Introduction
Tobacco consumption is an important threat to the health of the world population. Approximately 8 million people worldwide die annually from diseases related to tobacco consumption. 1 According to the WHO, 2 in 2016 there were 1.1 billion smokers worldwide, 6% of whom were women of childbearing age. In the USA and European countries, approximately 10% of pregnant women are smokers. Reference Napierala, Mazela and Merritt3 Even though there are several studies showing the harmful effects of maternal smoking during the perinatal period, many women do not stop smoking at this period. Cigarette smoke contains approximately 4000 chemicals, Reference Canales, Chen and Kelty4 nicotine being considered as its main psychoactive component. Reference Hukkanen, Jacob and Benowitz5 Studies have shown that nicotine exposure during the fetal (via the placenta) or the postnatal (PN; via breast milk) periods negatively affects the development of the cardiovascular and nervous system of the offspring. Reference Luck and Nau6–Reference Iyen, Vaz and Taggar8
For years, our research group has been investigating a metabolic programming model of maternal nicotine-only exposure during the breastfeeding period and its impacts on the offspring’s metabolism. Reference Miranda, Gaspar de Moura and Lisboa9 In this experimental model, adult male rats showed increased body mass, increased total adiposity, and adipocyte hypertrophy, both in the visceral and subcutaneous compartments. Reference Oliveira, Moura and Santos-Silva10,Reference de Oliveira, Moura and Santos-Silva11 We also detected hormonal changes in these animals, such as increased circulating corticosterone, insulin, and vitamin D levels. Reference de Oliveira, Moura and Santos-Silva11,Reference Pinheiro, Oliveira and Trevenzoli12 The females of this programming model have also been studied Reference Nobre, Lisboa and Santos-Silva13 and showed unchanged body mass, visceral fat, and corticosterone levels.
The white adipose tissue is an endocrine organ, responsible for the production of several adipokines that act on the regulation of metabolism and food intake. This tissue has two large deposits that have distinct morphophysiological characteristics: the visceral adipose tissue (VAT) is located in the intra-abdominal region and is more metabolically active, secreting several proinflammatory cytokines, Reference Paiman, de Mutsert and Widya14 while the subcutaneous adipose tissue (SAT) is considered an energy reservoir and has less inflammatory activity, with greater leptin production, which is considered a protective factor regarding the risk of cardiometabolic diseases. Reference Yang and Smith15 In addition, in humans, a subdivision has been described in the SAT (superficial and deep) and these subdivisions also have different functions. For instance, the deep SAT has a morphophysiology similar to VAT. Reference Nazare, Smith and Borel16 Rodents do not have this type of division; SAT is divided into compartments. It is well established, for example, that the inguinal SAT district has high activity in lipid metabolism and thermogenesis. Reference Rotondo, Ho-Palma and Remesar17,Reference Loncar18
This tissue is also the target for several hormones that regulate its growth and functioning through the processes of adipogenesis and lipogenesis/lipolysis. Reference Esteve Ràfols19 Glucocorticoids (GC), insulin, and vitamin D (1,25-dihydroxyvitamin D) are the main hormones that act on adipocyte differentiation as well as on lipid deposition in the adipose tissue. Reference Peckett, Wright and Riddell20–Reference Nimitphong, Holick and Fried22
GCs in excess have well-established adipogenic effects, Reference Peckett, Wright and Riddell20 as characterized by studies using dexamethasone or cortisol, which indicated that these substances stimulate the differentiation of pre-adipocytes into mature adipocytes. Reference Hauner, Entenmann and Wabitsch23,Reference Lee, Gong and Burkey24 It has been reported that dexamethasone increases the expression of several proteins related to lipid metabolism in 3T3L1 cells, such as lipoprotein lipase (LPL), apolipoprotein D, fatty acid synthase (FAS), fatty acid desaturase 1, diacylglycerol O-acyltransferase 2 (DGAT1), pyruvate carboxylase, long-chain-fatty-acid-CoA ligase (ACSL1), sterol regulatory element binding protein 1, and perilipin. Reference Lee, Gong and Burkey24 These actions are mediated by the interaction of the GC with its receptor, glucocorticoid receptor alpha (GRα), which is expressed in various tissues, including the adipose tissue. Reference Lee and Fried25 Another factor that determines the extent of the effects of GC on tissues is the expression and activity of the 11beta-hydroxysteroid dehydrogenase type 1 (11βHSD1), Reference Peckett, Wright and Riddell20 an enzyme that converts inactive cortisone to cortisol in humans Reference Stimson and Walker26 and 11-dehydrocorticosterone into the active corticosterone in rodents. Reference Terao and Katayama27
Insulin is an anabolic hormone closely related to the increase in adipose tissue mass, stimulating the synthesis and deposition of fatty acids. Reference Dimitriadis, Mitrou and Lambadiari21 In healthy individuals, the adipose tissue is sensitive to insulin and its action on glucose uptake and anabolism ensures the normal functioning of the tissue. Reference Søndergaard and Jensen28,Reference Huang, Liu and Guo29 In obesity, the adipose tissue is remodeled and the production of pro-inflammatory cytokines is increased, causing insulin resistance. Reference Reilly and Saltiel30 This dysfunction is a precursor to some diseases, such as DM2 and cardiovascular diseases, among others. Reference Brown and Walker31–Reference Diehl, Mullins and Kapogiannis33
Vitamin D, a steroid hormone synthesized in the skin and activated in the kidney, can be stored in the adipose tissue, Reference Abbas34 acting on its function. In human pre-adipocytes, vitamin D stimulates differentiation due to increased expression of adipogenic markers such as LPL and fatty acid-binding protein 4. Reference Lee and Fried25,Reference Narvaez, Matthews and Broun35 In human patients, low levels of vitamin D are associated with obesogenesis. Reference Bouillon, Carmeliet and Lieben36 In rodent models, some authors have not detected a relationship between obesity and serum levels of vitamin D. Reference Novaes Soares, Silva Tavares Rodrigues and Cherem Peixoto37,Reference Bonnet, Hachemi and Karkeni38 It has been reported that blocking the vitamin D receptor (VDR) results in reduced fat mass in animals, while increased signaling via VDR causes increased adiposity. Reference Bouillon, Carmeliet and Lieben36 However, our research group evidenced higher 1α-hydroxylase, which converts 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, in adipose tissue associated with lower VDR in obese animals, suggesting a vitamin D resistance in adipocytes. Reference Nobre, Lisboa and da Lima39,Reference Nobre, Lisboa and Peixoto-Silva40 Thus, the absence of vitamin D action can be obesogenic.
Previously, in an experimental model of neonatal tobacco smoke exposure, we have reported GC and vitamin D levels in visceral adipocytes of the adult progeny of both sexes. Reference Novaes Soares, Silva Tavares Rodrigues and Cherem Peixoto37 Both smoke-exposed males and females had increased visceral fat and normal serum vitamin D. Corticosteronemia was lower in males but higher in females, although GC metabolism and receptor were unchanged. Male offspring showed increased 1α-hydroxylase, acetyl-CoA carboxylase, and FAS in visceral adipocytes. In summary, smoke exposure during lactation induced abdominal obesity in both sexes, through distinct mechanisms, while vitamin D activation and lipogenesis were more influenced in males than in females.
Based on the findings obtained from the neonatal tobacco smoke exposure model and on previous data obtained with the use of the neonatal nicotine exposure model, which showed increased adiposity and hormonal dysfunction in the adult male offspring, in the current study we decided to evaluate the peripheral metabolism (activation and receptor) of GC and vitamin D, as well as insulin signaling in the VAT and SAT. Our hypothesis is that the changes in hormone levels in this programming model modify the status (cellular effects) of the three hormones in both fat depots, favoring the processes of lipogenesis and adipogenesis and the obese phenotype. In addition, we assessed the levels of insulin and vitamin D in the adult female offspring for the first time, which may help to understand why they do not develop fat accumulation.
Materials and methods
Ethics and animal procedures
Protocols followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Brazilian Federal Law n° 11.794/2008. All experiments were approved by the Institutional Ethical Committee for the Use of Laboratory Animals of the Biology Institute of the State University of Rio de Janeiro (authorization project: CEUA/007/2017).
Wistar rats were housed in a temperature (22 ± 1°C) and humidity (50%–55%) controlled room on a 12-h light/dark cycle (lights on at 7:00 a.m.) with standard chow (Nuvilab®, São Paulo, Brazil) and water available ad libitum. Three-month old, virgin female rats were caged with male rats at a ratio of 3:1 for mating per 1 week. Pregnant rats were individually housed until delivery.
Model of neonatal nicotine exposure
After parturition, 20 lactating dams were randomly assigned to each group and litters were normalized to six pups (3 male and 3 female): Nicotine (N; n = 10) – at PN day 2 (PN2), dams were lightly anesthetized with thiopental (30 mg/kg; Thiopentax, Itapira, SP, Brazil), a 3 × 6 cm area on the back was shaved, and an incision was performed to allow for the subcutaneous insertion of the osmotic minipumps (OMP, Alzet, 2ML2, Los Angeles, CA, USA). OMP were prepared according to the manufacturer’s recommendation with nicotine free-base (Sigma, St Louis, MO, USA) diluted in a saline solution (NaCl 0.9%) to deliver a dose rate of 6 mg/kg of nicotine per day during 2 weeks. Reference Oliveira, Moura and Santos-Silva10 Cotinine, which is a nicotine metabolite considered a marker of its exposure, was detected in the dams’ milk and serum and in the pups’ serum. Reference Oliveira, Pinheiro and Santos-Silva41 This protocol generates blood cotinine concentrations similar to those found in typical smokers. Reference Ypsilantis, Politou and Anagnostopoulos42 According to the literature, a nicotine dose of 6 mg/kg/d via an OMP implanted in pregnant rats produces plasma nicotine concentrations of 3–4 times larger than those detected in typical smokers Reference Benowitz, Kuyt and Jacob43–Reference Hussein, Farkas and Mackinnon46 and 10 times higher than those detected in smoking pregnant women. Reference Hussein, Farkas and Mackinnon46 (Control (C; n = 10) – dams were implanted with minipumps containing only saline.
For the present study, one pup/group/litter/sex was randomly chosen, that is, two pups from the same litter (one male and one female). The remaining offspring were used in other experiments.
At weaning (PN21), offspring were separated by sex, being kept in cages containing three to four animals, and freely received standard rodent chow and water. The offspring’s food intake and body mass were monitored once a week until PN180, when the animals were euthanized (Fig. 1). From PN150 to PN180, the estrous cycle was observed; both N and C females had regular 4–5-d cycles.

Fig. 1. Experimental timeline. Neonatal exposure to nicotine. PN, postnatal; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
Total fat mass evaluation
Nuclear magnetic resonance (NMR) for small living animals was performed for the evaluation of total fat mass at PN178. For this, rats were scanned using the Whole Body Composition Analyzer NMR equipment (Bruker’s Minispec LF90 TD-NMR, Rheinstetten, Germany), as previously reported. Reference Rodrigues, Moura and Bernardino47 A quality control check of internal voltages, temperature, magnets, and NMR parameters was performed using a standard provided by the manufacturer. Non-anesthetized animals were placed in a clear plastic cylinder and restrained by insertion of a tight-fitting plunger into the cylinder. The cylinder was inserted in the sample chamber of the NMR, where it remained for the duration of the scan, which took around 2 min. The technician was blind as to group assignment. Data were expressed as % fat mass.
Euthanasia and tissue collection
At PN180, the rats were anesthetized with thiopental (30 mg/kg body mass), after a 12-h fasting period. Glucose level was determined from the tail vein blood using a glucometer (ONETOUCH ULTRA®; Johnson & Johnson, São Paulo, Brazil). Then, animals were euthanized by cardiac puncture. The following fat compartments were collected and stored at −80°C: 1) the inguinal subcutaneous fat (located between the skin and muscle; collected from the groin region); 2) the perivisceral fat from the abdominal region (mesenteric – located in the vicinity of the stomach and intestine; retroperitoneal – located posterior to the peritoneum; gonadal – located around the internal sex organs). Together, the three visceral compartments represent the total visceral fat mass, which was weighed and expressed as visceral fat mass/body mass. Blood samples were centrifuged (1500 × g for 20 min at 4°C) to obtain plasma, which was kept at −20°C.
Plasma hormone analyses
Corticosterone concentrations were determined using a radioimmunoassay kit (ImmuChem TM 125I, coated tube; ICN Biomedicals, Inc., NY, USA). The other hormones were quantified by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions: Rat/Mouse Insulin ELISA kit (EMD Millipore Corporation, Billerica, MA, USA), total 25-hydroxyvitamin D (MyBioSource, San Diego, CA, USA), and adrenocorticotropic hormone (Rat ACTH ELISA kit; Wuhan Fine Biotech Co., Ltd., Hubei, China). The measurement of metabolite 25-hydroxyvitamin D was performed because this hormone generally determines the overall vitamin D status. All samples were measured in duplicate within a single assay and inter-assay and intra-assay coefficients of variation were below 5%.
Western blotting analysis
Western blotting was used to evaluate protein content in the VAT (specifically retroperitoneal depot) and in SAT (specifically inguinal depot). We measured the protein contents of the following markers: 11βHSD1, GRα, insulin receptor beta (IRβ), phosphorylated insulin receptor substrate 1, insulin receptor substrate 1 (IRS1), phosphorylated serine/threonine kinase (Thr 308) (pAKT(Thr 308)), serine/threonine kinase 1/2/3 (AKT1/2/3), glucose transporter type 4 (GLUT4), 1α-hydroxylase and VDR.
Tissues were frozen in liquid nitrogen and subjected to maceration in an extraction buffer (T-PER Tissue Protein Extraction) containing a protease inhibitor cocktail (Roche®). The homogenates were centrifuged at 12,851 × g for 30 min at 4°C (Eppendorf 5417R, Hampton, USA). The samples were treated with Laemmli sample buffer (w/v: glycerol, 20%; β-mercaptoethanol, 10%; 10% sodium dodecyl sulfate (SDS), 40%; 0.5 mol/l Tris at pH 6.8, 0.5%; deionized water and bromophenol blue). Total protein extracts (30 µg) were separated using 10% SDS-PAGE and then submitted to electrophoresis. The proteins were then transferred from the gel to a polyvinylidene difluoride (PVDF) membrane by Trans-Blot® turbo system (Bio-Rad® Laboratories, Hercules, CA, USA) and blocked with 5% BSA in Tween–Tris-buffered saline (TTBS; Tris–HCl, 1 mol/l; NaCl, 5 mol/l; Tween 20, 0.05%, v/v) for 45 min with continuous shaking. Membranes were incubated overnight with specific primary antibodies. PVDF membranes were washed with TTBS (0.1%), followed by 1 h incubation with appropriate biotin-conjugated secondary antibody. Then, membranes were incubated with streptavidin conjugated horseradish peroxidase (GE Healthcare, Buckinghamshire, UK). All antibodies are described in Table 1. Phosphorylated proteins were labeled first, then moderate membrane stripping was performed to label total protein. Immunoreactive proteins were visualized by chemiluminescence (ECL Plus kit; Amersham Biosciences, London, UK) using an ImageQuant LAS (GE Healthcare) in a single automatic exposure. Bands were quantified by densitometry using ImageJ software (Wayne Rasband, National Institutes of Health, MA, USA). Either β-actin or cyclophilin protein contents were used as loading control, depending on protocol. The membranes were cropped following the molecular weight pattern of each protein of interest. Each cropped membrane was incubated with a specific antibody for detection of each protein that was in a different molecular weight position. Representative western blots images show all bands; membrane cropped at specific molecular weights.
Table 1. Antibody list

11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; AKT1/2/3, serine/threonine kinase 1/2/3; CYP27B1, 25-hydroxyvitamin D-1 alpha hydroxylase; GLUT4, glucose transporter type 4; GRα, glucocorticoid receptor alpha; IRβ, insulin receptor beta; IRS1, insulin receptor substrate 1; pAKT(Thr 308), phosphorylated serine/threonine kinase (Thr 308); pIRS1, phosphorylated insulin receptor substrate 1; VDR, vitamin D receptor. β-actin or cyclophilin was used as controls in western blot.
Statistical analysis
The Kolmogorov–Smirnov one sample test (K–S) was used to assess the normality of the distributions of each of the variables. Data are compiled as means and standard errors of the means. Two-way univariate analyses of variance (mANOVA) were used to analyze all data. Exposure (nicotine or control) or sex (male or female) was used as the between-subjects factors. Significance is assumed at the level of P < 0.05. For interactions at P < 0.10 (two-tailed), we also examined whether lower-order main effects were detectable after subdivision of the interactive variables. Reference Snedecor and Cochran48 Post-hoc analyses were conducted separated by sex. Effect size data are provided as η 2 (small >0.1, medium >0.3, large >0.5). Table 2 shows significant effects and interactions for the two-way ANOVAs.
Table 2. Two-way univariate ANOVA results

ACTH, adrenocorticotropic hormone; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Results
Biometric parameters
Nicotine-exposed males showed higher body mass (+10%, F (1,18) = 11.4, P = 0.003, η2 = 0.39) and total visceral fat mass/body mass (+19%, F (1,18) = 7.3, P = 0.015, η2 = 0.29) compared to control animals, whereas the fat percentage tended to be increased (+18%, F (1,18) = 3.9, P = 0.063, η2 = 0.18) as shown in Table 3. In females, these parameters were not affected by nicotine exposure (Table 2).
Table 3. Effects of neonatal exposure to nicotine on biometric and plasma parameters of offspring in adulthood
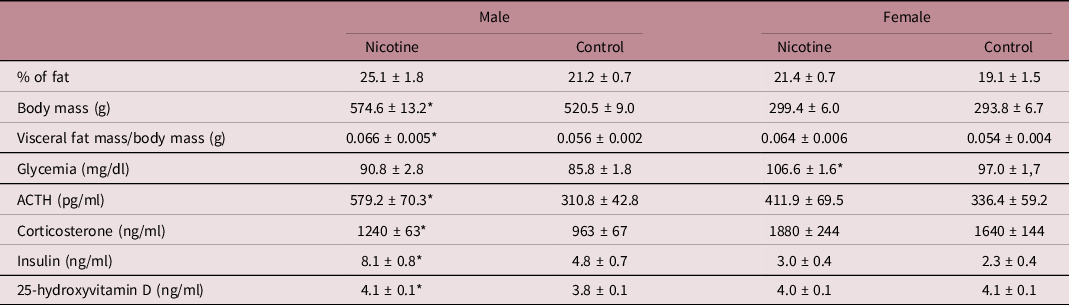
Values are expressed as mean ± SEM. Body composition and glycemia, n = 10; hormonal analysis, n = 8–10 rats/group (1 per litter and per sex).
* vs control (P < 0.05).
GC status
As depicted in Table 3, nicotine-exposed males had higher plasma adrenocorticotropic hormone (ACTH) (+86%, F (1,17) = 11.1, P = 0.004, η2 = 0.40) and corticosterone (+29%, F (1,14) = 9.1, P = 0.009, η2 = 0.39) when compared to control animals. These hormones were not altered in nicotine-exposed females when compared to control ones. Corticosterone (Table 2) showed a sex effect (F (1,27) = 21.9, P < 0.001, η2 = 0.45): females (1751 ± 135 ng/ml) had higher (+59%) values than males (1101 ± 57 ng/ml). Concerning the GC activation enzyme and receptor in fat depots, neither sex showed changes in 11BHSD1 and GRα protein contents (Fig. 2) in the VAT and SAT due to nicotine exposure.

Fig. 2. Glucocorticoid metabolism (activation enzyme and receptor). VAT, visceral adipose tissue; 11β HSD1, 11beta-hydroxysteroid dehydrogenase type 1(a: male n = 6–7 and female n = 6–7); GRα, glucocorticoid receptor alpha (b: male n = 6–7 and female n = 7); SAT, subcutaneous adipose tissue; 11β HSD1 (c: male n = 5–7 and female n = 6–7); GRα (d: male n = 6–7 and female n = 6–7). Representative western blots images show all bands and cropped membrane in specific molecular weight. Values are expressed as mean ± SEM; n represents 1 rat/group per sex per litter. * vs control (P < 0.05).
Insulin status
Despite normoglycemia, nicotine-exposed males showed hyperinsulinemia (+70%, F (1,14) = 9.4, P = 0.008, η2 = 0.40; Table 3) and lower protein content of IRS1 (−30%, F (1,11) = 5.2, P = 0.043, η2 = 0.32) in VAT when compared to control animals (Fig. 3a). The male insulin signaling pathway in SAT was not altered by nicotine exposure (Fig. 3c).

Fig. 3. Insulin signaling pathway. VAT, visceral adipose tissue; IRβ, insulin receptor beta (a: male n = 5–6 and b: female n = 4); pIRS1, phosphorylated insulin receptor substrate 1 (a: male n = 6–7 and b: female n = 6–7); IRS1, insulin receptor substrate 1 (a: male n = 6–7 and b: female n = 6–7); ratio pIRS1/IRS1 (a: male n = 6–7 and b: female n = 6–7); phosphorylated serine/threonine kinase (Thr 308) (pAKT(Thr 308)) (a: male n = 7 and b: female n = 5–7); serine/threonine kinase 1/2/3 (AKT1/2/3) (a: male n = 6–7 and b: female n = 5–6), ratio pAKT/AKT (a: male n = 6–7 and b: female n = 5); GLUT4, glucose transporter type 4 (a: male n = 6 and b: female n = 5–6); SAT, subcutaneous adipose tissue (c: male and d: female); IRβ, insulin receptor beta () (c: male n = 6 and d: female n = 5–6); pIRS1, phosphorylated insulin receptor substrate 1 (c: male n = 6–7 and d: female n = 5–6); IRS1, insulin receptor substrate 1 (c: male n = 6–7 and d: female n = 5–7); ratio pIRS1/IRS1 (c: male n = 5–7 and d: female n = 4–6); phosphorylated serine/threonine kinase (Thr 308) (pAKT(Thr 308)) (c: male n = 5–6 and d: female n = 6–7); serine/threonine kinase 1/2/3 (AKT1/2/3) (c: male n = 5–6 and d: female n = 6–7); ratio pAKT/AKT (c: male n = 5–6 and d: female n = 6–7); GLUT4, glucose transporter type 4 (c: male n = 4–7 and d: female n = 5–6). Representative western blots images show all bands and cropped membrane in specific molecular weight. Values are expressed as mean ± SEM; n represents 1 rat/group per sex per litter. * vs control (P < 0.05) and & vs control = approaching significance (P > 0.05 and <0.06).
Nicotine-exposed females showed hyperglycemia (+10%, F (1,18) = 16.1, P = 0.001, η2 = 0.47; Table 3), trend to higher pAKT protein content and pAKT/AKT ratio (+63%, F (1,10) = 4.7, P = 0.056, η2 = 0.32; +142%, F (1,8) = 4.8, P = 0.059, η2 = 0.38, respectively) in VAT (Fig. 3b), while lower IRβ, IRS1, and GLUT4 protein content (−41%, F (1,9) = 7.8, P = 0.021, η2 = 0.46; −63%, F (1,10) = 5.9, P = 0.035, η2 = 0.37; and −49%, F (1,9) = 13.3, P = 0.005, η2 = 0.60, respectively) in SAT compared to their controls (Fig. 3d).
Vitamin D status
As depicted in Table 3, plasma 25-hydroxyvitamin D concentrations were higher (+7%, F (1,18) = 5.4, P = 0.033, η2 = 0.23) only in nicotine-exposed males when compared to control animals. Neither sex showed differences in 1α hydroxylase and VDR protein contents in VAT and SAT when compared to their respective controls (Fig. 4).

Fig. 4. Vitamin D metabolism (activation enzyme and receptor). VAT, visceral adipose tissue; 1α-hydroxylase (a: male n = 6–7 and female n = 6–7); VDR (b: male n = 4 and female n = 4–6); SAT, subcutaneous adipose tissue; 1α-hydroxylase (c: male n = 6 and female n = 6); VDR (d: male n = 4–5 and female n = 6). Representative western blots images show all bands and cropped membrane in specific molecular weight. Values are expressed as mean ± SEM; n represents 1 rat/group per sex per litter.
Discussion
Nicotine is an endocrine disruptor Reference Cramer, Harlow and Xu49,Reference Heindel and vom Saal50 and an obesogenic factor that is present in cigarettes, as demonstrated by studies of early exposure to nicotine during gestation Reference Somm, Schwitzgebel and Vauthay51 or breastfeeding. Reference Oliveira, Moura and Santos-Silva10 One day of a rat’s life corresponds to approximately 9 d of a human’s life. Reference Quinn52 In this sense, the lactation period in rats, which lasts for 21 d, corresponds to around 6 months of breastfeeding in humans. In the present study, the exposure to nicotine for 14 d of lactation in rats corresponds to approximately 3–4 months of maternal smoking during breastfeeding in humans. During this period, the OMP implanted in the dams released daily doses of 6 mg/kg of nicotine, reaching maternal cotinine concentrations of 240 ng/ml in the blood and 226 ng/ml in the milk, Reference Oliveira, Pinheiro and Santos-Silva41 values that are compatible with cotinine levels observed in addicted smokers. Reference Ypsilantis, Politou and Anagnostopoulos42 In our model, the dam’s blood corticosterone was unchanged, indicating no maternal stress. Reference Oliveira, Pinheiro and Santos-Silva41 Also, the dam exposed to nicotine had no body mass alteration but had higher blood prolactin and higher milk production. Reference Oliveira, Pinheiro and Santos-Silva41
Here, we evidence that nicotine is capable of programming the status of hormones directly associated with the function of white adipose tissue. We showed that nicotine actions vary as a function of adipose tissue compartment and sex. The current study corroborates previous findings of our group, which indicated that only males show an increase in body adiposity. Reference Oliveira, Moura and Santos-Silva10–Reference Pinheiro, Oliveira and Trevenzoli12 Despite being mildly overweight, these animals showed significant VAT and SAT hypertrophy. Reference de Oliveira, Moura and Santos-Silva11 According to Goossens, Reference Goossens53 hypertrophic adipocytes show an impaired ability to quickly store dietary fat because they are already overloaded with lipids, resulting in a redirection of lipids to other organs, generating ectopic deposition. In addition, this hypertrophic tissue is characterized by infiltration of adaptive and innate immune cells and altered adipokine secretion, a condition that leads to the development of peripheral insulin resistance. The problem with VAT dysfunction is that metabolites and free fatty acids can drain directly from VAT into the portal circulation, disrupting liver function. Reference Vishvanath and Gupta54 A review from Srdić et al. Reference Srdić, Stokić and Korać55 indicated that eutrophic women with metabolic diseases have increased visceral adipocyte size. The authors correlated the metabolic problems with the aforementioned increase. Thus, in our study, we think that the morphological alteration observed in males is associated with their dysfunctional phenotype.
Since only males had high levels of corticosterone, insulin, and vitamin D, it is possible that these hormonal disruptions are at the center of this issue. The female progeny exposed to early nicotine through milk is eutrophic at adulthood and does not exhibit changes in body adiposity. Contrasting with what we observed here, Zhang et al. Reference Zhang, Li and Fan56 showed that adult females that were nicotine-exposed during the perinatal period show higher body mass, higher visceral and subcutaneous fat, higher adipocyte area (not evaluated here), and hyperinsulinemia. The differences in outcomes between studies regarding adult females can be explained mainly by differences in the nicotine exposure period (our group: only during lactation vs Zhang’s group: during both gestation and lactation). Concerning the blood hormone profile, nicotine-exposed female’s normocorticosteronemia is in accordance with our previous data. Reference Pinheiro, Oliveira and Trevenzoli12 Females have a higher corticosterone than males, but not ACTH. This finding has already been previously described in the literature Reference Critchlow, Liebelt and Bar-Sela57,Reference Atkinson and Waddell58 and is possibly explained by a direct estrogen action on the cortex of adrenal gland, which has estrogen receptors. Reference Hutson, Gurrala and Ogola59,Reference Lo, Chang and Wang60
For the first time, we show unchanged plasma insulin and 25-hydroxyvitamin D levels in this sex. Despite this, females show hyperglycemia and distinct alterations in protein of insulin signaling pathways in the VAT and SAT, which is intriguing.
The high levels of both ACTH and corticosterone in nicotine-exposed males per se help to explain obesity because it is known that excess cortisol, as in patients with Cushing’s disease, leads to abdominal obesity. Reference Yu, Kim and Lee61,Reference Albani, Ferraù and Ciresi62 Also, increased tissue sensitivity to GC (conversion and action) has pro-adipogenic and lipogenic actions. Reference John, Marino and Sanchez63 Here, the protein content of 11βHSD1 and GC receptor was not altered in either sex or fat depots. This is also intriguing because nicotine-exposed males showed hypercorticosteronemia and increased VAT mass. Thus, apparently, the local generation and action of corticosterone were not programmed by nicotine exposure during lactation and do not seem to have an important role in either sex or fat depots. In addition, it is known that the lipogenic effect of GC can also occur through its interaction with the mineralocorticoid receptor (MR) in the white adipose tissue, which concomitantly expresses MR and GR. Reference John, Marino and Sanchez63 Thus, it is also possible that the increase in visceral adiposity in nicotine-exposed males is due to the action of corticosterone on the MR. A limitation of our study was that MR was not measured. 11βHSD1 and 11βHSD2 activities, which respectively catalyze the activation and inactivation of GC, were also not evaluated.
The major circulating form of vitamin D is 25-hydroxyvitamin D; then, currently, its circulating level is considered the best indicator of vitamin D supply to the body. Low levels of vitamin D, especially in humans, Reference Walsh, Bowles and Evans64,Reference Savastano, Barrea and Savanelli65 as well as vitamin D resistance, especially in animal models, Reference Nobre, Lisboa and da Lima39,Reference Nobre, Lisboa and Peixoto-Silva40 can be obesogenic. In the current study, only nicotine-exposed males were shown to have increased plasma 25-hydroxyvitamin D concentrations, which corroborates our previous finding concerning the circulating levels of adult offspring exposed to nicotine during lactation. Reference Nobre, Lisboa and Santos-Silva13 However, this finding, together with normal 1α hydroxylase and VDR, does not help explaining the greater adiposity of males.
Concerning the glycemic homeostasis, the offspring have different sex-dependent profiles. Nicotine-exposed males have normoglycemia with hyperinsulinemia, indicating that the pancreas has increased insulin production in an attempt to maintain blood glucose levels at normal levels. Reference Cignarelli, Genchi and Perrini66 In contrast, nicotine-exposed females have hyperglycemia with normoinsulinemia, suggesting a more advanced stage of dysregulation of glycemic homeostasis, in which the pancreas does not seem to produce insulin properly. In fact, pancreas functional incapacity has been demonstrated in animals exposed to nicotine early in life. Reference Bruin, Gerstein and Morrison67 Besides, hyperglycemia may be due to reduced insulin sensitivity in peripheral tissues. Our results concerning insulin signaling in fat depots help to explain these ideas. The insulin pathway was more influenced by neonatal nicotine exposure in female adult rat offspring when compared to males. Nicotine-exposed males showed lower content of IRS1 in the VAT. This reduction has a moderate effect size, which was possibly insufficient to inhibit the anabolic effects of insulin on VAT, thereby contributing to the increase in adiposity in this deposit. On the other hand, studies have shown that downregulation of IRS1 is associated with insulin resistance, without hyperglycemia, Reference Araki, Lipes and Patti68,Reference Copps and White69 a phenotype expressed by nicotine-exposed males in the current study. Besides, our data are in agreement with the study from Fan et al., Reference Fan, Ping and Zhang70 in which adult males exposed to nicotine during pregnancy and lactation showed normoglycemia and hyperinsulinemia. Regarding the SAT, we suggest that the preserved anabolic action of insulin favors the increase in the fat mass of males.
Nicotine-exposed females showed a trend to higher pAKT protein content and pAKT/AKT ratio in the VAT, and lower IRβ, IRS1, and GLUT4 protein content in the SAT, suggesting increased insulin sensitivity in the VAT, but insulin resistance in the SAT. In fact, AKT is closely related to metabolism, survival, and cell proliferation; AKT hypophosphorylation has been associated with diabetes. Reference Ebner, Lučić and Leonard71 Even without an increase in body fat in females, the SAT shows an insulin resistance response, which may be due to some inflammatory process or redox imbalance Reference Luc, Schramm-Luc and Guzik72 that were not evaluated in the present study. An important issue is the fraction represented by the SAT; in non-obese rats, the inguinal compartment is the one with the highest percentage and absolute mass. Reference Arriarán, Agnelli and Remesar73 It has been reported that the SAT dysfunction favors the deposition of ectopic fat, promoting some metabolic complications of obesity. Reference Smith and Kahn74 Thus, the deregulation of this fat depot in females may lead to the accumulation of ectopic fat in other tissues, such as liver, muscle, and pancreas. Specifically, regarding the pancreatic tissue, in the presence of hyperglycemia, normal insulin suggests a deficiency in its production and secretion, which can later become hypoinsulinemia.
In a model of tobacco smoke exposure during the lactation period, Reference Novaes Soares, Silva Tavares Rodrigues and Cherem Peixoto37 our group studied GC and vitamin D blood levels, metabolism, and receptor in the VAT of the adult progeny (both sexes). In that study, neither insulin signaling nor the SAT depots were investigated. The two similarities between the previous study Reference Novaes Soares, Silva Tavares Rodrigues and Cherem Peixoto37 and the current model are the male adiposity (increased) and vitamin D concentrations in females (unchanged). Thus, the late obesogenesis induced by maternal smoking in early life may be due to the presence of nicotine, although other components present in the cigarette smoke may act together with nicotine on the hormone status in the adipose tissue.
Taken together, our present findings indicate that exposure to nicotine exclusively during the lactation period affects the hormone status and fat depots of the offspring during adult life, but in a sex-dependent manner. In males, obesogenesis is probably more related to corticosterone levels, whereas in the females, which do not have alteration in body mass or adiposity, but have hyperglycemia, insulin signaling seems to have distinct effects in the VAT and SAT. These differences can be explained, at least in part, by sex hormones, since they are responsible for body fat distribution, regulate adipogenesis, and modulate inflammation. Reference Griffin, Lanzetta and Eter75 Recently, our group demonstrated that male nicotine offspring have lower testosterone levels while the female offspring have no difference in plasma levels of testosterone and estradiol. Reference Miranda, de Moura and Soares76 Males with hypogonadism have reduced androgen and increased adiposity. Epidemiological studies show a positive correlation between hypogonadism and obesity, such as type 2 diabetes mellitus and metabolic syndrome. Reference Carrageta, Oliveira and Alves77 Thus, in our model, the hypotestosteronemia detected in NIC males can also be contributing to the dysfunction of fat depots.
The phenotype of adult male animals exposed to nicotine early in life can be a result of the programming of nicotinic cholinergic receptors (nAChRs), which are widely distributed in the organism. Reference Wu, Wang and Ji78 Particularly, α7nAchR activation exerts both central and systemic anti-inflammatory effects. Reference Souza, Amaral and Souza79,Reference Li, Hao and Gao80 There is still no consensus in the literature regarding its regulation by nicotine since both up- and downregulation after nicotine exposure have already been reported. Reference Sanderson, Drasdo and McCrea81,Reference Elsonbaty and Ismail82 It is possible that overweight and higher body adiposity in nicotine male offspring are due to downregulation of α7nAchR, favoring an inflammatory profile, which induces leptin and insulin resistance development, leading to changes in energy balance and disorders in peripheral tissues, such as the adipose one. Reference Milanski83 In fact, we have previously shown that nicotine-exposed male offspring develop hypothalamic astrogliosis and microgliosis. Reference Younes-Rapozo84 Also, these males show a higher preference for a high-sugar content diet than controls. Reference Pinheiro, Moura and Manhães85 Concerning females, we have recently shown that these animals, despite having normal body mass, show reduced nAChRs as well as leptin resistance in the hypothalamus, suggesting a central inflammatory process, as observed in nicotine-exposed males. Reference Peixoto86 A limitation of the current study is that we have not assessed α7nAchR in the hypothalamus and adipose tissue.
These experimental data, as well as other studies from our group that used animal models, contribute to the understanding regarding the underlying mechanisms that link early nicotine exposure to increased risk for obesity and chronic diseases at adulthood, as reported in epidemiological data. Nowadays, many smokers have turned to electronic cigarettes to quit smoking. Despite being a preclinical study, our results suggest that the use of electronic cigarettes or nicotine patches to replace cigarettes, which are sometimes used by pregnant and lactating women, should not be recommended since it can lead to obesity in the offspring, especially in males, and hyperglycemia in females.
Acknowledgements
The authors are grateful to Mr Ulisses Risso Siqueira, Mrs. Fabiana Gallaulckydio, and Mr. Leandro Fraga Bezerra for animal care and technical assistance in the laboratory.
Financial support
This research was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Conflicts of interest
None.
Ethical standards
Protocols followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Brazilian Federal Law n° 11.794/2008. All experiments were approved by the Institutional Ethical Committee for the Use of Laboratory Animals of the Biology Institute of the State University of Rio de Janeiro (authorization project: CEUA/007/2017).
Authors’ contribution
VST Rodrigues: conceptualization, investigation, methodology, formal analysis, writing – original draft; RA Miranda: methodology, investigation; PN Soares: methodology, investigation; TC Peixoto: methodology, investigation; E Oliveira: methodology, investigation, visualization, resources; AC Manhães: methodology, validation, formal analysis, visualization; EG Moura: conceptualization, visualization, resources; PC Lisboa: conceptualization, validation, formal analysis, visualization, resources, funding acquisition, data curation, writing – original draft, supervision, project administration.










