Introduction
Globally, cardiovascular disease (CVD) is the leading cause of death, accounting for over 17 million deaths in 2015 1 and concerningly a shift to an earlier age of onset is also now being reported. Reference Arora, Stouffer and Kucharska-Newton2 The onset of CVD is traditionally associated with poor lifestyle factors such as poor diet (excessive calories and detrimental composition), smoking and physical inactivity. Reference Howard and Wylie-Rosett3 These modifiable risk factors have traditionally been viewed as the primary cause of CVD. However, it is now well accepted that poor in utero conditions, particularly those leading to low birth weight (LBW), predispose offspring for a greater risk of developing adult-onset chronic disease, Reference Gillman and Rich-Edwards4,Reference McMillen and Robinson5 prior to these traditional postnatal risk factors having an impact.
LBW is used in the clinical setting as a crude proxy for a suboptimal in utero environment and a failure to meet genetic growth potential before birth, an outcome that often occurs in association with a number of factors including placental insufficiency (PI), living at high altitude or maternal undernutrition. Reference McMillen and Robinson5–Reference Morrison7 In these scenarios, the developing fetus undergoes organ-specific developmental changes that favour in utero survival. However, when these occur in organs such as the heart, they inevitably set the stage for a metabolic mismatch in postnatal life and consequential cardio-metabolic dysfunction. Reference Darby, Varcoe, Orgeig and Morrison8 In humans, the predisposition to adult-onset CVD in LBW offspring Reference Barker, Osmond, Golding, Kuh and Wadsworth9,Reference Barker10 makes them particularly vulnerable to additional lifestyle risk factors or “second hits” in postnatal life. These secondary postnatal stressors have the potential to firstly unmask an underlying phenotype and secondly accelerate disease progression. Reference Heindel, Balbus and Birnbaum11–Reference Cheong, Wlodek, Moritz and Cuffe16 Most notably, consumption of a western diet (WD; high in saturated fatty acids and processed carbohydrates) independently corresponds with an increased risk of developing pathological left ventricular hypertrophy (LVH), even without the confounding effects of the poor cardiac outcomes associated with being born small. Reference Liu, Nettleton, Bertoni, Bluemke, Lima and Szklo17,Reference Maugeri, Hruskova and Jakubik18
Various animal models have been used to demonstrate that decreased nutrient and/or oxygen supply in utero results in reduced cardiomyocyte endowment and remodelling Reference Dong, Ford, Fang, Nijland, Nathanielsz and Ren19–Reference Louey, Jonker, Giraud and Thornburg22 as well as impaired left and right ventricular function in postnatal life Reference Kuo, Li, Huber, Schwab, Nathanielsz and Clarke23,Reference Kuo, Li, Li, Huber, Nathanielsz and Clarke24 well before a WD could play a role in cardiac pathology. These morphological and functional responses may be underpinned by alterations to molecular signalling pathways within the heart. For example, impaired in utero substrate supply results in the activation of the insulin-like growth factor-2 receptor (IGF2R) and consequently pathological LVH that persists beyond birth due to increased histone acetylation in the IGF2/IGF2R promoter region. Reference Wang, Tosh and Zhang20,Reference Darby, McMillen and Morrison25–Reference Wang, Zhang and McMillen27 In addition, lambs born with a LBW not only have this aforementioned IGF2R-mediated LVH but also display altered myocardial glucose metabolism Reference Wang, Lim, McMillen, Duffield, Brooks and Morrison28 , highlighting the importance of studying cardiac growth and metabolic pathways concurrently in the setting of LBW.
When uncomplicated by in utero cardiac programming, the independent detrimental cardiac consequences of WD consumption appear to be related in part to alterations in cardiac substrate utilisation and energy metabolism. Indeed, increased dietary fat content is related to myocardial remodelling, reduced cardiac power and impaired diastolic function alongside either increased cardiomyocyte fatty acid uptake, accumulation of myocardial triglyceride content or increased mRNA expression of genes involved in fatty acid utilisation. Reference Aguila and Mandarim-de-Lacerda29–Reference Wilson, Tran, Salazar, Young and Taegtmeyer32 This suggests that WD-induced deleterious effects on the heart are driven by myocardial lipid accumulation and/or lipotoxicity. However, whilst these independent metabolically driven myocardial consequences of WD consumption are relatively well established, little is understood as to how an already reprogrammed and sensitised myocardium as a result of in utero substrate restriction responds when a WD acts as a second hit across the life course.
Importantly, there is a sex-dependent component to developmental programming of the cardiovascular system. Reference Wadley, McConell, Goodman, Siebel, Westcott and Wlodek33–Reference Thompson, Turan and Aberdeen36 Thus, should the cardiac outcomes remain metabolically driven, there is evidence to suggest that the consequences of a WD second hit may be differentially regulated according to offspring sex. When born LBW, young adult male, but not female, guinea pigs maintained on a control diet, display a metabolic gene expression profile associated with cardiac lipid accumulation. Reference Botting, Loke, Zhang, Andersen, Nyengaard and Morrison37 Such a cardio-metabolic profile may be at particular risk of a secondary metabolic stressor, suggesting that male LBW offspring may be particularly vulnerable to poor cardiac outcomes following WD consumption.
Guinea pigs exhibit many strengths as a preclinical animal model of the research investigating the developmental origins of health and disease. Specific to this study, cardiac maturation in utero occurs over a proportionally similar timeframe to humans. Reference Morrison, Botting and Darby38 Herein, we exposed both LBW male and female offspring to a postnatal WD from weaning and examined this impact on their hearts as young adults. We postulated that when assessed in young adulthood, the molecular pathways regulating cardiac growth and/or metabolism would be dysregulated by both LBW and WD in male offspring, but that only LBW females exposed to a WD would have altered expression of key cardiac growth and metabolic signalling molecules.
Methods
Animal care, maintenance and surgeries were conducted in accordance with standards and policies of the Canadian Council on Animal Care and the Western University Animal Care Committee reviewed, approved and monitored all procedures. All investigators understood and followed the ethical principles outlined in, Reference Grundy39 and study design was informed by guidelines (ARRIVE Reference Kilkenny, Browne, Cuthill, Emerson and Altman40 and DOHaD research Reference Morrison, Botting and Darby38,Reference Dickinson, Moss and Gatford41 ).
Animals and surgery
Time-mated pregnant Dunkin-Hartley guinea pigs (Charles River Laboratories, Wilmington, MA, USA) were housed in a temperature (20 ± 2°C) and humidity (30%–40%) controlled environment, with a 12 h light–dark cycle and had access to guinea pig chow (LabDiet diet 5025: 27% protein, 13% fat and 60% carbohydrates as % of energy) and tap water ad libitum. Uterine artery ablation surgery was performed at mid-gestation (32 days, term 69 days) to reduce placental blood flow and subsequently fetal growth as previously described. Reference Turner and Trudinger42,Reference Sarr, Thompson, Zhao, Lee and Regnault43 Briefly, a midline incision was made below the umbilicus in order to expose the bicornate uterus, and the number of fetuses was noted. At that time, arterial vessels were identified, and every second branch per placental unit was “picked up” by a pair of micro forceps, and a diathermy pen was applied to ablate the vessel (Bovie Medical, Clearwater, FL). In general, approximately 50% of arterial branches supplying each fetoplacental unit were ablated.
At birth, all pups were weighed and at the end of the pupping period, guinea pig pups were defined as normal birth weight (NBW) if their body weights were within the 25th and 75th percentile of all pups born, and LBW if their body weights were below the 25th percentile. Reference Thompson, Gros, Richardson, Piorkowska and Regnault44 Based on this, NBW pup weights were greater than 90 g, and LBW weights were below 85 g, similar to pup weight range allocations in uterine artery ligation, maternal feed restriction and maternal hypoxia guinea pig models. Reference Botting, Loke, Zhang, Andersen, Nyengaard and Morrison37,Reference Briscoe, Rehn and Dieni45,Reference Kind, Clifton and Grant46
Nutritional regime
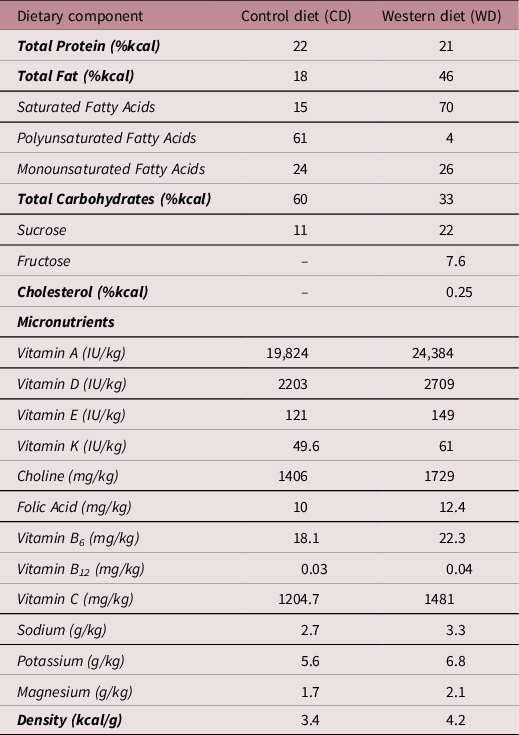
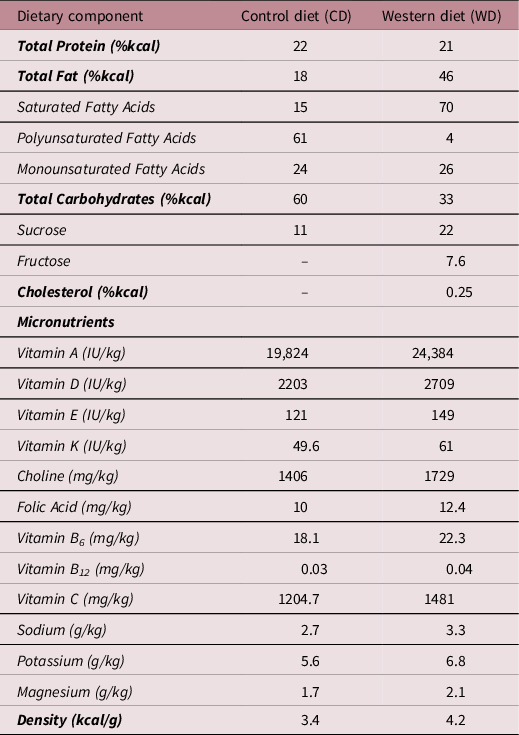
At 15 days of age, pups were weaned and housed in individual cages in a temperature (20 ± 2°C) and humidity (30%–40%) controlled environment with a 12 h light–dark cycle. At this time, pups were randomly assigned to either the control diet (CD; Table 1, TD.110240, Harlan Laboratories, Madison, WI, USA) or the WD (Table 1, TD.110239, Harlan Laboratories) thus creating 4 experimental groups with only one sex per litter per diet allocated to each group: NBW-CD (n = 16; male, n = 8; female, n = 8), NBW-WD (n = 15; male, n = 7 ; female, n = 6), LBW-CD (n = 13; male, n = 6; female, n = 7) and LBW-WD (n = 11; male, n = 5; female, n = 6).
Table 1. Comparison of the dietary composition of the control and western diet
Postmortem and tissue collection
At 143 ± 2d postnatal age (young adulthood; expected lifespan in captivity = 4–8 years), guinea pigs were weighed and killed via CO2 inhalation. The heart was collected, trimmed of connective tissue and weighed. The left ventricle was then excised before being flash frozen in liquid N2 and stored at −80ºC for subsequent molecular analyses.
Quantification of mRNA transcripts within the left ventricle
Total RNA extraction
Total RNA was extracted from ∼50–60 mg of the ventricular samples (total n = 55), using Qiagen QIAzol Lysis Reagent and Qiagen RNeasy purification columns (Qiagen Pty Ltd., Doncaster, Vic, Australia). Reference McGillick, Orgeig, McMillen and Morrison47,Reference Soo, Hiscock, Botting, Roberts, Davey and Morrison48 All extracted RNA samples were checked for integrity by running them on an agarose gel stained with ethidium bromide. Total RNA was quantified by spectrophotometric measurements at 260 and 280 nm.
cDNA synthesis
The optical density 260:280 nm ratios were used to calculate the correct dilutions of extracted RNA to be used for cDNA synthesis. The cDNA was synthesised according to manufacturer’s guidelines with Superscript III First Strand Synthesis System (Invitrogen), using 2 μg of total diluted RNA, random hexamers, dNTP, DTT and Superscript III in a final volume of 20 μL. A no template control (NTC) containing no RNA transcript and a no amplification control (NAC) containing no Superscript III were used to check for reagent contamination and genomic DNA contamination, respectively.
Quantitative real-time RT-PCR
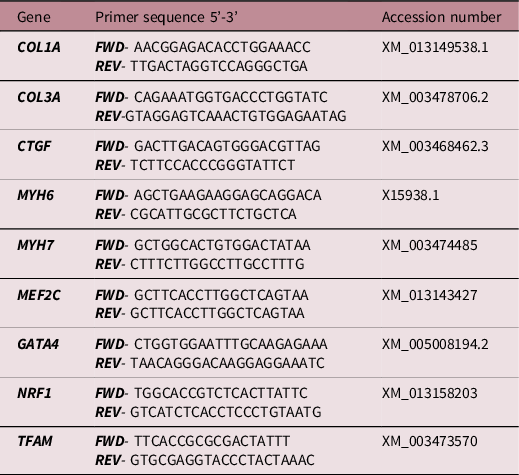
All essential information regarding our procedure is included as per the MIQE guidelines. Reference Bustin, Benes and Garson49 The expression of genes was measured by qRT-PCR as previously described (Botting et al., 2018). Briefly, those regulating IGF signalling (IGF1, IGF1R, IGF2, IGF2R), glucose transport (GLUT1, GLUT4), mitochondrial biogenesis (PPARGC1A, TFAM, NRF1), fatty acid transport into the mitochondria (CPT1B) and fibrosis (Table 2) were measured by qRT-PCR.
Table 2. Primer sequences designed and validated for quantitative real-time RT- PCR analysis
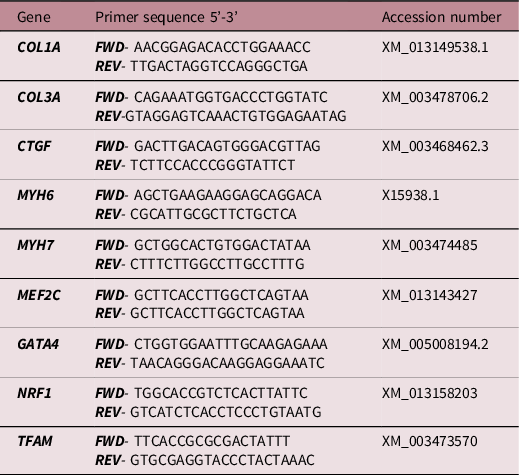
FWD, forward; REV, reverse.
Each sample was run in triplicate for each target and housekeeper gene, and the mRNA amplification for each sample was determined using Fast SYBR® Green Master Mix (Applied Biosystems) in a final volume of 6 μl on a ViiA7 Fast real-time PCR system (Applied Biosystems). Each well on the qRT-PCR plate contained 1 μl of cDNA, 3 μl Fast SYBR Green Master Mix (2×) and 2 μl of forward and reverse primers mixed with differing amounts of H2O depending on the required final primer concentrations. NTC for each primer set were included on each plate to check for nonspecific amplification. The threshold was set within the exponential growth phase of the amplification curve and the corresponding Ct values were obtained to quantitate each reaction.
The mRNA transcript abundance for each target gene was then normalised to the abundance of the three housekeeper genes (TBP, YWAHZ and B2M) using Data Assist 3.0 analysis software (Applied Biosystems) and expressed as mean normalised expression. Reference McGillick, Orgeig, McMillen and Morrison47,Reference Hellemans, Mortier, De Paepe, Speleman and Vandesompele50
Protein extraction and western blotting
Protein extraction
Left ventricle tissue (˜100 mg) was sonicated (John Morris Scientific, SA, Australia) in a lysis buffer compromising of 1 ml/100 mg tissue of 1 mmol/L Tris HCl (pH = 8, 5 mol/L NaCl, 1% NP-40, 1 mmol/LNa orthovanadate, 30 mmol/L NaF, 10 mmol/L Na tetrapyrophosphate, 10 mmol/L EDTA) and a protease inhibitor tablet (complete Mini; Roche). Samples were then centrifuged at 14,300 g and 4°C for 14 min (Eppendorf Centrifuge 5415, Crown Scientific, Vic, Australia). A Micro BCA Protein Assay Kit (PIERCE, Thermo Fisher Scientific Inc., Rockford, USA) was used to determine the protein content of each sample. Bovine serum albumin (BSA; 2 mg/mL stock solution) was used to form a standard curve. Extracted protein samples (50 μg) were subject to SDS page and stained with Coomassie blue. Samples that displayed differences in the abundance of major proteins were excluded.
Western blotting
Equal concentrations/volumes of each sample were subjected to SDS page. Reference Wang, Lim, McMillen, Duffield, Brooks and Morrison28,Reference Lie, Hui and McMillen51 Proteins in each sample were then transferred onto a nitrocellulose membrane (Hybond ECL, GE Health Care, NSW, Australia), which were subsequently stained with Ponceau S (0.5% Ponceau in 1% acetic acid) in order to determine the efficacy of the transfer. The membranes were briefly washed with 7% acetic acid and then subjected to three 5 min washes in Tris-Buffered Saline (TBS). The membranes were cut according to the size of the proteins and blocked in 5% BSA in Tris-Buffered Saline with 1% Tween (TBS-T) for 1 h at room temperature. The membranes then underwent X3 5 min washes in TBS-T and were incubated with their respective primary antibody Troponin I (ab19615, Abcam Cambridge, MA, USA), phospho-Troponin I (#4004, Cell Signalling Technology), AKT-1 (#4691, Cell Signalling Technology) or phospho-AKT (T308, #9275, Cell Signalling Technology) at 4°C overnight with agitation. Membranes were again washed 3× for 5 min in TBS-T before being incubated with the appropriate Horse Radish Peroxidase labelled secondary IgG antibody for an hour at room temperature. Enhanced chemiluminescence using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, IL, USA) was used to detect the blots. The Western blot was imaged either using ImageQuant LAS 4000 (GE Healthcare, VIC, Australia) or a VersaDoc Imaging System (Bio-Rad Laboratories Mississauga, ON, Canada), and the protein abundance was quantified by densitometry using either Image quant software (GE Healthcare, VIC, Australia) or ImageLab software (Bio-Rad Laboratories, Mississauga, ON, Canada). The abundance of proteins was then expressed relative to the loading control β-Actin (ATCB).
Statistical analysis
To control for the effect of sex but not eliminate it, data from males and females were separated, and a 2-way ANOVA (factors; birth weight (NBW, LBW) and diet (CD, WD)) was performed within each sex using STATA® (StataCorp, College Station, TX, USA). When a significant interaction between birth weight and diet was observed, data were split by birth weight, and a one-way ANOVA was performed with Bonferroni’s correction for multiple comparisons. Data are presented as mean ± SEM, and a probability of 5% (P < 0.05) was considered significant for all analyses.
Results
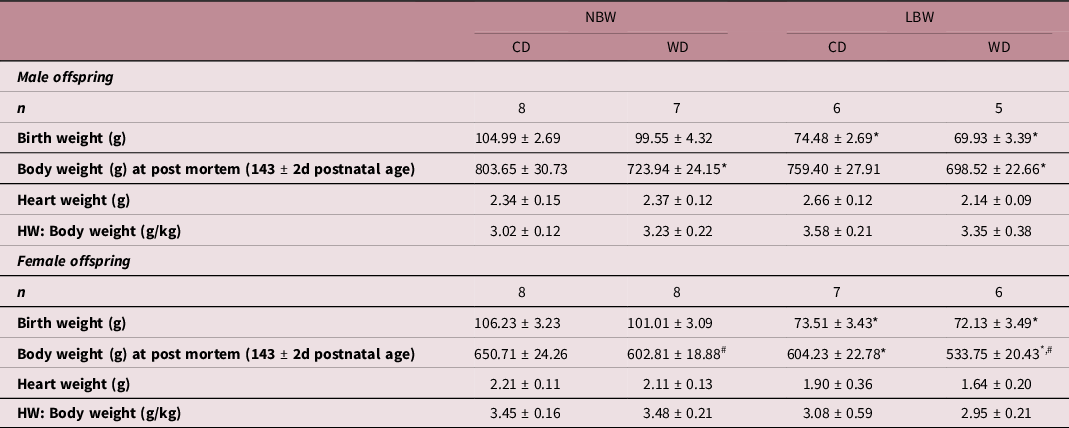
In both males and females, offspring assigned to the LBW group had significantly lower birth weights than those assigned to the NBW group (Table 3). At tissue collection (˜145 days, young adulthood), WD fed male offspring had lower body weights than CD fed male offspring; however, there was no effect of birth outcome upon male offspring body weight. There was a significant effect of LBW and postnatal diet on female offspring body weight. LBW female offspring had lower body weights, and WD fed female offspring had lower body weights irrespective of BW. There was no effect of either BW or postnatal diet on heart weight when normalised to body weight in both male and female offspring (Table 3).
Table 3. Characteristics of male and female guinea pigs across experimental groups

Data analysed by two-way ANOVA within each sex and presented as mean ± SEM. P < 0.05.
* , effect of birth weight;
# , effect of postnatal diet. NBW, normal birthweight; LBW, low birthweight; CD, control diet; WD, western diet; HW, heart weight.
Impact of BW and postnatal diet upon IGF system
There was no effect of BW or WD on the mRNA expression of IGF1 in male or female offspring (Fig. 1a & 1b). NBW-WD and LBW-WD males had higher mRNA expression of IGF1R (Fig. 1c), but the effect of WD was not observed in female offspring (Fig. 1d). There was a significant interaction between BW and diet for the mRNA expression of IGF2, such that there was an effect of postnatal WD in LBW male offspring. LBW-WD males had increased mRNA expression of IGF2 (Fig. 1e). There was no effect of BW or WD on IGF2 mRNA expression in female offspring (Fig. 1f). The expression of IGF2R in male offspring was significantly increased in LBW and also when exposed to WD (Fig. 1g). There was no effect of LBW or WD on the mRNA expression of IGF2R in female offspring (Fig. 1h).
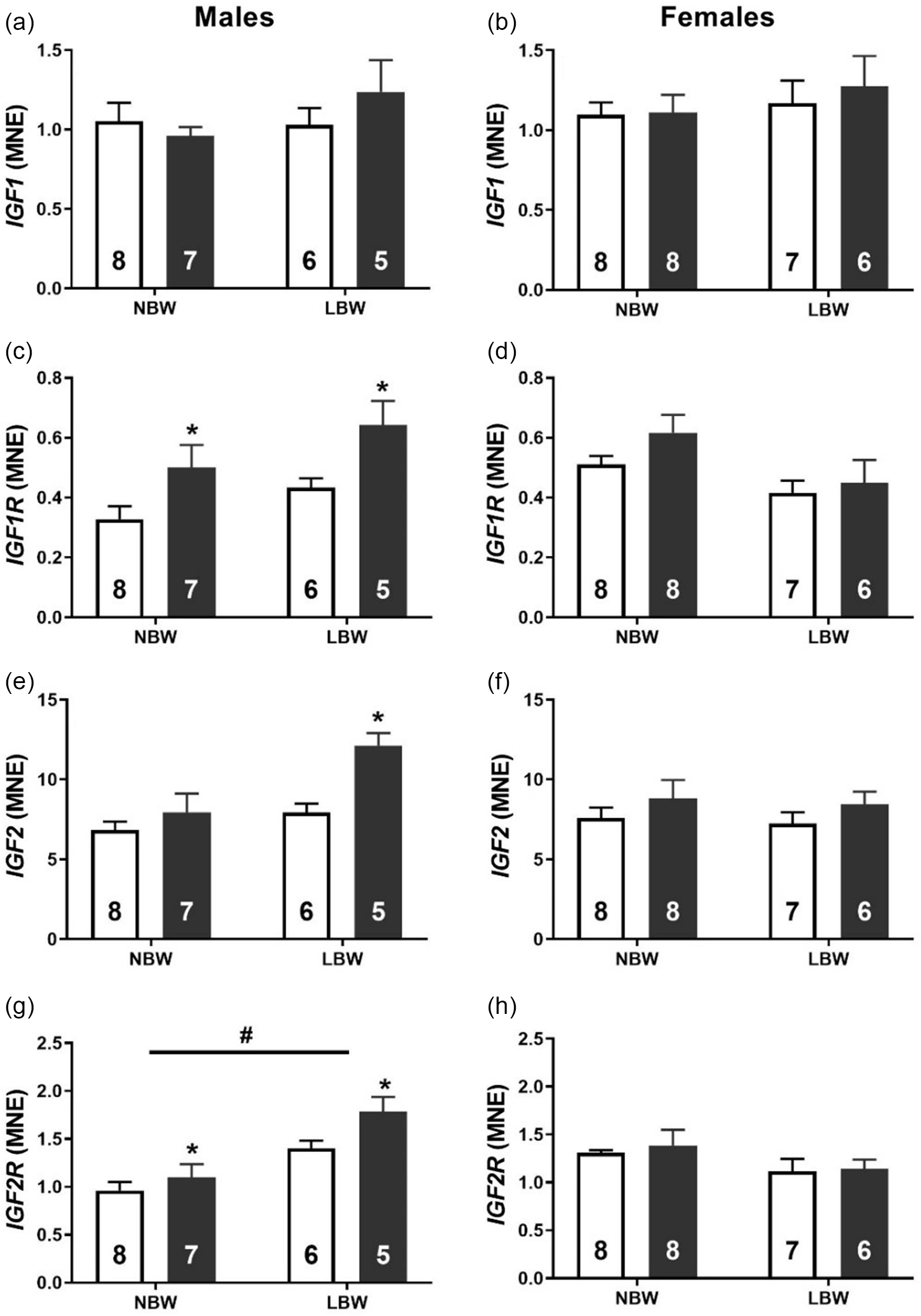
Fig. 1. Normalised mRNA expression of IGF1 (a, b), IGF1R (c, d), IGF2 (e, f) and IGF2R (g, h) in males (left panel) and females (right panel). Data analysed by two-way ANOVA within each sex. Post hoc analysis performed with Bonferroni’s correction for multiple comparisons. Data presented as mean ± SEM. Unfilled bars, control diet; filled bars, WD. #, effect of birth weight (NBW vs LBW); * postnatal diet (control vs WD); P < 0.05.
WD effects upon hypertrophy-associated transcription factors and markers of myocardial fibrosis in LBW offspring
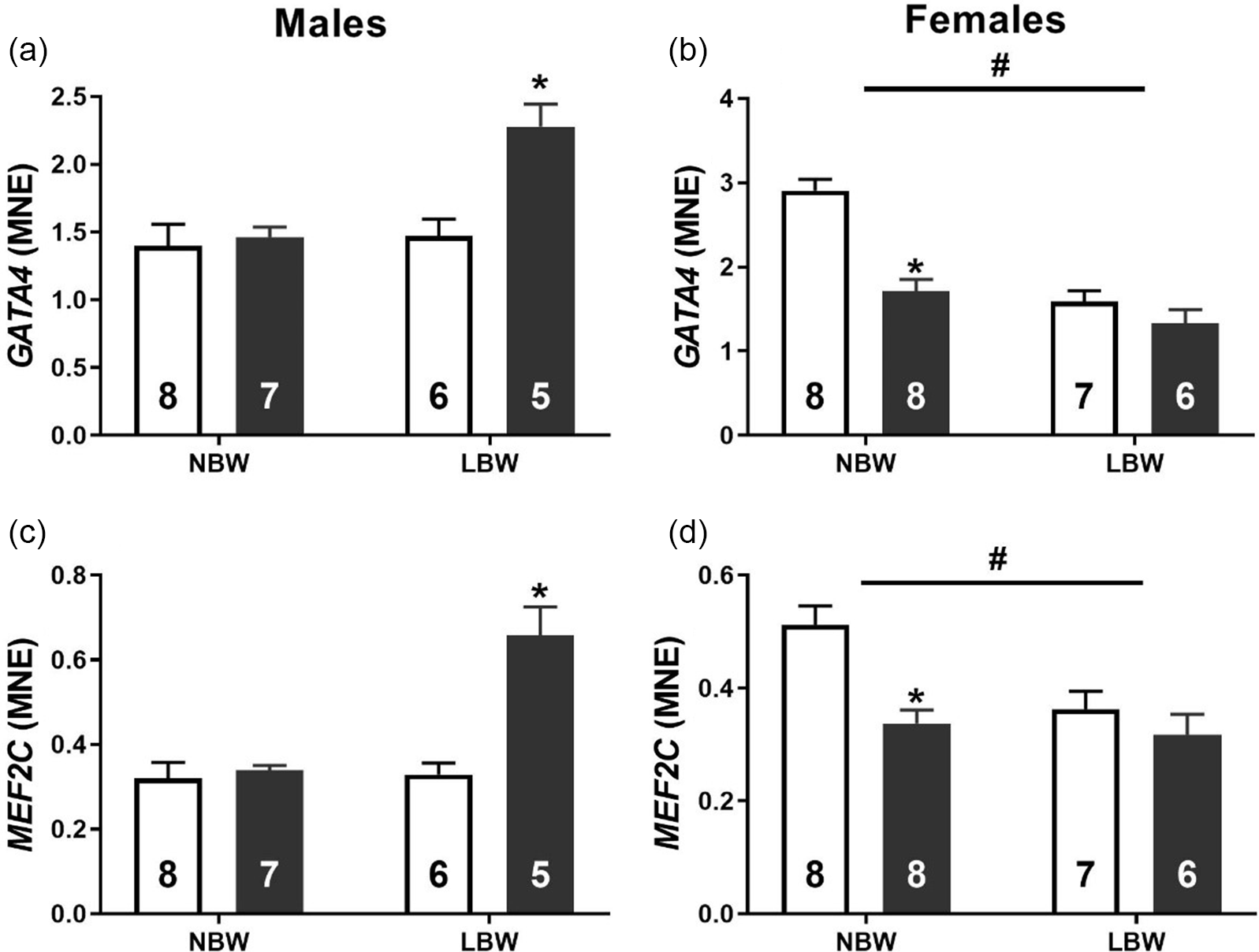
WD consumption was associated with an increased mRNA expression of transcription factors GATA4 (Fig. 2a) and MEF2C (Fig. 2c) in LBW male offspring. Conversely, LBW was associated with decreased mRNA expression of GATA4 (Fig. 2b) and MEF2C (Fig. 2d) in female offspring. NBW-WD female offspring had decreased the mRNA expression of GATA4 and MEF2C. In both male and female offspring, there was a significant interaction between BW and postnatal diet on the mRNA expression of CTGF. WD had no effect on the expression of CTGF in NBW males but significantly increased expression in LBW males (Fig. 3a). WD significantly decreased the mRNA expression of CTGF in NBW females but had no effect in LBW females (Fig. 3b).
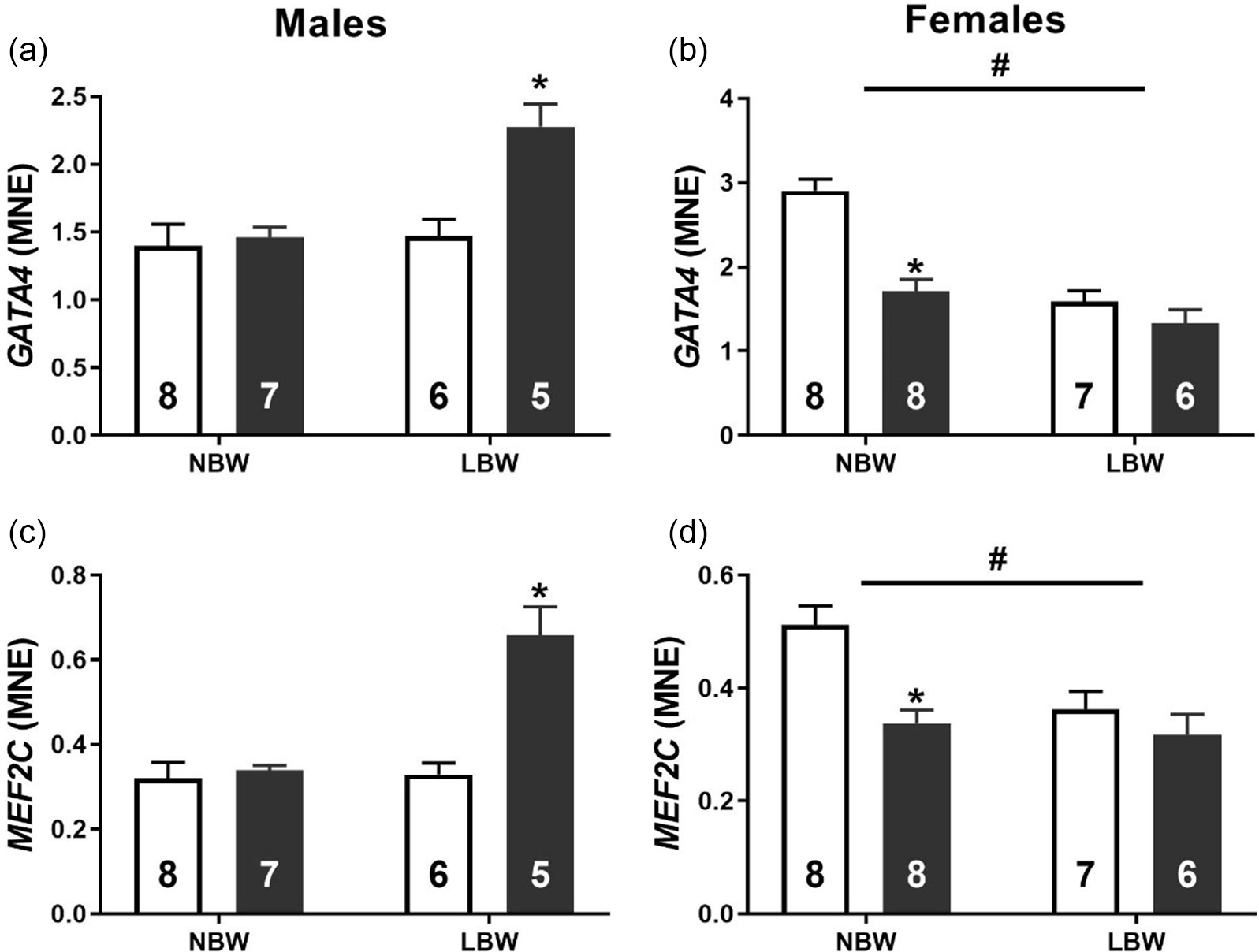
Fig. 2. Normalised mRNA expression of GATA4 (a, b) and MEF2C (c, d) in males (left) and females (right). Data analysed by two-way ANOVA within each sex. Post hoc analysis performed with Bonferroni’s correction for multiple comparisons. Data presented as mean ± SEM. Unfilled bars, control diet; filled bars, WD. #, effect of birth weight (NBW vs LBW); *, postnatal diet (control vs WD); P < 0.05.
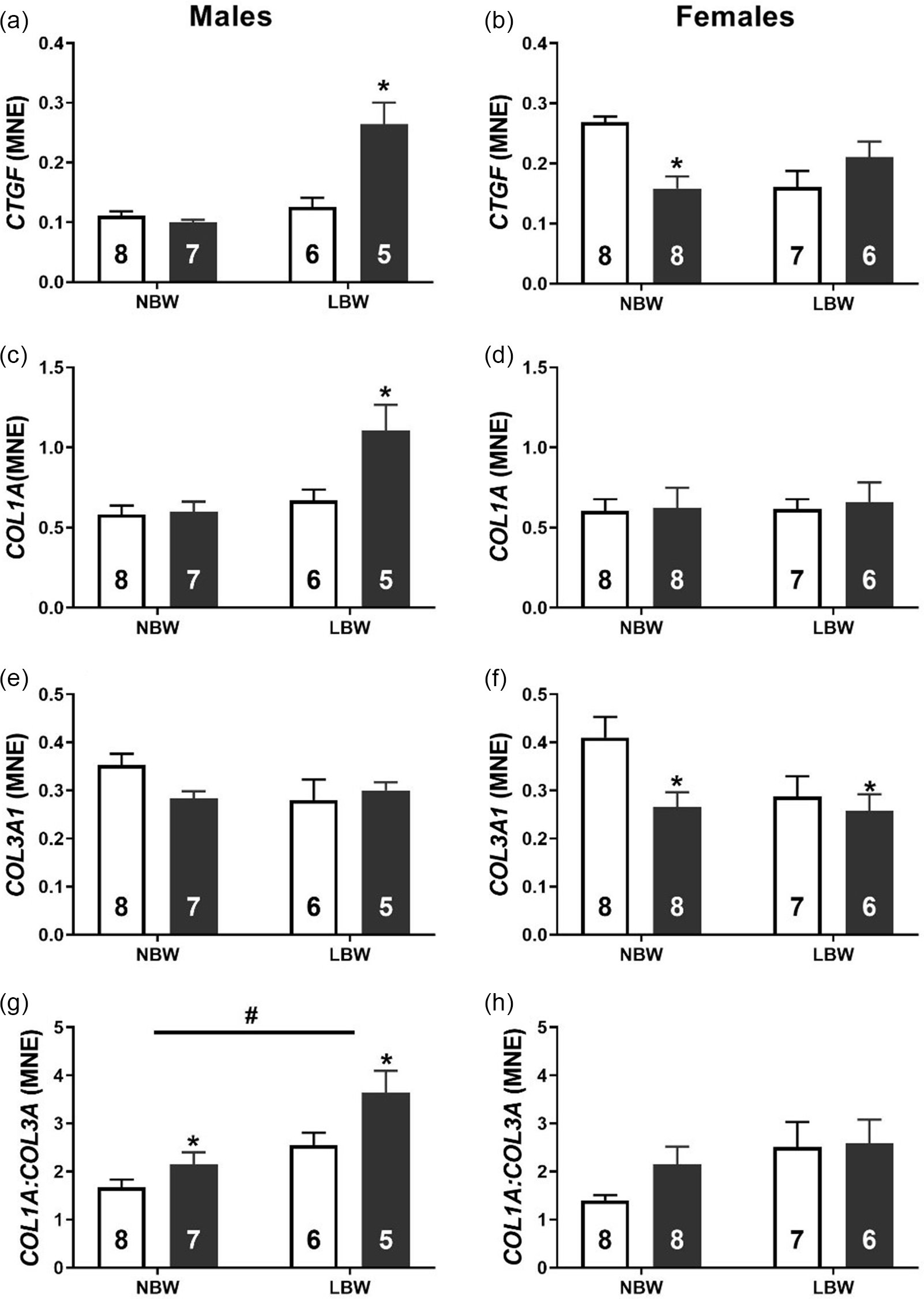
Fig. 3. Normalised mRNA expression of CTGF (a, b), COL1A (c, d), COL3A1 (e, f) and the ratio of COL1A:COL3A1 (g, h) in males (left panel) and females (right panel). Data analysed by two-way ANOVA within each sex. Post hoc analysis performed with Bonferroni’s correction for multiple comparisons. Data presented as mean ± SEM. Unfilled bars, control diet; filled bars, WD. #, effect of birth weight (NBW vs LBW); *, postnatal diet (control vs WD); P < 0.05.
There was a significant interaction between BW and postnatal WD on the mRNA expression of COL1A in male offspring such that WD increased COL1A expression in LBW, but not NBW males (Fig. 3c). There was no effect of either BW or postnatal diet on COL1A expression in females. There was no effect of BW or postnatal diet on the mRNA expression of COL3A1 in male offspring. However, WD decreased the expression of COL3A1 in both NBW and LBW females. Both LBW and WD increased the COL1A:COL3A1 ratio in male offspring (Fig. 3f). There was no effect of either BW or postnatal diet on COL1A:COL3A1 in females (Fig. 3g).
The mRNA expression of cardiac alpha (α)-myosin heavy chain (MYH6) was increased by both LBW and WD in male offspring. There was no effect of BW or postnatal diet on MYH6 expression in females (Fig. 4b). There was no effect of BW or postnatal diet on the mRNA expression of cardiac beta (β)-myosin heavy chain (MYH7) in male offspring (Fig. 4c). MYH7 expression was decreased in LBW females and NBW-WD females (Fig. 4d). The ratio of MYH7:MYH6 was decreased in LBW male offspring (Fig. 4e). There was no effect of either BW or postnatal diet on MYH7:MYH6 in females (Fig. 4f).
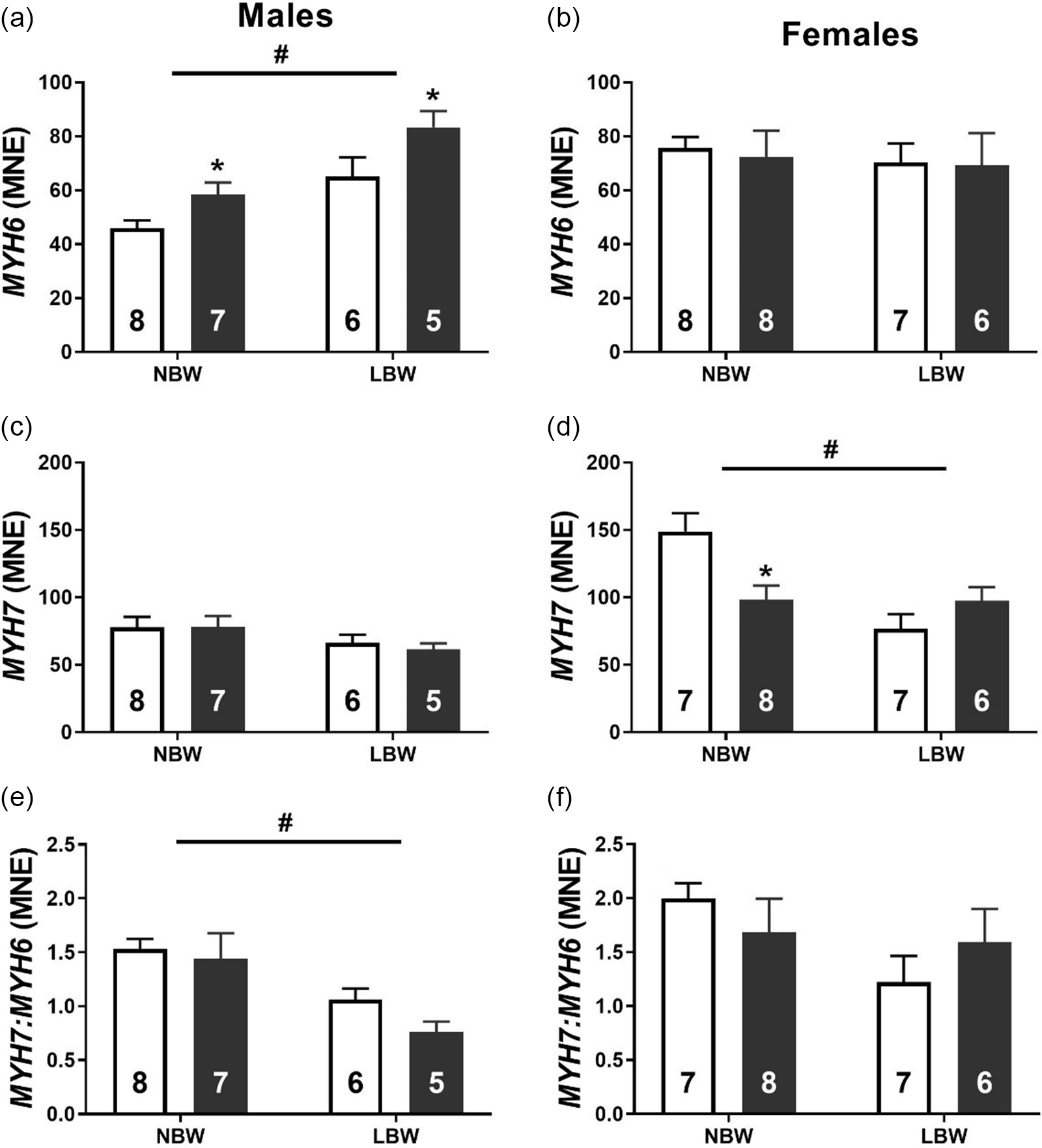
Fig. 4. Normalised mRNA expression of MYH6 (a, b), MYH7 (c, d) and the ratio of MYH7:MYH6 (e, f) in males (left side) and females (right side). Data analysed by two-way ANOVA within each sex. Post hoc analysis performed with Bonferroni’s correction for multiple comparisons. Data presented as mean ± SEM. Unfilled bars, control diet; filled bars, WD; #, effect of birth weight (NBW vs LBW); *, effect of postnatal diet (control vs WD); P < 0.05.
The phosphorylation of Troponin I was decreased in LBW-WD male offspring (Fig. 5a). There was no effect of WD on the phosphorylation of Troponin I in female offspring, but LBW females had higher phosphorylation of Troponin I than NBW female offspring (Fig. 5b).
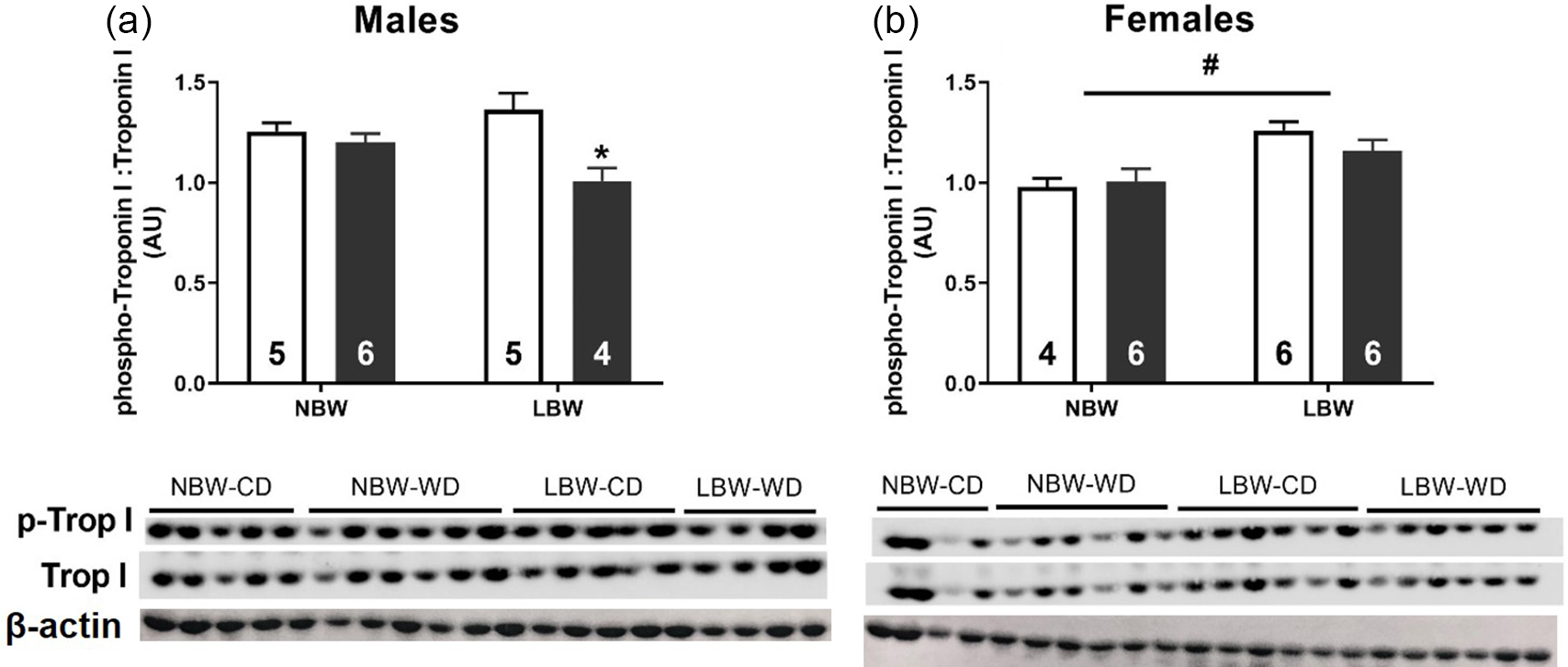
Fig. 5. Protein phosphorylation of Troponin I males (a) and females (b). Data analysed by two-way ANOVA within each sex. Post hoc analysis performed with Bonferroni’s correction for multiple comparisons. Data presented as mean ± SEM. Unfilled bars, control diet; filled bars, WD; #, effect of birth weight (NBW vs LBW); *, effect of postnatal diet (control vs WD); P < 0.05.
Effect of LBW and WD on signalling molecules involved in cardiac metabolism
Glucose transporters and AKT status
Postnatal WD decreased the mRNA expression of the insulin-independent glucose transporter GLUT1 in male offspring (Fig. 6c). There was no effect of postnatal diet or BW on GLUT1 expression in female offspring (Fig. 6d). Both WD and LBW decreased GLUT4 expression in male offspring (Fig. 6e). There was no effect of postnatal diet or BW on GLUT4 expression of female offspring (Fig. 6f). WD decreased T308 phosphorylation of AKT in both NBW and LBW female (Fig. 6b) but not male (Fig. 6a) offspring.
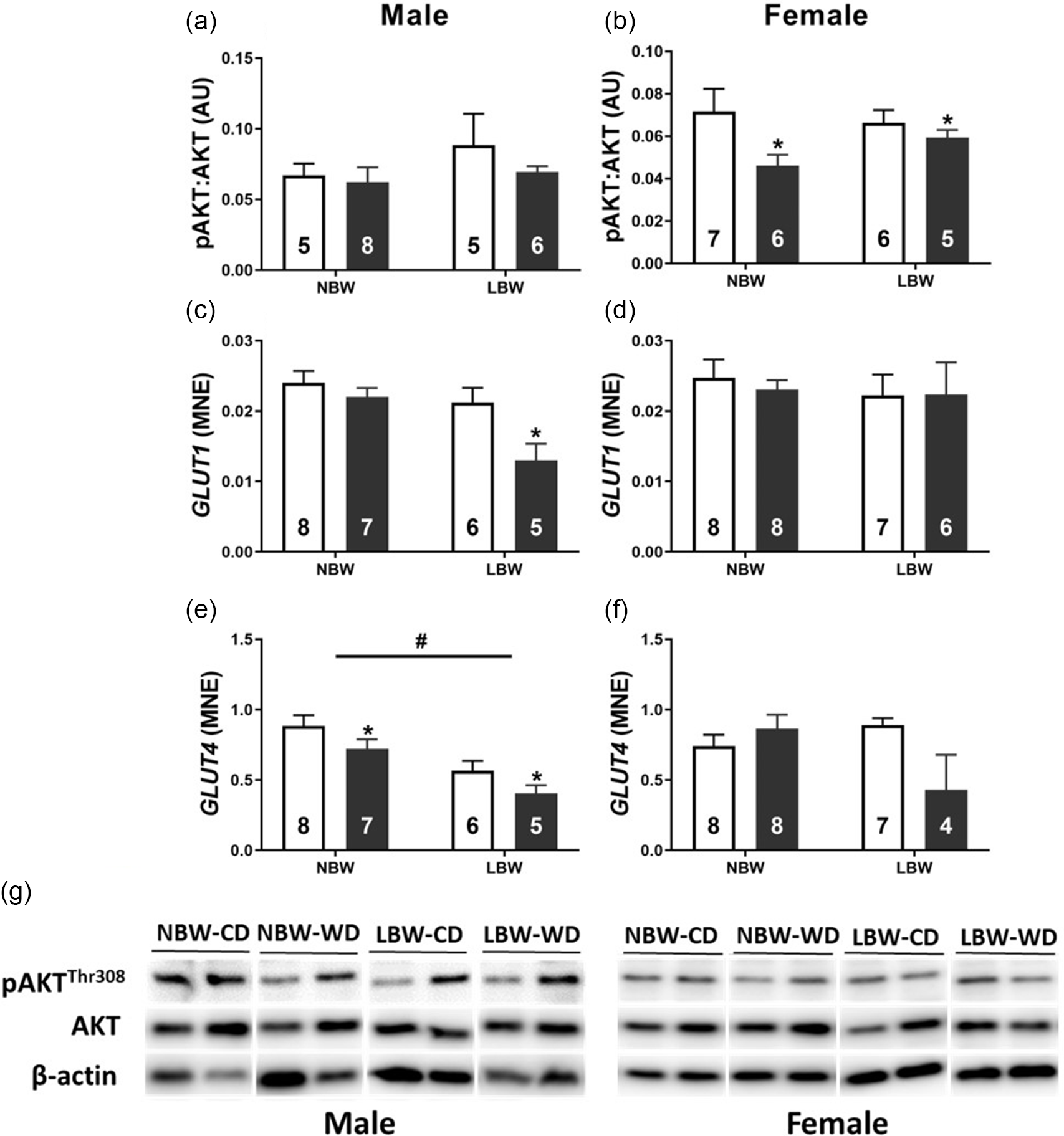
Fig. 6. Normalised protein abundance of phosphorylated AKT (T308; a, b) and mRNA expression of glucose transporters GLUT1 (c, d) and GLUT4 (e, f) in males (left panel) and females (right panel). Representative western blot images (g). Data analysed by two-way ANOVA within each sex. Post hoc analysis performed with Bonferroni’s correction for multiple comparisons. Data presented as mean ± SEM. Unfilled bars, control diet; filled bars, WD;. #, effect of birth weight (NBW vs LBW); *, postnatal diet (control vs WD); P < 0.05.
Expression of signalling molecules and transporters regulating fatty acid oxidation
Both LBW and WD impacted the mRNA expression of key genes involved in fatty acid oxidation. PPARGC1A was increased by LBW and WD in male offspring (Fig. 7a). There was no effect of WD on PPARGC1A expression in female offspring, but LBW females had decreased mRNA expression of PPARGC1A (Fig. 7b). LBW and WD increased the mRNA expression of CPT1B in male offspring (Fig. 7c). There was no effect of WD on CPT1B mRNA expression in females; however, LBW females had decreased CPT1B mRNA expression irrespective of postnatal diet (Fig. 7d). NRF1 was increased by WD in LBW male offspring (Fig. 7e) and decreased by WD in NBW female offspring (Fig. 7f). The mRNA expression of TFAM was increased by WD in both LBW male and female offspring (Fig. 7g, 7h).
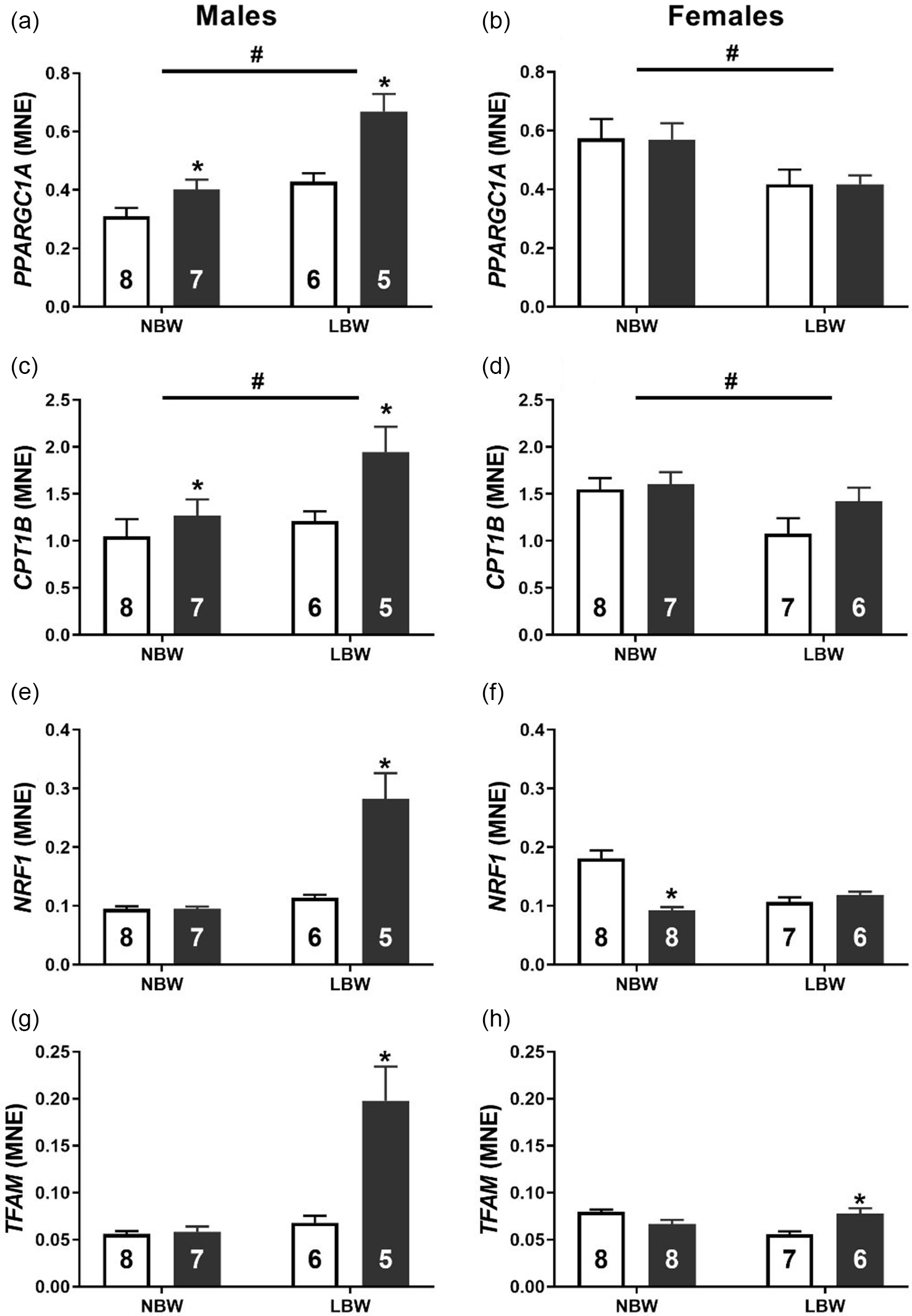
Fig. 7. Normalised mRNA expression of transcriptional regulator PPARGC1A (a, b) and fatty acid transporter CPT1B (c, d), mitochondrial transcription factors NRF1 (e, f) and TFAM (g, h) in males (left panel) and females (right panel). Data analysed by two-way ANOVA within each sex. Post hoc analysis performed with Bonferroni’s correction for multiple comparisons. Data presented as mean ± SEM. Unfilled bars, control diet; filled bars, WD. #, effect of birth weight (NBW vs LBW); *, postnatal diet (control vs WD); P < 0.05.
Discussion
Multiple large epidemiological and mechanistic animal studies have shown that cardiac health, contractile function and molecular markers of poor cardiac outcomes in adult life are associated with suboptimal in utero conditions. Reference Kuo, Li, Huber, Schwab, Nathanielsz and Clarke23,Reference Kuo, Li, Li, Huber, Nathanielsz and Clarke24,Reference Barker, Winter, Osmond, Margetts and Simmonds52–Reference Kajimura, Spiegelman and Seale58 Specifically, those in utero environments where fetal nutrient and/or oxygen delivery is restricted have the potential to cause structural changes and epigenetic modifications to the cellular machinery within the heart. Reference Wang, Tosh and Zhang20,Reference Wang, Zhang and McMillen27 It is often difficult to tease apart the in utero derived predisposition to CVD from the confounding lifestyle risk factors of the human environment. These confounding lifestyle risk factors have been termed ‘second hits’ on the path to programmed disease progression. Reference Cheong, Wlodek, Moritz and Cuffe16 Herein, we utilised a pre-clinical guinea pig model of PI resulting in LBW induced through uterine artery ablation to unmask the potentially additive and interactive “second hit” effect of a postnatal WD on the expression of signalling molecules regulating cardiac metabolism, growth and contractile function.
The adult myocardium responds to stress such as increased afterload, infarction or metabolic dysfunction by reorganising cellular machinery involved in metabolism and contractile function. Reference Taegtmeyer, Sen and Vela59 Concerningly, there is evidence that in response to in utero substrate restriction, the fetal myocardium has already undergone some level of remodelling well before the potential impact that a second hit could have after birth. Interestingly, regardless of the particular substrate (oxygen/glucose) that is restricted in utero, this cardiac remodelling appears to be governed by the activation of the IGF2R hypertrophic signalling pathway. Reference Wang, Tosh and Zhang20,Reference Darby, McMillen and Morrison25,Reference Wang, Zhang and McMillen27 In the present study, we found that neither BW nor postnatal diet influenced absolute or relative heart weight in either sex in young adulthood. Despite this lack of change in heart weight, LBW male offspring had elevated expression of IGF2R, and this effect was exacerbated by WD. The culmination of pathological hypertrophic signalling and stimuli downstream of IGF2R activation occurs in the nucleus where amongst others, the transcription factors GATA4 and MEF2 act to upregulate the “fetal gene program”. Reference Frey and Olson60,Reference McMullen and Jennings61 Here the gene expression pattern in the heart mimics that which would occur during cardiac development in utero. The finding that WD upregulated GATA4 and MEF2C expression in LBW male offspring suggests that LBW males may be more susceptible to an increased activation of this pathological hypertrophic signalling that may eventually lead to LVH. Furthermore, one hallmark of pathological cardiac hypertrophy is the presence of myocardial fibrosis. Following the same mRNA expression pattern as the hypertrophic signalling molecules, we observed that WD was associated with an increased CTGF and COL1A mRNA expression in LBW male but not female offspring. Further, the ratio of COL1A:COL3A1 was increased by both LBW and WD and that the phosphorylation of cardiac Troponin I was decreased in male LBW-WD offspring, suggesting a stiffer less compliant myocardium that is prone to contractile deficits. Reference Wei, Chow, Shum, Qin and Sanderson62 COL1A is approximately 37% stiffer than COL3A, and it is thus thought that the ratio of the collagen subtypes contributes to cardiac stiffness. Reference Collier, Watson and van Es63,Reference Norton, Tsotetsi, Trifunovic, Hartford, Candy and Woodiwiss64 Indeed, alterations to the COL1A:COL3A1 ratio are linked to early diastolic dysfunction with preserved ejection fraction; the incidence of this type of heart failure is climbing and predicted to soon be the leading type of heart failure. Reference Kasner, Westermann and Lopez65,Reference Owan, Hodge, Herges, Jacobsen, Roger and Redfield66 Similar increases in ventricular fibrosis have been observed in both fetal sheep and male, but not female, non-human primates exposed to maternal nutrient restriction. Reference Darby, McMillen and Morrison25,Reference Muralimanoharan, Li and Nakayasu35
Male and female LBW offspring were significantly smaller than NBW offspring at birth; however, in young adulthood and independently from postnatal diet, this difference in body weight was no longer significant. This lack of weight difference in young adulthood highlights a period of accelerated catch up growth, a known feature of IUGR. Reference Cho and Suh67,Reference Wollmann68 LBW offspring that display this catch-up growth are often predisposed to abdominal fat mass accumulation and insulin resistance in later life. Reference Hales and Barker69,Reference Barker, Martyn, Osmond, Hales and Fall70 Such conditions are themselves associated with the development of CVD. However, pre-clinical signs of cardiac dysfunction and CVD progression, which may occur earlier in the life course, often precede this fat deposition and global markers of metabolic dysfunction. Reference Christopher, Huang and Berthiaume71 For example, cardiac insulin resistance is associated with WD consumption and precedes clinical markers of whole-body insulin resistance. Reference McFarlane, Banerji and Sowers72,Reference Park, Cho and Kim73 Unpublished data from our laboratory show that although systemic glucose uptake is not different between NBW and LBW offspring, cardiac-specific glucose uptake is impaired by LBW. GLUT1 is the basal glucose transporter within the myocardium and differs to GLUT4 in that its ability to translocate to the membrane in order to transport glucose is not reliant on insulin binding. Reference Aerni-Flessner, Abi-Jaoude, Koenig, Payne and Hruz74,Reference Kraegen, Sowden and Halstead75 Herein, LBW was associated with decreased mRNA expression of the insulin-dependent glucose transporter GLUT4 in male offspring. Thus, suggesting that glucose uptake is potentially reduced. In addition, the decrease in GLUT4 mRNA expression was further exacerbated when these LBW males were exposed to a WD. Thus, it appears that in males whilst the expression of the insulin-dependent glucose transporter, GLUT4, is decreased by both LBW and WD; the basal glucose transporter GLUT1 is only decreased by the combined impact of LBW and WD. GLUT4 translocation to the cell membrane is dependent on the phosphorylation of AKT at Threonine 308 and Serine 473. Thus, it is interesting to note that despite the LBW- and WD-induced reductions in glucose transporter expression within the male myocardium, AKT phosphorylation at Threonine 308 was not influenced by WD in either NBW or LBW males but was reduced by WD in females irrespective of BW. This may signify that although glucose transporter mRNA expression is influenced by WD in male offspring, the translocation of these transporters to the membrane in males may in fact be maintained. On the other hand, glucose is not the sole metabolic substrate of the heart and nor is the phosphorylation of AKT solely associated with the translocation of glucose transporters. Reference Luiken, Coort, Koonen, Bonen and Glatz76
At birth, when there is a sudden increase in oxygen availability for oxidative phosphorylation, fatty acids become the preferred energy substrate of the heart. Reference Lopaschuk, Collins-Nakai and Itoi77,Reference Lopaschuk and Jaswal78 Previous work from our laboratory suggests that the hearts of guinea pig IUGR born male offspring are predisposed to increased fatty acid uptake and lipid accumulation Reference Botting, Loke, Zhang, Andersen, Nyengaard and Morrison37 ; a metabolic profile that may align with a cardiac lipotoxicity. Other than glucose transporter translocation, AKT phosphorylation is also associated with the translocation of the long-chain fatty acid transporter, CD36, to the cardiomyocyte cell membrane. Reference Luiken, Coort, Koonen, Bonen and Glatz76 Although we were unable to determine the cellular localisation or mRNA expression of CD36 in the present study, it is possible that the maintenance of AKT phosphorylation in WD fed males may represent an increased abundance of CD36 on the cell membrane and thus an elevated uptake of long-chain fatty acids into the cardiomyocyte. This is of concern as studies in insulin-resistant rats show a high-fat diet impairs cardiac function secondary to increased CD36-mediated fatty acid uptake. Reference Ouwens, Diamant and Fodor31
In order to correctly metabolise an abundance of intracellular fatty acids and avoid potentially toxic accumulations of lipid-derived sub species such as diacylglycerols and ceramides, the cardiomyocyte must rearrange its cellular machinery and increase the expression and activity of those molecules involved in fatty acid utilisation. Reference Goldberg, Trent and Schulze79 The proteins encoded by PPARGC1A, NRF1 and TFAM act as transcription factors to upregulate the mRNA expression of genes involved in mitochondrial biogenesis and oxidative phosphorylation. Reference Scarpulla80,Reference Attardi and Schatz81 An increase in their expression alongside the upregulation of CPT1B suggests an increase in oxidative phosphorylation and reliance on fatty acid utilisation. This may be a response to a potential increase in fatty acid uptake or a result of a programmed deficit in mitochondrial number. Reductions in skeletal muscle mitochondrial content have been observed as a result of both naturally occurring growth restriction in piglets and as a result of either protein restriction or PI in rats. Reference Park, Kim and Kim82–Reference Liu, Chen and Yao84 Furthermore, depletions in mitochondrial content are associated with increased expression of both NRF1 and TFAM, Reference Miranda, Foncea, Guerrero and Leighton85 both of which were observed in the present study. Mitochondrial number is regulated by energy demand. Reference Attardi and Schatz81 Therefore, one hypothesis may be that the LBW heart has a persistent mitochondrial deficit and due to increased fibrosis, the male heart requires more ATP than the current mitochondrial complement can produce in order to contract efficiently, potentially setting the stage for cardiac failure in later life. Nevertheless, the aberrant mRNA expression profile of genes linked to oxidative phosphorylation and mitochondrial biogenesis at this later life time point (young adult hood) suggest an in utero experience (in attempt to adapt to altered substrate availability) that leads to permanent changes to cardiac metabolism that are observable as early as young adulthood.
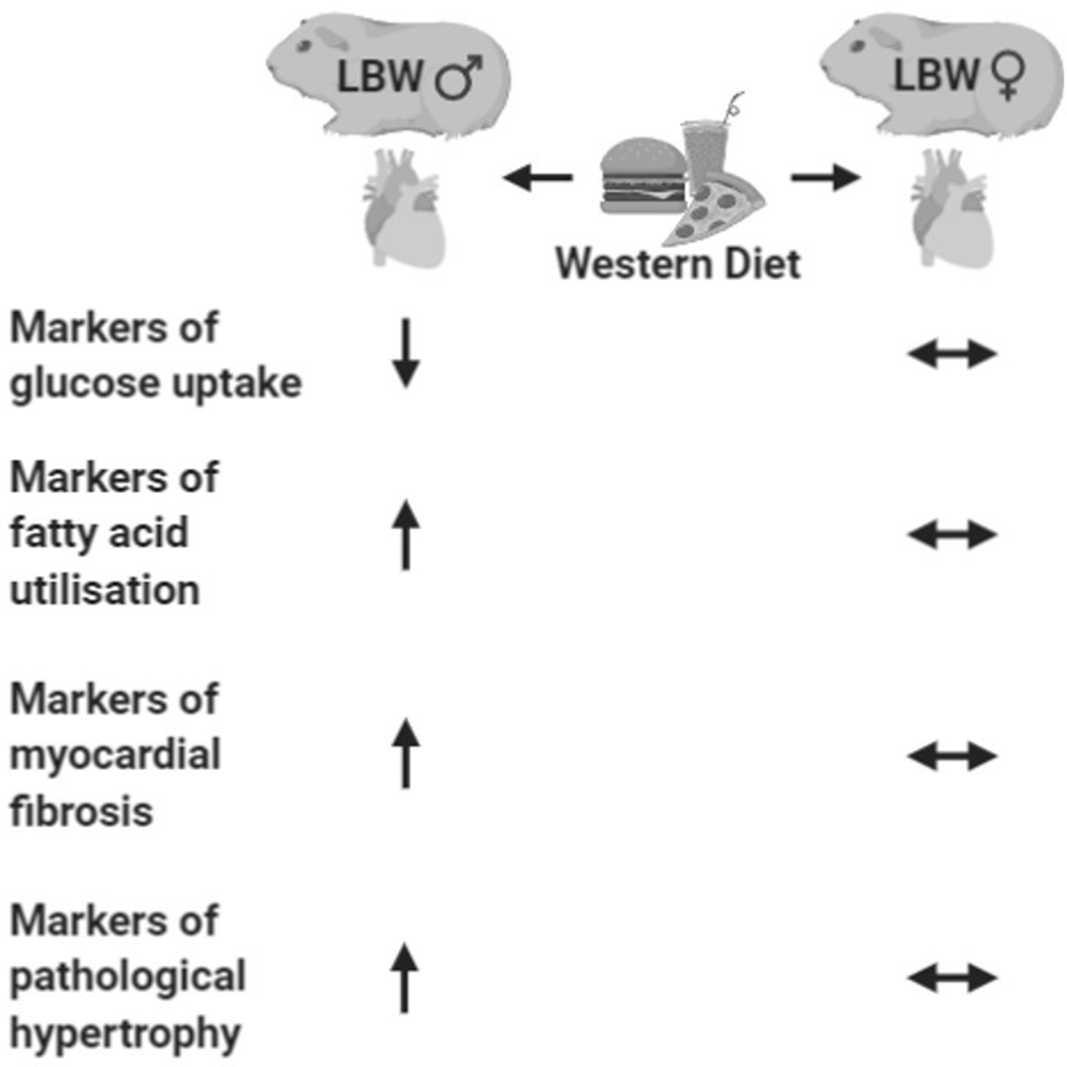
In summary, we postulated that detrimental characteristics of both cardiac hypertrophic, fibrotic and metabolism responses would be evident in a sex-dependent manner as a result of LBW and would only emerge in female LBW offspring when challenged with a WD. The second hit of the postnatal WD unmasked a gene expression profile in LBW male offspring that is associated with pathological cardiac hypertrophy and myocardial fibrosis and increased fatty acid uptake and utilisation (Fig. 8). Female offspring did not respond in the same way as their male counterparts. Notably, we have shown that as early as young adulthood, the young adult male myocardium is more vulnerable to the independent and additive and interactive effects of in utero programming by PI-induced LBW and a chronic “second hit” in the form of a postnatal WD. These findings are critical to better define the effect of sex in populations of earlier onset CVD where symptoms and markers thereof may not yet be considered clinically significant. Moreover, elucidating the interactions between an in utero derived predisposition to CVD and a postnatal second hit synonymous with an increasingly relevant lifestyle is paramount in understanding the development and progression of CVD in the LBW adult offspring.
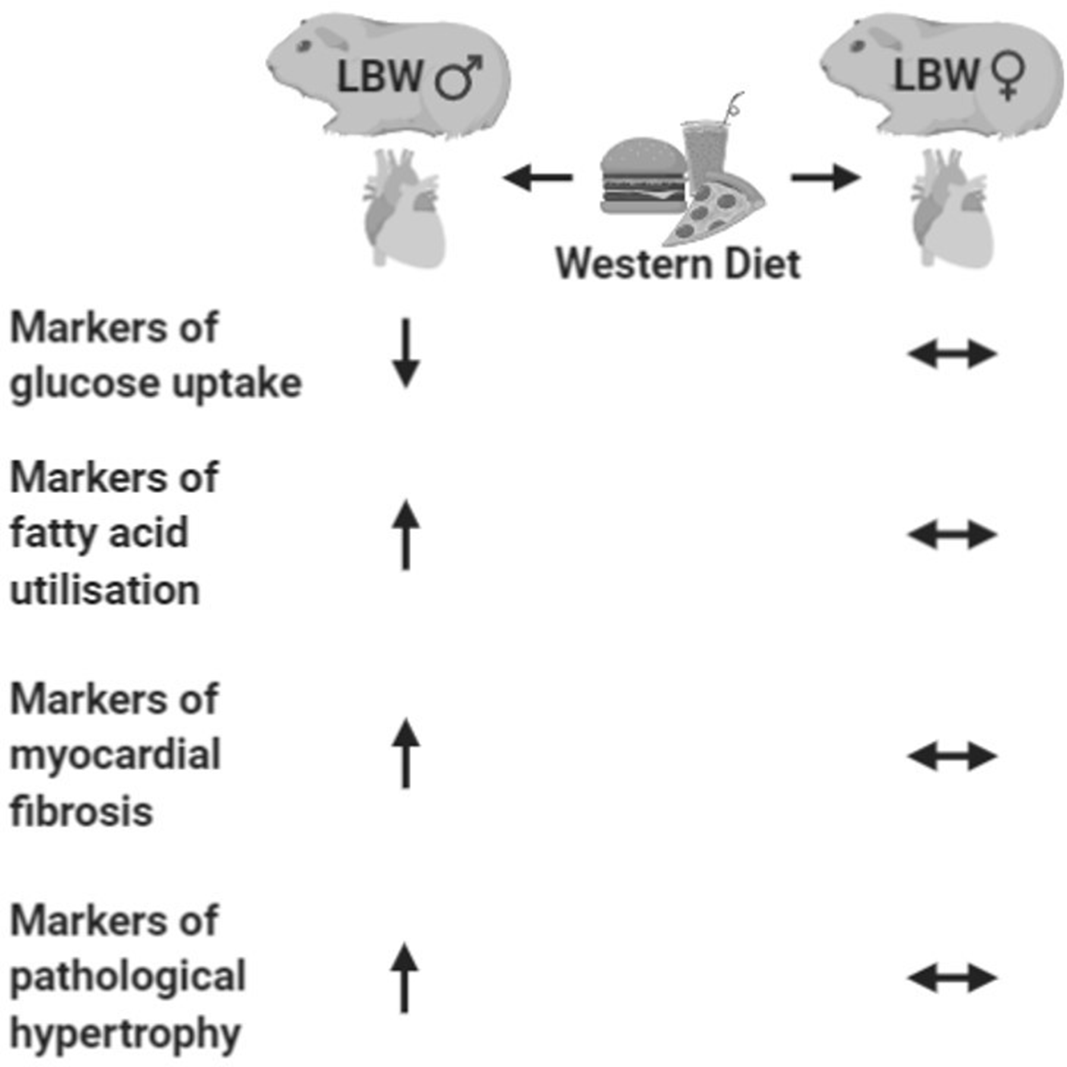
Fig. 8. Summary of the cardiac consequences of postnatal western diet in LBW male (left) and female (right) guinea pigs in young adulthood.
Acknowledgements
We gratefully acknowledge and thank Kristyn Dunlop and Ousseynou Sarr for animal support as well as Stacey Holman, Emma Bradshaw and Megan Quinn for their assistance in Western Blot and qRT-PCR procedures.
Financial support
JLM was funded by a NHMRC Career Development Fellowship (APP1066916) and an ARC Future Fellowship (Level 3; FT170100431). JRTD was funded by an Australian Government Research Training Program (RTP) scholarship. Animal studies were supported by the Canadian Institute of Health Research (TRHR: Operating Grant # MOP-209113).
Conflict of interest
The authors have no conflict of interest.
Author contributions
JRTD: acquisition, analysis and interpretation of data and drafting the manuscript. JC: acquisition, analysis and interpretation of data and drafting the manuscript. TRHR: conception/design of the work, acquisition, analysis and interpretation of data and revising the manuscript critically for important intellectual content. JLM: conception/design of the work, analysis and interpretation of data and revising the manuscript critically for important intellectual content.