life exposure to endocrine-disrupting chemicals may lead to morphophysiological and epigenetic changes in later life.Reference Brehm, Rattan, Gao and Flaws37–Reference Dávila-Esqueda, Jiménez-Capdeville and Delgado39 Micro-RNA-224 (miRNA-224) is an epigenetic factor that regulates the expression of steroidogenic genes, as CYP19A1, and acts in folliculogenesis. Prenatal exposure to EDCs could elevate the expression of miRNA-224 and lead to ovarian pathological conditions.Reference Lite, Ahmed, Santosh and Seetharaman40 Thus, it is suggested that the prenatal and/or pubertal exposure to EE2 can lead to long-term pathologic effects by epigenetic changes. In this case, new studies should be developed to understand the epigenetic changes that lead to folliculogenesis deregulation showed in our study.
This study demonstrated that exposure to low doses of EE2 in prenatal and/or pubertal periods promoted changes in folliculogenesis in mature gerbils. The treated groups showed a higher primordial follicle count, especially in mature gerbils that received treatment during puberty. On the other hand, the pre-antral follicle (primary and secondary) count was reduced in these groups.
Ovarian folliculogenesis is influenced by an interaction between granulosa cells, theca cells, and hormones.Reference Eppig, Pendola, Wigglesworth and Pendola41 The role of estrogen in folliculogenesis is still under study, but it is known that 17β-estradiol stimulates follicular growth and mitosis of granulosa cells, in addition to playing a role in the maturation of antral follicles and in ovulation.Reference Bendell and Dorrington42–Reference Emmen, Couse, Elmore, Yates, Kissling and Korach44 On the other hand, it has been shown that exposure to high levels of ethinylestradiol can alter the follicle morphology, with formation of follicular cysts and follicles with several oocytes.Reference Zhang, Taya, Nagaoka, Yoshida and Watanabe8,Reference Tarumi, Itoh and Suzuki45,Reference Sotomayor-Zárate, Dorfman, Paredes and Lara46
Primordial follicles are formed in the latter half of the fetal life and in the neonatal period in mice, while folliculogenesis occurs throughout puberty and sexual life. The formation of primordial follicles consists of breaking the germ cell nests and depends on apoptosis for their correct formation.Reference Zhang, Chen and Du9,Reference Sawyer, Smith, Heath, Juengel, Wakefield and McNatty47,Reference Pepling and Spradling48 In neonatal period, exposure to high levels of estrogen reduces the process of apoptosis, impairing the formation of normal primordial follicles.Reference Zhang, Chen and Du9,Reference Chen, Jefferson, Newbold, Padilla-Banks and Pepling49
In this study, the mature gerbils treated with low doses of EE2 in prenatal period did not present significant changes in the number of primordial follicles, suggesting that changes in folliculogenesis could be more evident in cases of early neonatal exposure or in the administration of high doses of estrogen as reported by Zhang and colaborators.Reference Zhang, Nagaoka and Usuda7 Thus, it is suggested that exposure to low doses of EE2 in prenatal period did not affect the mechanism of formation of these follicles.
In addition, the quantity of primordial follicles was higher in mature gerbils treated during puberty. These results could reflect the decrease in follicular activation after pubertal exposure. Some studies have shown that estrogen compounds promote follicle activationReference Rodríguez, Santambrosio, Santamaría, Muñoz-de-Toro and Luque50,Reference Wang and Roy51 and others showed the opposite effect.Reference Kezele and Skinner5,Reference Karavan and Pepling52 These different results may be due to species differences and different mechanisms of action of exogenous estrogen compounds. In this study, it was suggested that exposure to low doses of EE2 during puberty could reduce follicle activation with higher count of primordial follicles in mature gerbils.
Folliculogenesis can be divided into pre-antral – culminating in the formation of the secondary follicle – and antral, forming the mature follicle. Estradiol plays an important role in antral folliculogenesis and ovulation; however, its role in preantral folliculogenesis is still not defined.Reference Edson, Nagaraja and Matzuk53 Previous studies showed that exposure to low doses of EE in the prenatal period promoted an increase in the number of primary follicles in other periods of development, suggesting that the compound promotes changes in folliculogenesis.Reference Patel, Brehm, Gao, Rattan, Ziv-Gal and Flaws54
In this study, the reduction of preantral follicles in the mature animals exposed to EE2 in prenatal period and at puberty suggested that low doses of EE2 may promote changes in preantral folliculogenesis. Thus, it is suggested that the exposure to contraceptives in developmental periods, such as prenatal and puberty, may affect ovarian function in old age.
In addition to changes in folliculogenesis, exposure to low doses of ethinylestradiol in the prenatal period promoted an increase in the quantification and morphometry of the corpus luteum in mature gerbils, while higher serological levels of estradiol are also being observed in these animals. On the other hand, exposure to EE2 during the puberty period determined a reduction in the corpus luteum morphometry in mature gerbils, also accompanied by higher serological levels of estradiol.
Previous studies have already demonstrated the influence of ethinylestradiol on the stages of estrous cycle of female rodents, with delay or late onset being observed in females exposed to certain concentrations of exogenous estradiol analogs.Reference Shirota, Kawashima, Nakamura, Kamiie, Shirota and Yoshida55
Among the endocrine mechanisms involved in the development and maintenance of the corpus luteum, the elevated plasma estrogen concentration from the preovulatory follicle stands out as a preponderant factor, as it determines a preovulatory peak of LH and, consequently, ovulation and luteinization. Likewise, estradiol is also related to the degeneration of the structure of the corpus luteum, since they are related to the secretion of PGF2-a, a substance responsible for, among other effects, decreasing blood flow to the corpus luteum and degeneration of its capillaries.Reference Lv and Shi13,Reference Shirota, Kawashima, Nakamura, Kamiie, Shirota and Yoshida55,Reference Silvia, Lewis, McCracken, Thatcher and Wilson56
In gerbils, females that have not copulated present a rapid involution of the corpus luteum within 3 to 6 days after their formation. However, remaining luteal cells may be present for longer periods of time.Reference Fischer and Fisher57,Reference Meckley and Ginther58 Considering the short estrous cycle of gerbils, the cross-sectional analysis of the corpus luteum may reflect the presence of newly formed corpus luteum and corpus luteum remaining from previous cycles.
Thus, it is possible to assume that exposure to EE2 during the prenatal period may contribute to a way of favoring the luteinization phase and the formation of the corpus luteum in mature animals. It is also possible that this exposure disrupts the normal estrous cycle of gerbils, as demonstrated in other rodents,Reference Shirota, Kawashima, Nakamura, Kamiie, Shirota and Yoshida55 favoring the slower evolution of the corpus luteum. In addition, there was a proliferation of other ovarian structures in 12-month-old gerbils exposed to EE2 during puberty, such as the interstitial gland, which could justify the significant reduction in the morphometry of the corpus luteum in this group, not accompanied by a significant reduction in the quantification of these structures.
In addition to the above, this study did not reveal changes in follicular atresia in 12-month-old female gerbils exposed to EE2 during the prenatal period and/or puberty. Previous studies have shown that exposure to exogenous estrogens promoted an increase in follicular atresia in a dose-dependent manner.Reference Kaptaner and Unal59–Reference Talsness, Grote and Kuriyama61 Thus, based on the results of this study, it is suggested that low doses of EE2 are not sufficient to promote significant increases in follicular atresia.
The interstitial glands, described in the human ovary in 1902,Reference Limon62 are originated from cells of the theca interna of atretic follicles.Reference Guraya and Greenwald63 Initially, the primary interstitial gland is located below the epithelial surface, in the primary portion of the ovarian cortex, even before puberty. During old age, the gland increases in size, and new areas are formed along the ovarian stroma, characterizing the secondary interstitial gland.Reference Díaz-Hernández, Caldelas, Montaño and Merchant-Larios31
The cells of the interstitial gland have an important steroidogenic function, being capable of producing high androgen levels.Reference Adashi64,Reference Erickson, Magoffin, Dyer and Hofeditz65 The postmenopausal ovary is hormonally active, being viewed as the expression of steroidogenic enzymes and the production of steroids such as testosterone and estradiol.Reference Brodowski, Brodowska, Laszczyńska, Chlubek and Starczewski66–Reference Laszczyńska, Brodowska, Starczewski, Masiuk and Brodowski69 The secondary interstitial gland, formed during old age, has an even more evident steroidogenic function, being essential for the maintenance of ovarian function in the post-menopausal period.Reference Díaz-Hernández, Caldelas, Montaño and Merchant-Larios31
The morphological analysis conducted in this study suggests that the interstitial gland had lipidic and glycoproteic components, considering the findings in HE and PAS stains. In this study, all groups had a secondary interstitial gland, although this was more evident in the EE/PUB and EE/PRE-PUB groups.
Treatment with low doses of EE during puberty increased the number of the interstitial gland cells in 12-month-old gerbils. This increase was even more pronounced in the EE/PUB group, in which higher levels of testosterone and estradiol were detected, showing the importance of the gland in steroidogenesis. These results suggest that the use of contraceptives during puberty can contribute to the maintenance of ovarian steroidogenic function in old age. However, it is important to note that prolonged exposure to estradiol increases the risk of some diseases, including breast and endometrial cancer.Reference Brinton, Trabert and Anderson70,Reference Yue, Wang and Li71 On the other hand, estrogen has a cardioprotective function,Reference Iorga, Cunningham, Moazeni, Ruffenach, Umar and Eghbali72 and the maintenance of steroidogenic function during senescence may hope to reduce the risk of cardiovascular diseases.
However, the intense activity of the interstitial gland can lead to the accumulation of reactive oxygen species, contributing to the change in folliculogenesis and impairing the viability of ovarian follicles.Reference Ramalho-Santos and Amaral73
Lipofuscin is a pigment formed by deposits of oxidized protein and lipid,Reference López-Otín, Blasco, Partridge, Serrano and Kroemer74 visualized in HE staining as brownish-yellow deposits. Popularly known as the “aging pigment,” lipofuscin is formed from the oxidation of cellular components.Reference Jung, Bader and Grune75
In this study, exposure to EE during puberty increased the deposits of lipofuscin in ovarian tissue of mature animals. Pigmentation was present mainly in the cells of the interstitial gland. The pigment was easily identified by brownish-yellow deposits in HE and PAS reactivity.
Previous studies showed that the highest deposits of lipofuscin in ovarian tissue occurred in nulliparous mice.Reference Urzua, Chacon, Espinoza, Martínez and Hernandez76 Nulliparity is associated with higher levels of estradiol when compared to multiparity.Reference Bernstein, Pike, Ross, Judd, Brown and Henderson77 In our study, mature gerbils exposed to EE2 during puberty, especially in EE/PUB group, had higher serological levels of estrogen and also higher deposition of lipofuscin.
Lipofuscin has chemical properties that can culminate in disorders of cellular metabolism. The pigment is also associated with deposits in macrophages, imbalance of folliculogenesis and ovarian function, increased oxidative stress and DNA damage.Reference Urzua, Chacon, Espinoza, Martínez and Hernandez76,Reference Wu, Van der Hoek, Ryan, Norman and Robker78 Thus, it is suggested that the greater deposits of lipofuscin, associated with exposure to EE, may be a predisposing factor to ovarian dysfunction and inflammatory and neoplastic pathologies in the ovarian tissue. Other studies should be developed to investigate the association of exposure to EE2 and the pathologic conditions.
This study showed that longest exposure to low doses of EE2, in the case of the EE/PRE-PUB group, led to a significant increase in the height of the ovarian superficial epithelium in 12-month-old gerbils. Previous studies have shown that high levels of estrogen promote the proliferation of ovarian epithelium and increase the risk of ovarian epithelial cancer.Reference Murdoch and Van Kirk79 The interaction between estrogen and its receptors promotes genetic instability due to frequent replication and recombination of DNAReference Syed, Ulinski, Mok and Ho80; the stimulation of mitotic pathwaysReference Hall and Korach81; and the release of transcription factors that culminate in epithelial proliferation, angiogenesis, and remodeling of the tissue matrix.Reference Moll, Katsaros and Lazennec82,Reference O’Donnell, Macleod, Burns, Smyth and Langdon83 Previous studies showed that the administration of high doses of estrogen promoted the excessive proliferation of ovarian epithelium of 12-month-old female rats.Reference Perniconi, Simões, Simões, Haidar, Baracat and Soares84 Our study reinforces that low doses of ethinylestradiol may lead to thickening of ovarian epithelium. Thus, other studies should be developed to evaluate the effects of low doses of estrogen during prenatal and puberty in the risk of ovarian cancer and other proliferative disorders.
Another important change observed in this study was the thicker tunica albuginea in all experimental groups. The thickening of tunica albuginea is expected during the proestrus,Reference Joshi, Nanda and Saigal85 in agingReference Joshi, Nanda and Saigal85 and in pathological conditions such as polycystic ovary syndrome.Reference Bulut, Kurdoglu, Dönmez, Kurdoglu and Erten86 Furthermore, it has been shown that prolonged exposure to exogenous androgens promotes thickening of the tunica albuginea.Reference Amirikia, Savoy-Moore, Sundareson and Moghissi87
However, the effects of exposure to estrogenic compounds on stromal proliferation are still unclear. Previous studies have shown the synergistic role of estrogen with paracrine factors derived from oocytes, especially from the transforming growth factor-beta (TGF-β) family, in folliculogenesis.Reference Emori and Sugiura88 Thus, it is suggested that the proliferation of the tunica albuginea is secondary to this interaction, since these factors also stimulate fibrogenesis and proliferation of fibroblasts. However, new studies must be developed to better clarify the alteration found.
Considering the widespread use of synthetic estrogens in contraceptive pills, including during initial gestational periods when the mother is still unaware of the pregnancy, and the greater environmental exposure to this compound, adequate clinical management of exposure to endocrine disruptors is essential in order to avoid pathological conditions.
Conclusions
The present study reinforces the importance of careful assessment of exogenous estrogen exposure in ovarian morphology and function. Exposure to low doses of 17α-ethinylestradiol during prenatal and pubertal periods promoted changes in folliculogenesis, luteinization, and endogenous levels of estradiol and testosterone, hyperplasia of the interstitial gland, higher deposits of lipofuscin, increased height of superficial ovarian epithelium, and increased thickness of the tunica albuginea in mature gerbils.
Our results reinforce the need to study exposure to ethinyl estradiol during different stages of development in the context of pathological changes in ovarian morphology and function. Other researches still need to be developed to identify molecular pathways that promote the morphological changes found in the ovary of mature gerbils exposed to ethinyl estradiol during the prenatal and/or pubertal periods.
Acknowledgment
The authors acknowledge the Program of Initiation to Scientific, Technological Research and Innovation at UFG (PIVIC/2018-2019).
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Law nº 11.974/2008 that regulates care and use of laboratory animals and has been approved by the Committee for Animal Research (protocol nº 020/09 CEEA) of UNESP.










