Introduction
The incidence of atopic diseases has increased significantly during the last few decades.Reference Eder, Ege and von Mutius 1 Sensitization to allergens (i.e. atopy) is thought to occur early in life, perhaps in utero.Reference Holt, Naspitz and Warner 2 Thus, early exposure to exogenous factors that may lead to a bias toward a T-helper 2-dominated cytokine pattern, which is a hallmark for type 1 allergy, might play an important role in sensitization. The increased susceptibility to atopic disease is thought to be mediated at least in part by fetal programming.Reference Prescott and Dunstan 3 , Reference Hersoug and Linneberg 4 This concept states that the nutritional environment during early development affects health and disease in adulthood, probably via epigenetic mechanisms like DNA methylation, histone modifications and/or RNA silencing.Reference Dolinoy, Das, Weidman and Jirtle 5 Both animal experiments and epidemiological studies indicate that maternal diet during pregnancy and lactation or the neonatal diet can alter disease susceptibility in the offspring.Reference Burdge, Hanson, Slater-Jefferies and Lillycrop 6 , Reference Gluckman, Hanson, Cooper and Thornburg 7
Linoleic acid and α-linolenic acid are essential polyunsaturated fatty acids (eFAs) and must be acquired from the diet. They are the precursors for the long-chain polyunsaturated fatty acids (lcPUFAs) arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which affect immune function and so might influence susceptibility to immune disorders.Reference Enke, Seyfarth, Schleussner and Markert 8 Interestingly, the intake of eFAs and lcPUFAs has changed more or less concurrently with the changes in the prevalence of atopic disease. In short, the intake of ω-3-polyunsaturated fatty acids has decreased, whereas the intake of ω-6-polyunsaturated fatty acids has increased during the last decades.Reference Simopoulos 9
lcPUFAs can influence immune cell function via several mechanisms. lcPUFAs are the precursors for eicosanoids and other lipid mediators. They can not only alter plasma membrane lipid raft composition but also influence cell signaling, cell proliferation and apoptosis.Reference Brassard, Larbi and Grenier 10 – Reference Shaikh and Edidin 12 ω-3- and ω-6-lcPUFAs differ in their effects on immune cells. While ω-6-lcPUFAs give rise to pro-inflammatory eicosanoids and stimulate T-helper 2 cell proliferation, ω-3-lcPUFAs promote T-helper 1 and even regulatory T-cell type of reaction and are converted into less potent eicosanoids.Reference Enke, Seyfarth, Schleussner and Markert 8 , Reference Sierra, Lara-Villoslada, Comalada, Olivares and Xaus 13 Apart from these direct effects, which are only observed as long as the specific lcPUFAs are present, lcPUFAs have been shown to regulate gene transcription in a number of ways as well. lcPUFAs can influence signal transduction cascades and they can also alter the de novo synthesis of transcription factors. More importantly, lcPUFAs and their metabolites can serve as ligands for certain transcription factors and thus regulate gene expression. This could lead to long-term programming of immune function.Reference Duplus and Forest 14 – Reference Hihi, Michalik and Wahli 16
In humans, the long-term effects of pre- and postnatal exposure to ω-3-lcPUFAs through maternal supplementation have been observed on allergy development in the offspring. Neonates, whose mother was exposed to fish oil supplements, had decreased cord blood T-helper 2 cytokine expression.Reference Krauss-Etschmann, Hartl and Rzehak 17 In addition, fish oil supplementation during pregnancy lowered cord plasma interleukin (IL)-13 concentration.Reference Dunstan, Mori and Barden 18 IL-13 is a well-known inducer of classical type 1 allergy in humans and animals. In addition, offspring of mothers who took fish oil during pregnancy were less susceptible to egg sensitization and were less prone to develop severe atopic dermatitis at 1 year of age.Reference Dunstan, Mori and Barden 19 At 2 years of age, maternal dietary ω-3-lcPUFAs increased offspring's ex vivo interferon (IFN)-γ production,Reference Lauritzen, Hoppe, Straarup and Michaelsen 20 which is indicative of lower susceptibility to allergy development since IFN-γ is a crucial T-helper 1 type of cytokine. Consumption of fish by pregnant women was inversely related to the risk of developing asthma in their children at the age of fiveReference Salam, Li, Langholz and Gilliland 21 and to the risk of atopy-related outcomes in children at the age of 1 and 6 years, respectively.Reference Romieu, Torrent and Garcia-Esteban 22 Although these studies show the effects of early exposure to ω-3-lcPUFAs, the main ω-3 PUFA in the diet is eFA α-linolenic acid, while the major dietary ω-6 PUFA is linoleic acid. Little is known about the effects of early exposure to these eFAs on later immune responses. It seems important to investigate the effect of changes in early dietary exposure to ω-3 and ω-6 eFAs since such changes have occurred in the recent past in humans.Reference Simopoulos 9
Since mammals cannot synthesize polyunsaturated fatty acids de novo, both the fetus and neonate acquire eFAs and lcPUFAs via the maternal diet; first via placental transferReference Gottrand 23 and later via breast milk.Reference Gibson, Neumann and Makrides 24 – Reference D'Asti, Long and Tremblay-Mercier 28 Since lcPUFAs can alter gene expression when present in the developing fetus or neonate, they could very well have programming effects.
In this study, the programming effects of perinatal maternal eFA exposure on the offspring's immune response were studied, using the ovalbumin (OVA)-induced allergy model to examine the allergic responseReference Deurloo, van Esch, Hofstra, Nijkamp and van Oosterhout 29 and the flu-vaccination model to assess the vaccination response.Reference Vos, Haarman and Buco 30
Methods
Chemicals
OVA (Grade V), urethane, β-mercaptoethanol, lipopolysaccharide (Escherichia coli, B55:O55), concanavalin A (ConA) from Canavalia ensiformis type IV, and o-phenylenediamine dihydrochloride were obtained from Sigma (St. Louis, USA). Aluminum hydroxide was purchased from Pierce (Rockford, USA). Phosphate buffered saline (PBS) was obtained from BioWhittaker (Verviers, Belgium). Tween20 was purchased from Merck (Whitehouse Station, NJ, USA). Rat-anti-mouse-IgG1-biotin, rat-anti-mouse-IgG2a-biotin and rat-anti-mouse IgE were purchased from BD Pharmingen (Heerhugowaard, The Netherlands). Streptavidin-HRP was obtained from Biosource (Etten-Leur, The Netherlands). Bovine serum albumin grade V and anti-DIG-Fab fragments-HRP were obtained from Roche (Roche Diagnostics, GmbH, Mannheim, Germany). RMPI-1640 medium was obtained from Lonza (Lonza Group Ltd, Basel, Switzerland). Anti-mouse CD3mAb (clone 17A2rIgG2b) was purified from a hybridoma obtained from the American Type Tissue Collection (Manassas, VA, USA). Fetal calf serum (FCS), penicillin and streptomycin were from Life Technologies (Gaithersburg, MD, USA). All flow cytometry antibodies were obtained from BD Biosciences (Breda, The Netherlands), except for F4/80-FITC mAb, which was purchased from AbD Serotec (Oxford, UK). Anti-FoxP3-APC, Fix/perm- and permeabilization buffer were bought from EBioscience (San Diego, CA, USA). Influvac 2008/2009 was purchased from Solvay Pharma B.V. (Weesp, The Netherlands).
Animals
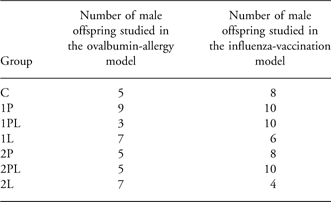
Twelve-week-old BALB/C and C57BL/6 mice were obtained from Harlan (Horst, The Netherlands) and housed at constant temperature (20°C) and humidity (40–60%) in a 12:12 h light/dark cycle in the animal facility of Utrecht University. On arrival, mice were put on control diet (Table 1). After 1 week, female mice were mated and randomly assigned to one of seven groups fed a control diet, a low ω-6/ω-3 eFω ratio diet (diet 1) or a high ω-6/ω-3 eFA ratio diet (diet 2), see Figure 1 and Table 2 for study design and Table 1 for diet composition. At weaning, blood samples were taken by submandibular puncture from both dams and male pups, which were subsequently fed a Western-style diet until the end of the experiment. Three or four weeks after weaning, the OVA-allergy model and the influenza-vaccination model, respectively, were started.
Table 1 Fatty acid composition of the different diets

Values are percentage of total fatty acids.

Fig. 1 Study design. Dams were fed control and/or one of the eFA diets during pregnancy (P), pregnancy and lactation (PL) or lactation (L). After weaning, all pups were fed the Western-style diet until the end of the experiment.
Table 2 Number of offspring in each group

OVA-induced allergy model
Male mice were sensitized to OVA at 6 and at 7 weeks of age by i.p. injection of 10 μg OVA adsorbed into 22.5 mg aluminum hydroxide in 100 μl saline. One day before the first sensitization, a blood sample was taken by submandibular puncture.Reference Deurloo, van Esch, Hofstra, Nijkamp and van Oosterhout 29 Acute allergic skin reaction (ASR) was measured at 9 weeks of age by s.c. injection of 1 pg OVA in 25 μl PBS into the pinna of one ear. The pinna of the other ear was injected with 25 μl PBS. Ear thickness, as a readout for a T-helper 2 mediated allergic reaction, was measured in duplicate before antigen challenge and 1 h afterward, using a digital micrometer (Mitutoyo Digimatic 293561; Veenendaal, The Netherlands). The ASR was calculated by subtracting the basal ear thickness from the value at 1 h after challenge, correcting for the ear swelling that occurred in the PBS-injected ear. At the age of 11 weeks, mice were euthanized by intraperitoneal injection of 1 ml 10% urethane and blood was collected via cardiac puncture.
Flu-vaccination model
Male mice were vaccinated at 7 and 9 weeks of age by s.c. injection of 100 μl Influvac 2008/2009. One day before the first sensitization, a blood sample was taken by submandibular puncture.Reference Vos, Haarman and Buco 30 Delayed-type hypersensitivity responses (DTH) were induced at the age of 11 weeks by s.c. injection of 25 μl Influvac into the pinna of one ear, while 25 μl of PBS was injected into the other ear. Ear thickness, as a readout for T-helper-1 dependent cellular immunity, was measured in duplicate before antigen challenge and 24 h afterwards, with a digital micrometer (Mitutoyo Digimatic). The DTH response was calculated by subtracting the basal ear thickness from the value at 24 h after challenge, correcting for the ear swelling that occurred as a result of s.c. injection of PBS. Afterward, mice were euthanized by intraperitoneal injection of 1 ml 10% urethane. Blood was collected via cardiac puncture.
Analysis of body composition
At 4 and 10 weeks of age, fat mass and lean body mass were measured by Dual Energy X-ray Absorptiometry (DEXA) under general anesthesia (isoflurane/N2O/O2) using a PIXImus imager (GE Lunar, Madison, WI, USA). In addition, the weight of each mouse was recorded weekly over the course of the experiment.
Animal care and use were performed in accordance with the guidelines of the Dutch Committee of Animal Experiments and the local university ethics committee.
Diets
All semi-purified diets were AIN93 based and obtained from Research Diet Services (Wijk bij Duurstede, The Netherlands). All diets consisted of 20% casein, 37% cornstarch, 13% maltodextrin, 10% dextrose, 5% Vitacel L600-20 (cellulose fiber), 5% mixture of vitamins and minerals and 10% fat. The fatty acid composition of the different diets is listed in Table 1. The ratio of ω-6 to ω-3 eFAs was 10.8 in the control diet, 1.4 in the low ω-6/ω-3 eFA ratio diet (diet 1), 30 in the high ω-6/ω-3 eFA ratio diet (diet 2) and 5.3 in the Western diet.
Fatty acid analysis of erythrocytes
Blood was collected into EDTA tubes and stored on ice. Samples were centrifuged at 14,000 rpm for 5 min and plasma was removed for antibody analysis. Erythrocytes were washed twice with PBS containing 5 mM EDTA and then resuspended in 200 μl PBS-EDTA and stored at −80°C until analysis. Erythrocyte lipids were extracted as described previouslyReference Bligh and Dyer 31 and the fatty acid profile was analyzed using GC.
Analysis of plasma antibody concentrations
Influvac-specific immunoglobulins and OVA-specific IgE were measured as described earlier.Reference Deurloo, van Esch, Hofstra, Nijkamp and van Oosterhout 29 , Reference Vos, Haarman and Buco 30 For determination of OVA-specific IgG2a and IgG1, plates were coated overnight at 4°C with 1 μg OVA in PBS. Subsequent steps were performed at room temperature. Between each step, the plates were washed with PBS, 0.05% Tween20. Then the plates were blocked and 100 μl diluted plasma was added. After 2 h of incubation, the plates were incubated for 1.5 h with 50 μl anti-IgG1-biotin or anti-IgG2a-biotin antibodies (diluted 1:1000). Later, the plates were incubated with a 1:20,000 dilution of streptavidin-HRP. Color development was performed with o-phenylenediamine-dihydrochloride (0.4 mg/ml) and 4 mM H2O2.. The reaction was stopped by adding 4 M sulfuric acid. The OD was read at 490 nm, using a Bio-Rad iMark plate reader (Bio-Rad Laboratories, Hercules, CA, USA). Results were analyzed with MicroplateManager PC software (Bio-Rad Laboratories). Concentrations in test sera were calculated in arbitrary units, relative to a standard curve of pooled plasma.
Ex vivo cytokine production
Splenocyte restimulation assay
For the OVA-allergy model, small pieces of spleen were gently pressed through nylon mesh filters (Falcon cell strainer; Becton Dickinson, Alphen a/d Rijn, The Netherlands). 100,000 cells were incubated with RMPI-1640 medium supplemented with 10% FCS, 100 U/ml penicillin and 100 μg/ml streptomycin (culture medium) with or without 50 μg/ml anti-mouse CD3mAb or 10 mg/ml OVA. After 5 days of culture at 37°C in a humidified environment containing 5% CO2, supernatants were harvested and stored at −80°C until further analysis. The supernatants were analyzed for IFN-γ, IL-2, IL-4, IL-5, IL-10, IL-12 and IL-13 using a mouse cytokine multiplex bead array according to the manufacturer's instructions (x-plex reagents; BioRad, Veenendaal, The Netherlands) on a Bio-Plex workstation (BioRad).
Whole blood assay
For the influenza-vaccination model, whole blood stimulations were carried out as described earlier.Reference Faber, Vos and Kegler 32 Briefly, blood was collected into heparin tubes. 50 μl blood was incubated with culture medium with or without 0.1 μg/ml Influvac or 40 μg/ml ConA for 20 h (Influvac) or 44 h (ConA) at 37°C in a humidified environment containing 5% CO2, after which supernatants were harvested and stored at −80°C until further analysis. Cytokine levels were measured using a commercial mouse cytokine multiplex bead immunoassay (Bio-Rad, Veenendaal, The Netherlands) according to the manufacturer's protocol on a Luminex workstation (Luminex BV, Oosterhout, The Netherlands).
Flow cytometric analysis
Single cell suspensions were prepared from the spleen and thymus tissue, as described for the splenocyte restimulation assay and, unless indicated otherwise, all incubations were performed at room temperature in the dark. 100,000 cells were blocked in PBS, 1% BSA, 5% FCS for 30 min. Next, the cells were spun down, taken up in FACS-buffer (PBS, 1% BSA) and incubated with fluorescent-labeled antibodies for 60 min. The following monoclonal antibodies were used: Armenian hamster anti-mouse CD3-PerCPCy5.5, rat anti-mouse CD4-FITC, rat anti-mouse CD8a-APC, rat anti-mouse CD69-PE, rat anti-mouse CD62L-PE, rat anti-mouse F4/80-FITC, rat anti-mouse CD11b- PerCP-Cy5.5, rat anti-mouse CD94-FITC, rat anti-mouse NK1.1-APC, rat anti-mouse CD-25-PE and rat anti-mouse FoxP3-APC. Then the cells were washed three times and resuspended in 100 μl FACS buffer.
After staining for surface markers, 200,000 cells that were used for analysis of CD3/CD4/CD25/FoxP3 expression were permeabilized using Fix/Perm buffer for 30 min at 4°C. Cells were incubated with Perm/Wash buffer for 10 min and spun down, twice. The cells were resuspended in 100 μl blocking buffer for 15 min, and subsequently, 1 μl anti-FoxP3 was added for 30 min. Cells were washed twice and resuspended in FACS buffer.
Flow cytometric analysis was carried out on either a Becton Dickinson FACS Calibur flow cytometer (Breda, The Netherlands) using FCS Express version 3 software (De Novo Software, Los Angeles, CA, USA) or a Becton Dickinson FacsCanto flow cytometer (Breda, The Netherlands) using BD FACSDiva Software version 6.1.2 (BD Biosciences, San Jose, California).
Statistical analysis
All data are expressed as mean ± s.e.m. Statistical analyses were performed using SPSS 15.0 (SPSS Benelux, Gorinchem, The Netherlands). Differences were considered significant at P < 0.05. Variables were checked for Gaussian distribution with the Shapiro–Wilkes test. Levene's test for equality of variance was used to estimate the probability that treatment groups had different variances. One-way analysis of variance (ANOVA) was used to detect differences between treatment groups if all conditions were met; otherwise, the non-parametric Kruskal–Wallis test was performed.
Results
In order to study the long-term programming effect of perinatal eFA supplementation on the immune system, pregnant and/or lactating dams were fed diets varying in the ω-6/ω-3 eFA ratio. Subsequently, the immune response in the adult offspring was analyzed. The vaccination modelReference Vos, Haarman and Buco 30 was used to investigate the programming effects on a T-helper 1 mediated immune response that is essential for infection resistance. The effects on allergy were examined using the T-helper 2 mediated OVA-induced allergy model.Reference Deurloo, van Esch, Hofstra, Nijkamp and van Oosterhout 29
Fatty acid composition of erythrocytes
In order to investigate whether eFAs from the maternal diet were transferred to the pups, red blood cell (RBC) fatty acid composition in both dams and pups was measured at weaning.
The dams on the low ω-6/ω-3-eFA ratio diet (diet 1) during pregnancy and lactation (group 1PL in Fig. 2) had significantly higher levels of α-linolenic acid (ALA) and its derivative EPA in their RBCs compared to all other groups except dams fed this diet during lactation only (Group 1L in Fig. 2). The DHA levels in dams in group 1PL differed only from the dams fed the high ω-6/ω-3-eFA ratio diet (diet 2), either during pregnancy (Group 2P), or during pregnancy and lactation (Group 2PL). No significant differences were found for other fatty acids. There was a non-significant trend toward lower AA, EPA and DHA levels in the BALB/C dams compared to the C57BL/6 dams (results not shown).

Fig. 2 Maternal red blood cell polyunsaturated fatty acid content at the time of weaning. Data from BALB/C and C57BL/6 mice were combined, since there were only a few dams in each group. Dams were fed the control diet (C), low ω-6/ω-3 eFA ratio diet (diet 1) or high ω-6/ω-3 eFA ratio diet (diet 2) during pregnancy (P), pregnancy and lactation (PL) or lactation (L). Values are given as means ± s.e.m. Groups which do not share a letter have a significantly different percentage of that fatty acid, P < 0.05. For instance, for ALA 1L (designated ab) does not differ from either 1P (a) or 1PL (b), but 1P (a) and 1PL (b) do have significantly different ALA levels.
The RBC ALA and EPA content of all pups mirrored that of their mother and the maternal diet during lactation. All pups on the low ω-6/ω-3-eFA ratio diet (diet 1) during lactation (Group 1L) and pregnancy and lactation (Group 1PL) had higher levels of these fatty acids compared to the other groups (Fig. 3).

Fig. 3 Red blood cell polyunsaturated fatty acid content at weaning in BALB/C (panel a) and C57BL/6 (panel b) offspring of mice fed the control diet (C), low ω-6/ω-3 eFA ratio diet (diet 1) or high ω-6/ω-3 eFA ratio diet (diet 2) during pregnancy (P), pregnancy and lactation (PL) or lactation (L). Values are given as means ± s.e.m. Groups which do not share a letter have a significantly different percentage of that fatty acid, P < 0.05.
In the C57BL/6 pups (Fig. 3, panel b), AA levels also reflected the maternal diet; pups on the low ω-6/ω-3-eFA ratio diet (diet 1) during lactation and pregnancy (Group 1PL) and lactation (Group 1L) had lower AA levels compared to the other groups (Fig. 3). Surprisingly, in the BALB/C pups (panel a), there was no difference in AA between groups at weaning. In contrast, the BALB/C pups’ DHA levels mirrored the maternal diet; control pups and pups on the high ω-6/ω-3-eFA ratio diet (diet 2) had similar DHA levels, which were lower than the levels of the pups on the low ω-6/ω-3-eFA ratio diet (diet 1). In the C57BL/6 pups, however, only the control pups had lower DHA levels compared to all other dietary groups.
Overall, at weaning, the BALB/C pups had lower EPA and DHA levels compared to the C57BL/6 pups on the same diet. No differences between groups or mice strains were found in linoleic acid levels (results not shown).
Before the first sensitization with OVA and first vaccination (3 and 4 weeks, respectively, after weaning), RBC fatty acid content was analyzed again to identify whether the earlier differences in fatty acid composition due to maternal diet were retained. At this time point, the levels of EPA and DHA in the BALB/C offspring of dams on the low ω-6/ω-3-eFA ratio diet during lactation (1L) and during pregnancy and lactation (1PL) were slightly but significantly higher compared to the other groups (results not shown). No significant differences in the levels of other fatty acids between the dietary groups were found in BALB/C mice at this time point (results not shown).
In contrast to the levels at weaning, at immunization BALB/C mice had higher AA and DHA levels compared to C57BL/6 mice on the same diet. RBCs of C57BL/6 mice on the control diet contained 4.3% ± 1.0 AA, while the RBCs of BALB/C mice on the control diet contained 14.0% ± 0.6 AA at the time of immunization, P < 0.05. At this time point, the RBC DHA level was 1.4% ± 0.3 in control-fed C57Bl/6 mice and 3.1% ± 0.3 in control-fed BALB/C mice, P < 0.05.
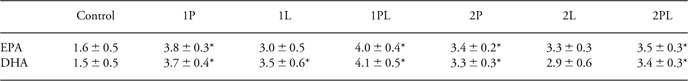
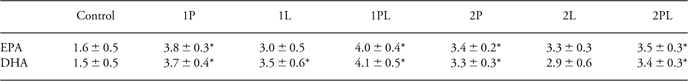
At the end of the experiment, after 8 weeks on the Western-style diet, no differences between groups in RBC fatty acid levels were found in the BALB/C mice. In the C57BL/6 mice, some groups had higher DHA and/or EPA levels compared to control mice (Table 3).
Table 3 Red blood cell EPA and DHA content
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Percentage of EPA and DHA of total fatty acids in red blood cells in offspring of C57BL/6 mice fed control diet (C), low ω-6/ω-3 eFA ratio diet (diet 1) or high ω-6/ω-3 eFA ratio diet (diet 2) during pregnancy (P), pregnancy and lactation (PL) or lactation (L), at the end of the experiment. Values are given as means ± s.e.m.
*Significantly different compared to control, P < 0.05.
Similar to what was observed in control mice before immunization, at the end of the experiment BALB/C mice had higher levels of AA and DHA in their RBCs compared to C57BL/6 mice on the same diet. As RBCs of control-fed C57BL/6 mice contained 4.4% ± 3.7 AA, three-fold more AA (14.5% ± 0.3) was found in RBCs from BALB/C mice on the control diet, P < 0.05. At this time point, the RBC from C57Bl/6 mice on the control diet contained 1.5% ± 0.8 DHA, while 3.8% ± 0.1 DHA was found in control-fed BALB/C mice, P < 0.05. No differences were found in EPA levels between the two mice strains (results not shown).
Acute allergic skin response and delayed type hypersensitivity (DTH) response
To analyze whether the maternal diet affected the immune response in her adult offspring, ear swelling responses were measured; the acute ASR (ear swelling at 1 h) in the OVA-sensitized mice as a validated readout for T-helper 2 mediated allergy and the DTH response (ear swelling at 24 h) as a validated readout for T-helper 1 mediated immunity in the influenza-vaccinated mice.
Offspring of dams fed the low ω-6/ω-3-eFA ratio diet during lactation (1L) showed an enhanced DTH response against the vaccine, compared to the control group (see Fig. 4a), indicating an improved cellular immunity to viral antigens. Interestingly, this group also showed the most prominent attenuation of the T-helper-2 dependent ASR (Fig. 4b). All eFA diets diminished the ASR, but the extent of the effect was highly dependent on the feeding period and was not similar between the two eFA diets. While the low ω-6/ω-3-eFA ratio diet significantly reduced the ASR in the offspring most when fed during lactation (1L), as compared to the other groups on this diet, the high ω-6/ω-3-eFA ratio diet was most effective when fed during pregnancy (2P), as compared to the groups on this diet during lactation (2L) and during pregnancy and lactation (2PL).

Fig. 4 T-helper 1 mediated delayed type hypersensitivity response (DTH; panel a) and T-helper 2 mediated acute skin response (ASR; panel b) in offspring of mice fed the control diet (C), low ω-6/ω-3 eFA ratio diet (diet 1) or high ω-6/ω-3 eFA ratio diet (diet 2) during pregnancy (P), pregnancy and lactation (PL) or lactation (L). Values are given as means ± s.e.m. Groups which do not share a letter have a significantly different earswelling response, P < 0.05. For instance, the ASR response of 1PL (designated bd) does differ from the control group (a) and from 1L (c), but not from 1P (b) or 2L (d).
Ex vivo cytokine production
In the OVA allergy model, ex vivo splenocyte cytokine production, and in the influenza-vaccination model, ex vivo whole blood cell cytokine production were analyzed. No significant differences were observed for any of the cytokines measured (data not shown), except for CXCL1 in the influenza-vaccination model. CXCL1 levels in the 1P (20.8 pg/ml ± 4.8) and the 2PL (18.0 pg/ml ± 3.7) groups were significantly lower compared to the control group (51.6 pg/ml ± 7.6). We observed a trend toward lower CXCL1 levels in other groups (data not shown), but these differences did not reach statistical significance.
Plasma antibody concentrations
Plasma concentrations of OVA-specific IgE, IgG2a and IgG1 were determined in OVA-allergic mice and of influvac-specific IgG2a and IgG1 in the vaccinated mice. Although detectable levels of immunoglobulins were observed in all immunized groups, no significant differences could be detected between groups (data not shown).
Flow cytometric analysis
To investigate whether the maternal diet influenced the offspring's immune cell populations, the percentages of different cell types in the offspring's thymus and spleen were determined.
Significant differences were found between different feeding periods of the same diet in the OVA-allergic mice, especially with the low ω-6/ω-3-eFA ratio diet. While the mice fed this diet during pregnancy (1P) had a similar pattern of immune cells in the thymus and spleen, as compared to control, the mice whose mother was fed this diet during pregnancy and lactation (1PL) and during lactation only (1L) had significantly different levels of almost all analyzed cell types (Tables 4 and 5). Offspring of dams fed the high ω-6/ω-3-eFA ratio diet during lactation (2L) had increased numbers of CD3+CD4+ T-helper cells in both the thymus and spleen, as compared to the groups fed this diet during pregnancy (2P) and pregnancy plus lactation (2PL; P < 0.05). In contrast, the percentage of macrophages (F4/80+ cells) was only elevated in spleens of the offspring of the dams fed the high ω-6/ω-3-eFA ratio diet during pregnancy and lactation (2PL) but not in the offspring of dams fed this diet during only pregnancy (2P) or only lactation (2PL; P < 0.05). All dietary groups, except for the offspring of dams fed the low ω-6/ω-3-eFA ratio diet during pregnancy (1P), showed an increase in regulatory T-cells (CD4+CD25+FoxP3+) in both the thymus and spleen, as compared to control.
Table 4 Thymocytes of ovalbumin (OVA)-sensitized mice

Cell subtypes in thymus of OVA-sensitized offspring of mice fed control diet (C), low ω-6/ω-3 eFA ratio diet (diet 1) or high ω-6/ω-3 eFA ratio diet (diet 2) during pregnancy (P), pregnancy and lactation (PL) or lactation (L). Values are given as mean percentage ± s.e.m.
*Significantly different from control, P < 0.05.
Table 5 Splenocytes of ovalbumin (OVA)-sensitized mice

Cell subtypes in spleen of OVA-sensitized offspring of mice fed control diet (C), low ω-6/ω-3 eFA ratio diet (diet 1) or high ω-6/ω-3 eFA ratio diet (diet 2) during pregnancy (P), pregnancy and lactation (PL) or lactation (L). Values are given as mean percentage ± s.e.m.
*Significantly different from control, P < 0.05. x = no data available.
In contrast to the thymocyte and splenocyte populations from the OVA-allergic mice, none of the studied cell populations were found to differ between groups in the influenza-vaccination model (data not shown).
Body composition
No differences were found in growth, body weight and fat percentage, as measured by DEXA, between groups at weaning (results not shown). In addition, no differences were found in body weight, weight gain and fat percentage at the end of the experiment (results not shown).
Discussion
This study indicates that the maternal diet, specifically the type of fatty acids that it contains, can influence the immune response in the offspring in early adulthood. Using validated animal models for allergy and vaccination responses, we show that a higher intake of ALA during the lactation period has a prominent effect on the offspring's immune response at a later time, resulting in a lower T-helper 2 mediated allergic response and an enhanced T-helper 1 mediated vaccination response. As dietary eFAs are readily converted into lc-PUFAs in mice, it is not possible to discriminate between the effects of eFAs and their derivatives, but the overall implication is that it is possible to program immune responses by altered dietary exposures early in life. The findings of this work suggest that in mice the window of greatest susceptibility to immune programming by diet is the lactation/suckling period.
The marked effect of the low ω-6/ω-3-eFA ratio diet during lactation suggests a requirement for transfer of relevant bioactive polyunsaturated fatty acids from the maternal diet to the offspring via the milk, as has been demonstrated earlier in other studies in rodents and man.Reference Gibson, Neumann and Makrides 24 – Reference D'Asti, Long and Tremblay-Mercier 28 , Reference Oosting, Kegler and Boehm 33 Indeed, in this study, increased levels of ALA and of its derivative EPA were found in the RBCs of the offspring of dams fed the low ω-6/ω-3-eFA ratio diet during lactation, indicating that such transfer occurred, as RBC fatty acid composition is considered to reflect the pattern of fatty acid intake.Reference Katan, Deslypere, van Birgelen, Penders and Zegwaard 34
In the OVA allergy model, feeding of eFA diets during pregnancy only had an effect on the ASR in the offspring, suggesting that there is transfer of eFAs from the maternal diet to the offspring via the placenta as well. Alternatively, eFA-fed dams, as compared to those fed the control diet, might transfer different immune-active compounds (for instance, cytokines) via the placenta or via milk to their offspring, which could result in the diminished OVA-induced immune response in the offspring. Group 1PL also showed a decreased ASR compared to the control mice; however, this group consists of only three mice, and therefore it should be realized that the low number of animals in this group makes statistical analysis difficult.
In the OVA allergy model, two groups (1L and 1PL) had higher levels of EPA and DHA in their RBCs at the time of sensitization. Although it is not entirely certain that the elevated EPA and DHA levels at the time of sensitization did not influence the ASR in these groups, it is very unlikely that the diminished ASR is a direct effect instead of a programming effect, since the offspring of the other experimental groups also had reduced ear swelling reactions, as compared to control mice, but with equally low EPA and DHA levels at the time of sensitization as the control mice. EPA and DHA levels before immunization did not correlate with the severity of the ASR. In addition, the EPA and DHA levels were higher in the 1PL group than the 1L group while the 1L group had a lower ASR, as compared to the 1PL group, suggesting that there is a programming effect instead of a direct effect of these lcPUFAs, as with a direct effect, one would expect the group with the highest levels of EPA and DHA to have the lowest ASR, which was not observed in this study.
Since the 1PL and 1L groups had higher EPA and DHA levels at the time of sensitization, the RBC AA/(EPA+DHA) ratio is also lower in these groups, as compared to the other groups. No differences in the RBC AA/(EPA+DHA) ratio at this time point were observed between the other groups. Furthermore, attenuation of allergic symptoms is not expected as a direct effect of ω-6 fatty acids, since these give rise to pro-inflammatory and pro-allergic eicosanoids, again suggesting that the observed diminished ASR is a programming effect.
In contrast to rodents, humans do not readily convert the eFAs into lcPUFAs. Therefore, most studies in humans are performed with lcPUFA supplementation. Analogous to reports from human epidemiologyReference Salam, Li, Langholz and Gilliland 21 , Reference Romieu, Torrent and Garcia-Esteban 22 and clinicalReference Dunstan, Mori and Barden 19 studies using lcPUFAs, we observed a significant effect of perinatal eFA exposure on allergy development.
In contrast to the studies in humans,Reference Lauritzen, Hoppe, Straarup and Michaelsen 20 we did not observe any effects on cytokine levels after ex vivo restimulation of splenocytes from adult offspring of dams fed the high ω-3-eFA diet. Possibly the difference in developmental stage (human infants v. adult mice) underlies this observation.
In contrast to ex vivo cytokine production, differences in immune cell subsets in the thymus and spleen were found. All experimental groups, except for 1P, had a greater proportion of CD3+CD4+CD25+FoxP3+ regulatory T-cells in the thymus and spleen. Group 1PL also showed an increase in the percentage of this cell population, as compared to the control mice; however, this group consists of only three mice, and therefore it should be realized that the low number of animals in this group makes statistical analysis difficult. Increased numbers of tolerogenic T-cells might very well contribute to the observed diminished allergic reaction in these groups. In addition, these groups had a lower proportion of CD19+ splenic B-cells, as compared to the control group, which might suggest that these mice had diminished capacity to produce antibodies, although no differences were found in plasma OVA-specific IgE, IgG2a and IgG1 concentrations. No changes in cell subsets could be seen in the group on the low ω-6/ω-3-eFA ratio diet during pregnancy, suggesting that in this group other mechanisms may underlie the attenuation of the ASR. The lack of a clear correlation between the ASR and the percentages of cell types, antibody and cytokine levels, however, does not mean that these differences are of no relevance. The immune system is very complex and immune responses are not controlled by one particular cell type, but rather by the interactions between several cell populations working in an integrated manner in response to an immunologic challenge. The differences in cell populations and the small and non-significant variations in antibody levels and splenocyte cytokine production might together underlie the observed differences in ASR. In addition, since these parameters were analyzed only at the end of the experiment, important shifts in certain cell types and cytokines at the time of sensitization might no longer be detectable.
Interestingly, the feeding period resulting in the most pronounced effects regarding the allergic response differed for the two maternal diets used in this study. The low ω-6/ω-3-eFA ratio diet was most effective when fed during lactation, while the high ω-6/ω-3-eFA ratio diet suppressed the ASR most significantly when fed during pregnancy, indicating that, although both diets have a programming effect on the offspring's allergic response, they might work via different mechanisms and at different ‘windows of opportunity’, as is indicated by the differences in cell subsets between mice fed the same diet but during different feeding periods.
In the influenza-vaccination model, an increase in vaccination responsiveness was observed in the offspring of mice fed the low ω-6/ω-3-eFA ratio diet during lactation. We assume this increase in the vaccination response to be a programming effect, since no significant differences in the RBC AA, EPA or DHA levels at the time of immunization were observed, indicating that the differences in the lcPUFA levels at the end of the experiment do not represent residual lcPUFAs from the maternal diet, but (possibly programmed) differences in PUFA metabolism. In contrast to the OVA-allergic mice, no differences were found in thymic or splenic cell subsets between the groups in this model. All dietary groups had lower levels of the neutrophil-attracting chemokine CXCL1Reference Moser, Clark-Lewis, Zwahlen and Baggiolini 35 , Reference Schumacher, Clark-Lewis, Baggiolini and Moser 36 after ex vivo stimulation of blood cells, as compared to control. The CXCL1 levels, however, did not correlate with the vaccination response. Group 2L also showed a decreased level of CXCL1, as compared to the control mice; however, this group consists of only four mice, and therefore it should be realized that the low number of animals in this group makes statistical analysis difficult. As for the OVA allergy model, in this model it is very likely that differences observed in the in vivo immunological response are due to differences in processes that took place at the time of vaccination. These differences may lead to subtle changes in the balance of various individual parameters, but altogether might lead to detectable changes if in vivo, that is, holistic, tests such as DTH measurement are used.
Interestingly, the observed increase in the vaccination response in the offspring of dams fed the low ω-6/ω-3-eFA ratio diet during lactation and the non-significant trend in the other groups on this diet, together with the increased number of regulatory T-cells found in the OVA allergy model, might contribute to the dampened Th2-allergic response in these groups.
Differences in proportions of cell subsets between the two models might be due to differences between the two mouse strains used in this study.Reference Heinzel, Sadick, Holaday, Coffman and Locksley 37 , Reference Mills, Kincaid, Alt, Heilman and Hill 38 In addition, both the antigen as well as the route of sensitization differed between the two models; the OVA-allergic mice were sensitized intraperitoneally with OVA, while the influenza-vaccinated mice were subcutaneously immunized with a seasonal influenza vaccine. Such technical differences may lead to differences in the resulting immune response.Reference Repa, Wild and Hufnagl 39
Surprisingly, the two mouse strains used in this experiment seem to handle dietary eFAs and their derivatives very differently, as is suggested by the differences in RBC AA and DHA content at weaning and at the end of the experiment between C57BL/6 mice and BALB/C mice from the same dietary groups. Possibly during lactation, C57BL/6 dams transfer more lcPUFAs to their milk than do BALB/C dams, which showed a trend toward lower levels of RBC lcPUFAs. Alternatively, C57BL/6 pups might incorporate these fatty acids better into their cells than their BALB/C counterparts.
After weaning, BALB/C mice might synthesize more AA and DHA from LA and ALA than do C57BL/6 mice, possibly to compensate for the lower quantities of these fatty acids they acquired from their mothers during the lactation period, resulting in higher levels, as compared to the C57BL/6 mice at the end of the experiment.
Apart from a programming effect on the immune response, an effect on fatty acid metabolism was observed. The offspring of C57Bl/6 mice on either eFA diet had higher levels of EPA and/or DHA at the age of 10 weeks, compared to controls. Although this was not investigated any further in this study, changes in metabolism by perinatal lcPUFA diet have been described by others.Reference Chapman, Morgan and Murphy 40 , Reference Merzouk and Khan 41
Our findings can contribute to the understanding of earlier studies in human subjects, where long-term beneficial effects of perinatal dietary intervention with ω-3-PUFAs on allergic responses have been observed.Reference Krauss-Etschmann, Hartl and Rzehak 17 – Reference Romieu, Torrent and Garcia-Esteban 22 In this study, we show that there is a difference in effectiveness of the diet which depends on the feeding period, and it is very likely that there is also an optimal ‘window of opportunity’ in man. Therefore, in future clinical studies, it may be useful to first determine the optimal timing for supplementation.
In summary, perinatal exposure to eFAs can program the offspring's immune response. The timing of maternal dietary intake of eFAs affects the extent to which the immune system is programmed. The lactation period appears to be the period that confers most susceptibility to immune programming. The underlying mechanisms leading to the observed differences in the allergic and vaccination responses need to be investigated further.
Acknowledgments
The authors like to thank M. Balvers for technical assistance. Alison Fear was supported by a BBSRC Strategic Research Studentship.
Statement of Interest
None.