Introduction
Bariatric surgery is the most effective treatment for the reduction of the comorbidities of obesity and diabetes, and its popularity is increasing. There are many positive benefits due to surgical weight loss procedures like Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) for both males and females, including loss of body weight and adiposity, improvements in plasma lipid profiles, and reduced need for pharmacologic control of blood glucose.Reference Colquitt, Pickett, Loveman and Frampton1, Reference Himpens, Dobbeleir and Peeters2 Additionally, both result in similar changes in gut hormone secretion in humans,Reference Peterli, Wölnerhanssen and Peters3 rodents,Reference Chambers, Jessen and Ryan4 and comparable remission of type-2 diabetes in humans.Reference Schauer, Kashyap and Wolski5, Reference Stemmer, Bielohuby and Grayson6
Women are overwhelmingly the most common recipients (>80%) of bariatric surgery, and approximately half are of child-bearing age.Reference Grayson, Schneider, Woods and Seeley7 In woman of child-bearing age, some additional reproductive improvements that have been reported after obtaining bariatric surgery include return of menstrual cycles,Reference Teitelman, Grotegut, Williams and Lewis8 recovery of luteal function,Reference Rochester, Jain and Polotsky9 and increased spontaneous (unassisted) pregnancies.Reference Tan and Carr10 Furthermore, after successfully achieving pregnancy, women who have had bariatric surgery have a lower risk of gestational diabetes, gestational hypertension, and preeclampsia.Reference Johansson, Cnattingius and Näslund11, Reference Galazis, Docheva, Simillis and Nicolaides12 Thus, women who undergo weight loss surgery have a significant health benefit for both the mother and child. Unfortunately, there are also numerous reports that highlight negative outcomes in women with previous bariatric surgery, including increased risk for small-for-gestational age babies or intrauterine-growth restriction,Reference Galazis, Docheva, Simillis and Nicolaides12–Reference Baker, Osman and Bodnar14 preterm birth,Reference Galazis, Docheva, Simillis and Nicolaides12 stillbirth, and neonatal death.Reference Johansson, Cnattingius and Näslund11, Reference Gonzalez, Rubio and Cordido15
Though rodent models of bariatric surgery also tout many of the same improvements in metabolic health as reported in the human,Reference Chambers, Jessen and Ryan4, Reference Grayson, Schneider, Woods and Seeley7, Reference Stefater, Pérez–Tilve and Chambers16, Reference Wilson-Perez, Chambers, Sandoval, Stefater, Woods, Benoit and Seeley17 previously, we reported that females that have received VSG prior to pregnancy have offspring that are born smaller and shorter, as well as experience catch-up growth during postnatal life.Reference Grayson, Schneider, Woods and Seeley7 During the first 15–16 days postnatal life, the rat pup is exclusively dependent on ingestion of maternal milk; independent food consumption begins around postnatal day 16 (PD16). Milk composition varies based on nutritional status of the dam and the diet consumed. Previously, studies have indicated that milk yield is reduced in a model of calorie restriction, with a more pronounced decrease occurring when dams are restricted both prior to and during lactation than those primarily restricted during lactation.Reference Parlee and MacDougald18, Reference Lactation19
In the present studies, we hypothesized that the VSG pups would have a reduced rate of growth and that this reduction was due to altered nutrient and hormone content of the milk. We probed whether milk obtained at PND15/16 from dams having received VSG prior to pregnancy and fed a HFD (H-VSG) produced milk of a similar quality to either body weight-matched, chow-fed dams (C-Sham) or diet-matched, HFD-fed obese (H-Sham) dams. We analyzed the growth trajectory during postnatal life, milk macronutrient content, fatty acid composition, and hormone levels.
Methods
Animals
All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee. In the present study, we report on lactating maternal body weights and food intake and milk samples collected from dams PND15–16. Other parameters of these females prior to their pregnancy and their offspring have been previously carefully reported.Reference Grayson, Schneider, Woods and Seeley7
As previously reported,Reference Grayson, Schneider, Woods and Seeley7 adult female long-Evan rats (Harlan Laboratories, Indianapolis, IN, USA; 225–250 g) were individually housed and maintained in a room on a 12/12-h light/dark cycle at 25 °C and 50%–60% humidity. Following acclimatization to the facilities, animals were given ad libitum access to water and either low-fat chow (#7012, Harlan Teklad, 3.41 kcal/g; 5.67% fat) or palatable high-fat diet (HFD) (#D03082706, Research Diets, New Brunswick, NJ, USA, 4.54 kcal/g; 41% fat) for 3 weeks prior to surgery. Animals were assigned in a counter-balanced fashion to three groups: (A) maintained on chow and having received Sham-VSG surgery (C-Sham), (B) maintained on HFD and having received Sham-VSG (H-Sham), and (C) maintained on HFD and having received VSG surgery (H-VSG). The subset of dams used in the present study was previously characterized and contributed to the body of work on VSG pregnancy by Grayson et al. Reference Grayson, Schneider, Woods and Seeley7 In the current study, C-Sham, N = 9; H-SHAM, N = 7; and H-VSG, N = 7 were used.
Surgery
Animals received either Sham-VSG or VSG performed by trained surgical core as described.Reference Grayson, Schneider, Woods and Seeley7 Animals recovered on Osmolite OneCal Liquid diet for 3 days with postoperative saline and analgesics.Reference Grayson, Schneider, Woods and Seeley7 Animals were mated 5 weeks after surgery.
Husbandry
As previously described,Reference Grayson, Schneider, Woods and Seeley7 singly housed males were caged with one female for 4–8 days. Females were returned to their own cages for the remainder of gestation when a significant increase in body weight was measured. Parturition was designated as postnatal day 0 (PND0). Dams were allowed to suckle their own litters. Litters were culled to four females and four males (when possible) on PND2. Pups were weighed on PND2, 7, 14, and 21.
Milk collection
On PND15–16 and 4 h into the light cycle, pups were removed from the dams for 4 h to increase milk reserves. After 4 h, dams were anesthetized with isoflurane and injected intramuscularly with 0.6 units of purified oxytocin (Agrilabs, St Joseph, MO, USA) to stimulate milk ejection. Milk samples were collected by a vacuum pump and frozen at −20 °C until further processing and analysis. Pups were returned to dams following the procedure.
Glucose determination
Milk and blood glucose were measured in duplicate using an AccuChek glucometer with corresponding strips.
Protein albumin determination
Concentrations were determined using a Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL, USA), and spectrometry was performed with a Tecan Infinite 200 PRO. A standard curve with known concentrations of albumin was used to extrapolate the milk protein concentration.
Free fatty acid analysis
Fatty acid analysis was performed at the Cincinnati Mouse Metabolic Phenotyping Center. A subset of milk samples, N = 6/group, were submitted for fractionation. The Shimadzu GC-2010 gas chromatograph (GC) was used to detect methyl esters of free fatty acids. First, samples were saponified with methanolic sodium hydroxide to obtain free fatty acids. Next, fatty acids were methlyated with 14% BF3 methanol to produce fatty acid methyl esters (FAMES). Finally, FAMES were extracted in hexane, and this solution injected into the GC for analysis. Fatty acids were detected by the GC FID, and a peak was formed on the monitor, indicating the presence of a specific fatty acid known to peak at that retention time point after injection. Quantitative analysis was also performed using an internal standard in each sample, which is a known amount of fatty acid normally not present in the sample (heptadecanoic acid).
Measurements of adipokines and analytes
The following analytes were measured according to the manufacturer’s specifications: Infinity Cholesterol Reagent (#TR13421, ThermoFisher, VA, USA), Infinity Triglyceride Reagent (#TR22421, ThermoFisher, VA, USA), insulin (#90060, Crystal Chem, Downers Grove, IL, USA), leptin (#MOB00, R & D System, Minneapolis, MD, USA), adiponectin (#EZRADP-62K, EMD Millipore, Burlington, MA), and rat immunoglobulin (Ig)A (#6410-10, Alpha Diagnostic International, San Antonio, TX) and IgG (#6420, Alpha Diagnostic International).
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 7.2 (GraphPad Software, San Diego, CA, USA). Differences between three treatments were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. To observe time-wise differences, two-way ANOVA with repeated measures was performed using Treatment Group and Time as factors. Further analysis was performed to determine specific differences by Student’s t-tests specifically because animals were body weight-matched or diet-matched. All results are given as means ± SEM. Results were considered statistically significant when P < 0.05.
Results
VSG dams maintained on HFD (H-VSG) prior to and following surgery weighed significantly less than Sham dams maintained on HFD (H-Sham) on postnatal days 15–16 (PND15–16) (P < 0.01) (Fig. 1a). Despite maintenance on diet of differing fat content, all three groups of animals ingested similar amounts of average calories during lactation (Fig. 1b). Body weight curves for the male pups from the dams whose milk was extracted demonstrated that offspring of H-VSG (oH-VSG) have reduced body weight growth during postnatal life in comparison with oC-Sham and oH-Sham (P < 0.05) (Fig. 1c). When the slope of the growth trajectory for the animals (Fig. 1c) is calculated, H-VSG animals have a significantly reduced growth trajectory in comparison with both C-Sham and H-Sham (P < 0.001) (Fig. 1d).

Fig. 1. Dam and offspring weight and growth trajectory. (a) Body weight of dams at PND16. (b) Average daily kcal intake of dams at PND15/16. (c) Body weight curve of offspring of dams from birth to PND21. (d) Calculation of the slope of the postnatal body weight curve of the offspring. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001.
No difference was detected for glucose, protein, cholesterol, or triglyceride content of the milk expressed from animals at PND15–16 by one-way ANOVA (Fig. 2a–d). However, elevated glucose concentrations were detected in H-VSG versus C-Sham (t-test, P < 0.05) (Fig. 2a). Reduced protein concentrations were identified in H-Sham versus H-VSG (t-test, P < 0.05) (Fig. 2b). Furthermore, H-VSG animals had significantly reduced triglyceride content in comparison with H-Sham animals (P < 0.01) (Fig. 2d).

Fig. 2. Macronutrients in milk from PND15/16 dams. (a) Glucose in mg/dl, (b) protein albumin in mg/dl, (c) cholesterol in mg/dl, and (d) triglycerides in mg/dl. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01.
Total lipid content of the milk was significantly reduced in C-Sham (P < 0.01) in comparison with H-Sham and H-VSG (Fig. 3a). As a percent of the total, C-Sham animals generally had increased caprylic, capric, lauric, stearic, and lineoleic acid (Table 1) compared to either H-Sham or H-VSG. However, C-Sham had reduced percentages of palmitic acid and oleic acid (Table 1). There were no differences in the levels of myristic acid (Table 1). Total numbers and percentages are reported in Table 1.
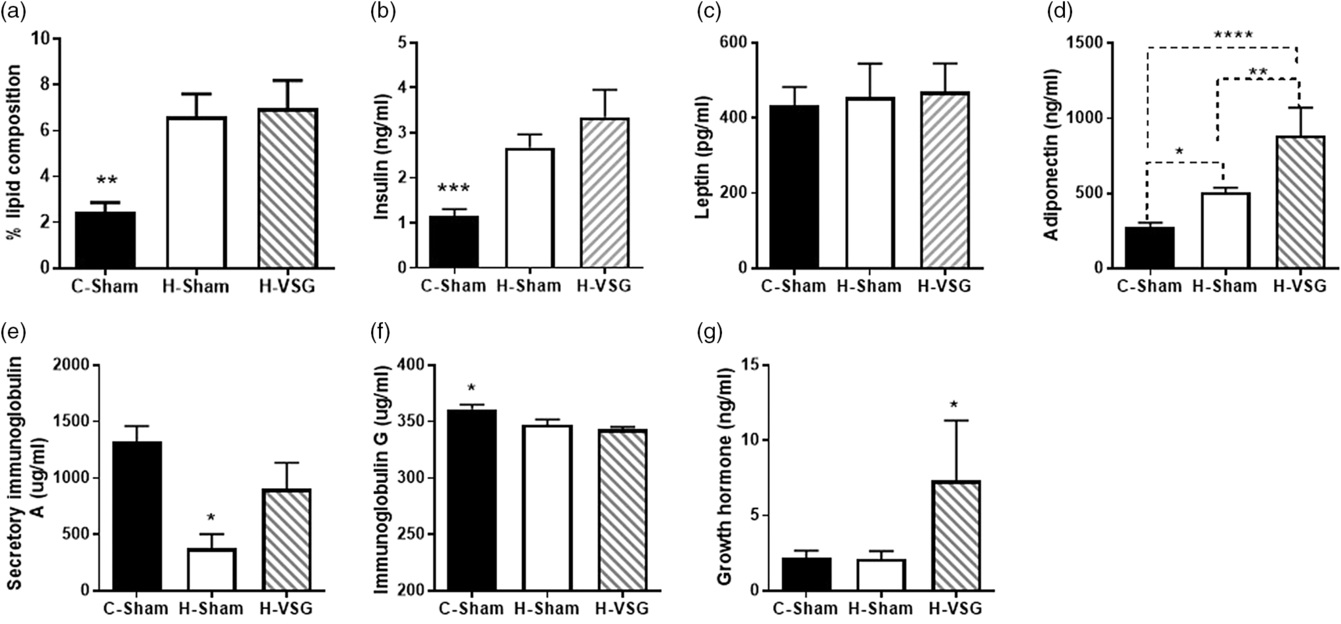
Fig. 3. Lipid and hormones in milk expressed from PND15/16 dams. (a) Percent lipid composition, (b) insulin in ng/ml, (c) leptin in pg/ml, (d) adiponectin in ng/ml, (e) secretory IgA in µg/ml, (f) IgG in µg/ml, and (g) growth hormone in mg/ml. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ****P < 0.0001.
Table 1. Data are presented as mean ± SEM

Milk from H-Sham and H-VSG dams had increased concentrations of insulin in comparison with milk from C-Sham dams (P < 0.001) (Fig. 3a). No difference in leptin concentrations was detected among the groups (Fig. 3c). H-VSG had the highest concentrations of adiponectin in the milk which was significantly greater than C-Sham (P < 0.001) and H-Sham (P < 0.01) (Fig. 3d). Furthermore, differences were also observed in adiponectin concentrations between C-Sham and H-Sham milk (P < 0.05) (Fig. 3d). H-Sham had the lowest concentrations of IgA in comparison with C-Sham and H-VSG (P < 0.05) (Fig. 3e). C-Sham had increased concentrations of IgG (P < 0.05) compared to H-Sham and H-VSG (Fig. 3f). Finally, H-VSG milk had the highest concentrations of growth hormone (P < 0.05) (Fig. 3g).
Discussion
We previously identified that offspring of VSG dams are born smaller and shorter and continue to experience catch-up growth during postnatal life.Reference Grayson, Schneider, Woods and Seeley7 We hypothesized in the current work that the milk of the VSG dams was substantively different than body weight-matched and diet-matched milk from control dams. We show first that the VSG offspring have a reduced growth trajectory suggesting that perhaps the quality of the milk produced by the dam is different. Focusing on the macronutrient content, hormones, and immunoglobulins, we demonstrated that the milk of the VSG dam is substantively different than the diet-matched H-Sham milk or the body weight-matched C-Sham milk.
Limited work has been done to determine the quantity and quality of milk produced by postbariatric mothers. In one study, breast milk from normal-weight, overweight, and obese mothers compared to postbariatric (RYGB and lap-band) mothers measured day 1 to week 6 postpartum demonstrated that postbariatric breast milk appears to be adequate in energy, macronutrients, and vitamin A.Reference Ameye, Devlieger and De Preter20 Overall, the milk from the bariatric mothers, who were still considered overweight, had similar macronutrient content to the various other BMI categories of women but showed elevation in carbohydrates and proteins.Reference Ameye, Devlieger and De Preter20 This is similar to what we report in the current study. Another study reported reduction in retinol and β-carotene in the postbariatric milk, but the authors posit that the control group was not adequate and may have had other nutritional deficiencies related to demographics that reduced the quality of the breast milk overall and diminished the ability to observe differences in the postbariatric milk.Reference Garretto, Kim and Quadro21
Variation in milk composition
Macronutrient composition differs when comparing preterm to term milk, with preterm milk being higher in protein and fat.Reference Ballard and Morrow22 However, despite the complex variation in maternal nutritional status, macronutrient composition is still often conserved.Reference Ballard and Morrow22 In our studies, the VSG dams not only have altered nutritional status but also a completely altered physiology; hence, we observe some unique differences in the milk from the VSG dams.
Proteins
The most abundant proteins in breast milk are casein, α-lactalbumin, lactoferrin, secretory immunoglobulin (Ig)A, lysozyme, and serum albumin.Reference Lönnerdal23 Although milk protein concentration is not widely affected by the diet of the mother, protein concentration is reduced with increased maternal body weight.Reference Bachour, Yafawi, Jaber, Choueiri and Abdel-Razzak24, Reference Rolls, Gurr, Van Duijvenvoorde, Rolls and Rowe25 Milk protein can also be reduced in mothers who produce greater volumes of milk potentially due to dilution of the concentration of protein.Reference Ballard and Morrow22 In our current study, the lowest milk albumin levels are in the H-Sham dams which weighed the greatest amount. The C-Sham and H-VSG, which generally had similar body weights, had equivalent concentrations of milk protein. These data are congruent with our previous studies where there were minimal amino acid differences between Sham and VSG rats.Reference Grayson, Schneider, Woods and Seeley7 By all indications, protein content appears adequate which was previously published in the postbariatric human milk study.Reference Ameye, Devlieger and De Preter20
Lipids in milk
Triglycerides account for 98% of the fats in milk. Therefore, maternal diet and thus, circulating plasma levels of triglycerides increase the availability of triglycerides for production of milk. In the current study, total triglyceride levels in VSG milk were significantly reduced in comparison with either C-Sham or H-Sham. This is not surprising; post-VSG rats have significantly reduced circulating fasting plasma triglyceride levelsReference Grayson, Gutierrez-Aguilar and Sorrell26 which are further reduced during VSG pregnancy.Reference Grayson, Schneider, Woods and Seeley7 Cholesterol makes up only 0.3% of milk fat content and in our studies, we did not observe altered cholesterol levels in VSG milk. In general, the fatty acid composition of the milk was affected by the diet of the dam rather than the surgery of the dam or body weight of the dam. Both H-Sham and H-VSG animals were maintained on a 41% saturated fat diet derived from butter-fat, whereas the chow diet had 5.67% saturated fat content derived from soybean oil. Therefore, the overall percentage of lipid and the type of the free fatty acid that appears in the milk are influenced by what is consumed by the dam and not by the metabolic status (i.e., level of adiposity) of the rat. The greater the fat intake of the dam, the greater the percentage of the large long-chain fatty acids. Our data support that the HFD-fed animals had significantly elevated levels of C:14–18 long-chain fatty acid levels in comparison with chow-fed C-Shams. Accordingly, this resulted in significant reduction of the short-chain fatty acid concentrations of milk samples in the H-Sham and H-VSG dams (C:8–10). De novo fatty acid synthesis in the mammary gland is reduced when more long-chain fatty acids are available in the diet. Taken together, the milk of the VSG provides a significant proportion of its caloric content in the form of fatty acids; in general, this is a function of the diet consumed by the dam and this level of fat content appears not to be altered by the surgical intervention of the dam. It is unfortunate that at the time of collection of these samples, we were not able to obtain samples from VSG rats that were maintained on chow since we were not generating these animals at the time of milk collection. Future research should examine this cohort.
Variations in hormonal regulators of appetite in breast milk
With respect to hormones expressed naturally in breast milk, it is unknown as to their role in programming health and disease in the offspring. Insulin measured in breast milk is often negatively correlated with body weight; thus, the highest levels of insulin were associated with lower infant body weight when relatively normal-weight mother–infant pairs were investigated.Reference Fields and Demerath27 However, in other studies by the same group, when insulin levels in breast milk were compared among mothers that had obese or lean BMIs, the highest insulin levels were obtained from obese mothers.Reference Fields, George and Williams28 Again, this is mimicked in our data. The lowest levels of insulin were measured in milk from lean C-Shams. H-Sham dams had significantly higher levels of insulin, but the VSG dams which had lower levels of adiposity produced significantly higher levels of insulin and had the smallest pups.Reference Fields, George and Williams28
Leptin concentrations in breast milk typically correlate positively with maternal plasma leptin concentrations and maternal BMI.Reference Miralles, Sánchez, Palou and Picó29 However, the relationship between leptin levels measured in milk and infant body weight outcomes are varied. Various studies have either shown significant correlation between higher maternal BMI and higher milk leptin levels, but the milk leptin levels at 1 month correlated inversely with infant adiposity and length at 6 months.Reference Fields, George and Williams28, Reference Miralles, Sánchez, Palou and Picó29 This potentially suggests that leptin or similar anorexic hormones produced in milk might modulate the subsequent growth of the offspring. However, another study reported no significant difference between milk leptin concentrations of obese and nonobese mothers,Reference Uysal, Önal, Aral, Adam, Dilmen and Ardicolu30 even though breast milk leptin concentrations were significantly correlated with mothers’ BMI.Reference Uysal, Önal, Aral, Adam, Dilmen and Ardicolu30 Another study comparing leptin levels from breast milk from overweight versus normal-weight mothers demonstrated that leptin levels were not predictive of the growth or body weight of the babies throughout infancy.Reference Khodabakhshi, Ghayour-Mobarhan and Rooki31 No consensus exists concerning the relationship of leptin. In the current study, leptin levels do not reflect the body weight differences of the dams at the time of milk collection or their weight prior to pregnancy.Reference Grayson, Schneider, Woods and Seeley7
Adiponectin is an adipocyte-derived hormone important in regulating plasma glucose and fatty acids. For both men and women, adiponectin has been shown to have a moderately negative correlation with BMI, percent body fat, sum of skin folds, waist circumference, fasting triglycerides, and plasma insulin and leptin.Reference Goropashnaya, Herron and Sexton32 There are two forms of adiponectin: high and low molecular weight versions; in human milk, adiponectin is invariably the high molecular weight version. Adiponectin levels measured in milk from both obese and normal-weight mothers show a significant negative correlation between milk adiponectin and the weight of the 4-month-old obese infants.Reference Khodabakhshi, Ghayour-Mobarhan and Rooki31 Another study reported that lower infant weights were associated with higher milk adiponectin.Reference Woo, Guerrero and Altaye33 In our present work, the most profound difference we observed in the milk was in the levels of adiponectin where H-VSG had the highest levels of adiponectin. Since circulating plasma adiponectin varies inversely with adiposity, it is not surprising that VSG has the highest amount of milk adiponectin;Reference Grayson, Schneider, Woods and Seeley7, Reference Spann, Lawson and Bidwell34 however, it is interesting that C-Sham dams produce the lowest levels of adiponectin in milk despite having lower levels of adiposity compared to H-Sham in every study that we have performed.Reference Grayson, Schneider, Woods and Seeley7, Reference Spann, Lawson and Bidwell34 In a study of maternal milk collected at 6 weeks postpartum, adiponectin levels were predictive for being overweight at 24 months of age; high milk adiponectin levels were thus a risk factor for overweight offspring later in life.Reference Weyermann, Brenner and Rothenbacher35 This is intriguing with the current VSG model since the highest adiponectin in milk is from H-VSG dams who we have shown give rise to adult pups with greater adiposity than either the C-Sham or H-Sham.Reference Grayson, Schneider, Woods and Seeley7
Growth hormone is also secreted into maternal milk and when the mother is supplemented with exogenous growth hormone, it can increase the rate of growth of her offspring. Here, we report that growth hormone in VSG milk is elevated. This may be a compensation for the low growth hormone produced within the dam (unpublished data) as a result of overall low total and active ghrelin levelsReference Grayson, Schneider, Woods and Seeley7 due to 80% resection of the stomach following bariatric surgery. Increases in growth hormone may help to produce “catch-up” growth that the VSG pups require because of their reduced birth weight and growth trajectory.
Milk immunoglobulins provide immune protection
The most common immunoglobulin in milk is secretory IgA though the other immunoglobulins (IgG and IgM) are also present. IgG is the only immunoglobulin that is able to pass free from the mother to the infant, and maternal immune health is transferred to the infant via immunoglobulins in the maternal milk. Typically, infants are born with low IgA and this is increased as the infant ages and is augmented through maternal milk. In a study that collected colostrum from lean, overweight, and obese mothers, IgA levels were higher in colostrum obtained from obese women.Reference Fujimori, França and Fiorin36 In our study, we did not collect colostrum but rather mature milk. No differences were reported in this study in IgM or IgG levels.Reference Fujimori, França and Fiorin36 In our rodent study, the obese H-Sham have lower levels of IgA and the highest levels of IgG were observed in the C-Sham rats. The lowest levels of IgG in milk were measured in the H-VSG animals. This is in direct contrast to the high levels of IgG that were measured in plasma at G19 during pregnancy.Reference Spann, Lawson and Bidwell34 It is possible that the IgGs in milk are depleted during early postnatal life or alternatively that IgGs do not pass normally to the infant through the placenta after VSG pregnancy.
Caveats and limitations to the current work
In the present study, a significant limitation of the study is the lack of a fourth group of dams that received VSG and then were maintained on chow after their surgery. This particular group would have strengthened our ability to parse out differences attributable to both surgery and diet. Also, in our study, we were not able to quantify the relative amount of milk produced by the dams to feed their pups. This could have been accomplished through a weigh-suckle-weigh paradigm. This paradigm can be somewhat disruptive to maternal behavior, and we were not able to carry this out in the present cohort. Thus, it is possible that VSG dams were not able to produce the same quantity of milk as the sham dams. Furthermore, it is possible that the water content of the milk was altered in VSG dams’ milk which we were not able to account for. Reduced capacity for milk could also result in reduced growth trajectory for the pups during postnatal life and would further reduce their ability to thrive. We also only chose one time point to measure concentrations of analytes within the milk. It would have been useful if we had collected colostrum since milk solids decrease substantially with the formation of mature milk. Furthermore, directly assaying fat-soluble vitamins in the milk would also have strengthened this study considering the impact on lipids of VSG. We also did not study and report maternal behavior patterns and the overall length of time that the dams spent suckling their young. Some cursory observations suggest that VSG dams do not spend as much time caring for their pups as Sham dams but this would need to be studied in a controlled paradigm to define the ways in which VSG offspring may be affected by this altered maternal behavior.Reference Spann, Lawson and Bidwell34
Conclusions
Overall, there appears to be a shift in the caloric content of VSG milk such that total calories from fat are reduced and total calories from glucose may be increased, thus producing milk with a reduced caloric content for the VSG offspring. The VSG offspring from dams maintained on HFD during pregnancy are born small-for-gestational age and also have a reduced growth trajectory requiring catch-up growth during postnatal life. This preclinical work is important to further support women seeking to improve their reproductive potential by obtaining bariatric surgery. Further work is necessary in humans who have received VSG or other bariatric procedures to determine if similarities in milk content are observed and whether macro- and micronutrient supplementation is advisable.
Acknowledgements
None
Financial Support
E.M.D. is supported by T32 HL105324. B.E.G. has received funding from the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number P20GM121334; Mississippi Center of Excellence in Perinatal Research (MSCEPR) COBRE. L.L.H. is supported by USDA grants Hatch WIS01732 and NIFA: 2016-67015-24584 and by NICHD: R01HD094759. Analytical and Assay Core at UMMC performed some work supported by Award Number P20GM104357 and HL51971. Plasma assays were completed by the Mouse Metabolic Phenotyping Center at the University of Cincinnati, Ohio (www.uc.edu/labs/mmpc.html) funded by U2C DK059630. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
E.M.D., L.L.H., B.W., and B.E.G. have no conflicts of interest to declare. R.J.S. receives research support from Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Zafgen, Kallyope, and MedImmune. He has equity in Zafgen. R.J.S. also acts as a consultant for Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Sanofi, Janssen/Johnson & Johnson, Kallyope, and Scohia.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals, IACUC and have been approved by the institutional committee at the University of Cincinnati.






