Introduction
The use of donor oocytes is an increasingly common strategy for the treatment of female infertility. The manipulation of gametes and culture of embryos, however, have the potential to negatively influence embryological development and perinatal outcomes compared to normally conceived children.Reference Hart and Norman 1 Moreover, Assisted Reproductive Technologies (ART) perinatal outcomes are worse than those observed from spontaneous conceptions with increased incidences of birth defects (BD), preterm delivery (PD), lower birth weights and mortality.Reference Marino, Moore and Willson 2 – Reference Helmerhorst, Perquin, Donker and Keirse 7 Additionally, poor neonatal outcomes have been linked with increased incidences of morbidity and mortality in later life in the general population,Reference Class, Rickert, Lichtenstein and D’Onofrio 8 – 10 and phenotypically normal ART offspring have also been linked to increased epigenetic changes throughout their genome.Reference Batcheller, Cardozo, Maguire, DeCherney and Segars 11
With adverse perinatal outcomes from the ART population already established, there is an opportunity to consider the pattern of outcomes within specific exposure groups to identify opportunities for intervention and to inform patient decision making. Donor oocyte conceptions form one such sub-group and has a novel characteristic within the ART population. The woman receiving treatment will be gestating an embryo derived from another woman’s oocyte which potentially represents an immunological challenge to the mother.
The incidence of preeclampsia is increased when donated oocytes are used in infertility treatments.Reference Tranquilli, Biondini and Talebi Chahvar 12 – Reference Salha, Sharma and Dada 14 Preeclampsia is argued to be an immune responseReference Saito, Shiozaki, Nakashima, Sakai and Sasaki 15 , Reference Sargent, Borzychowski and Redman 16 that can alter placentationReference Ahn, Park, Gilman-Sachs and Kwak-Kim 17 , Reference Schiessl 18 and is a leading cause of foetal and maternal morbidity and mortality.Reference Backes, Markham and Moorehead 19 Notably, the immune mechanism of preeclampsia is associated with factors such as intra uterine growth retardation (IUGR) and PD known to adversely affect the health of the child.Reference Challis, Lockwood and Myatt 20 , Reference Zetterström, Lindeberg, Haglund, Magnuson and Hanson 21 Individuals born following preeclampsia in their gestation have an increased risk of hypertension,Reference Geelhoed, Fraser and Tilling 22 – Reference Himmelmann 24 endothelial dysfunction,Reference Davis, Newton and Lewandowski 25 higher body mass index (BMI),Reference Davis, Lazdam and Lewandowski 26 epilepsy,Reference Wu, Sun and Vestergaard 27 increased hospitalization due to disease,Reference Wu, Nohr and Bech 28 and stroke.Reference Kajantie, Eriksson, Osmond, Thornburg and Barker 29 Preeclampsia has also been associated with an increased risk of an autism spectrum disorder in offspring.Reference Mann, McDermott, Bao, Hardin and Gregg 30 Millis found that the placenta suffered from altered methylation due to preeclampsia and that infants also had altered methylation of insulin-like growth factor 2,Reference Millis 31 which is associated with metabolic diseases in later life.Reference He, Zhang and Fang 32 Since the use of donor oocytes is associated with preeclampsia, it is reasonable to consider whether there is a concomitant increased risk for poor neonatal outcomes that is elevated above those found in ART offspring conceived with autologous oocytes.
Accordingly, there is a need to review and summarize the literature related to conceptions after oocyte donation to provide a knowledge base to inform reproductive technology practitioners and patients. The aims of this review were to summarize the published literature on neonatal outcomes such as birth weight, gestational age, and BD, for conceptions after oocyte donation in comparison to those conceived from autologous oocytes and spontaneous conceptions.
Methods
Literature search
A computerized literature search was conducted on articles published up to November 2012 in the online databases PubMed, EMBASE and Cochrane Reviews to identify studies containing data on neonatal health outcomes from donor oocytes.
The literature search comprised a three-stage process. First, relevant articles known to the authors were used to identify keywords along with a search of the U.S. National Library of Medicine MeSH (medical subject headings) database to identify appropriate search headings. Second, MeSH terms and other identified keywords were used to search the online databases. Search results were then analysed to determine specificity and to refine search terms, and filtered based on human not animal studies. Finally, the references of articles fitting the criteria were examined and online searches were implemented to find related articles.
Two main search categories were created and were termed ‘Techniques’, these were: oocyte donation and reproductive techniques. The term ‘reproductive techniques’ was chosen as a catch-all phrase. Two descriptors of human and donor were chosen to filter out animal studies and autologous treatments (own gametes). Three further qualifiers were implemented to each of the two techniques plus descriptor searches to refine the results. These qualifiers were: outcome*, morbidity, and adverse effects. The * wildcard was implemented to cover studies whose keywords implemented outcomes v. outcome. Search terms are also presented in Supplementary Table 1 for reference.
Eligibility and selection
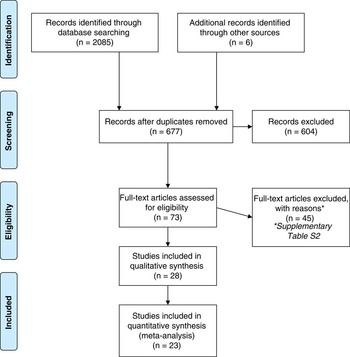
Article selection and eligibility was performed by D.H.A. and verified by S.de L. according to the following PICOS criteria: Participants; cohorts of neonates that had neonatal health data presented in a study. Interventions; the treatment group must be comprised of a cohort of neonates conceived through fertility treatment of their parents with donated oocytes. The treatment group data must be appropriately segregated from comparison group data and not subsumed into larger combined data sets. Comparators; comparison cohorts could include offspring conceived through fertility treatment with autologous oocytes or general population data of spontaneous conceptions. Outcomes; studies to be included must report neonatal health outcomes such as (but not restricted to), birth weights, PD and BD (as determined by the publishing authors). Studies that only focused on live-birth rates or were case studies of single or few outcomes were excluded. Study design; only those observational case-controlled studies that used a treatment-cohort and comparison-cohort, or those studies involving a treatment-cohort that was compared to published, or public health data for that specific region and within 10 years of the study were included in the review. This was to remove any natural variation occurring between populations and between given time points. Published articles were restricted to the English language to allow for careful analysis of the study to ensure accurate data extraction as terminology and reporting methods varied considerably. There was no time restriction posed on publication dates, however, EMBASE would only allow a search from 1980. Database search results were downloaded into Endnote then analysed by their titles and abstracts. Some articles were initially included as they could not be excluded based on their titles or abstracts, and were subsequently excluded following a full review of the text article (Fig. 1). Any disagreement was resolved via discussion.

Fig. 1 PRISMA flowchart for identifying studies for inclusion in the review.
Data extraction
The following data were recorded by D.H.A. from the eligible studies: citation data, country, comparison type (autologous cohort, published data), number of offspring in treatment/comparison group, if cryopreservation of oocytes was used, and specific neonatal health outcomes. Data were recorded onto a specially designed data extraction form. Specific health outcomes included birth weights, chromosomal or congenital malformations (BD), PD/gestational age, IUGR, and singletons v. multiple births (Table 1). Maternal age and parity were also extracted to assess confounding where applicable. Multiple birth is a known predictor of increased adverse outcomesReference Skora and Frankfurter 33 , Reference Barrington and Janvier 34 and was therefore a confounder to the analysis unless the data were stratified and extracted where presented. Oocyte cryopreservation is also a potential confounder as its effects on neonatal outcomes are poorly characterized, and therefore data were extracted where possible.
Table 1 Donor oocyte offspring health outcomes
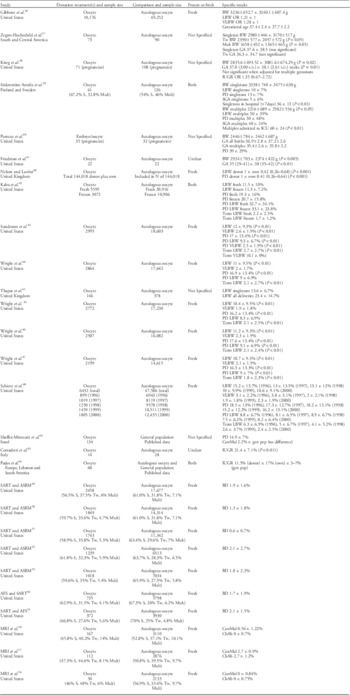
BW, birth weight; LBW, low birth weight <2500 g; VLBW, very low birth weight <1500 g; ConMal, congenital malformation; ChAb, chromosomal abnormalities; BD, birth defects; IUGR, intra-uterine growth retardation; PD, preterm delivery (<37 weeks); GA, gestational age (weeks); SGA, small for gestational age; S, singleton; Tw, twin; Mult, multiple (triplets or greater); OR, odds ratio; gen pop, general population.
Health outcomes for neonates conceived via donated oocytes. Sample sizes represented by statements such as ‘published data’ refers to the study citing data published elsewhere. Data are presented as donor v. control, P value for significance is only provided where P<0.05. Comparison groups are autologous cohorts unless otherwise noted. General population refers to data obtained from spontaneous conceptions that have either been presented in ‘published data’ by other authors, or were part of a comparison cohort with specific data presented in the study.
Data referring to previously published data or general population databases, but that did not record the actual outcome data, were not included in meta-analysis. No authors were contacted for missing or obscure data.
Meta-analysis
Meta-analyses of dichotomous outcomes data of low birth weight (LBW), very low birth weight (VLBW), PD and BD were performed according to Mantel-Haenszel methods, using a fixed effects model, risk ratios, 95% confidence intervals, and assessment of statistical heterogeneity using Cochrane’s Q test and I 2 statistic. Continuous mean birth weight and gestational age data were analysed by inverse variance with fixed effects and 95% confidence intervals to determine the mean difference. Heterogeneity of data were determined by the I 2 value and is considered significant when I 2>65%. Sensitivity analysis of studies that presented overlapping data were performed by conducting separate meta-analysis with those studies removed to determine any influence on data. All analyses were performed using Review Manager (RevMan) Version 5.2.8 (Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration, 2012). Publication bias was assessed by visual analysis of funnel plots constructed in RevMan for asymmetry.
Risk of bias in individual studies
Included studies were assessed for methodological quality and the risk of bias using a modification of the Joanna Briggs Institute Meta Analysis Statistics Assessment and Review Instrument (JBI-MAStARI), with critical appraisal criteria for comparable cohort/case control studies. 35 Studies were assessed on the following criteria; Are patients in cohorts representative of patients typically receiving fertility treatment (for example, do they only include patients who may have been treated for ovarian cancer)? Are the patients at a similar point in the course of their condition/illness (do they describe how many times the patient had received treatment)? Has bias been minimized in relation to selection of cases and of comparators? Were singleton v. multiple births identified and strategies to deal with them stated? (If singleton v. multiples was described but all of the outcome data relevant to the review was not stratified then=No); Are other confounding factors identified and strategies to deal with them stated? Was cryopreservation of oocytes or the use of fresh oocytes adequately described, and were they appropriately stratified if both were included? Are outcomes assessed using objective criteria? Were outcomes measured in a reliable way? Was appropriate statistical analysis used?
Results
Searching of the three databases yielded 2085 articles with another six identified by hand-searching references lists. Duplicate removal reduced potential articles to 677. After screening the article titles and abstracts, 73 full-text publications were reviewed leaving a total of 28 studies to be included in the review and, of these, 23 were included in a meta-analysis (Fig. 1).
A summary of the characteristics and outcomes of all studies included in this review is presented in Table 1. Studies removed and the reasons for exclusion are presented in Supplementary Table S1.
There was a large amount of heterogeneity in the presentation of published outcomes. Subsequently, meta-analysis was only performed for outcomes that presented comparable statistical outcomes. The five studies not included in meta-analysis did not present comparable outcome data and have been summarized in the text.
Oocyte donation outcomes
A total of 28 studies investigating the health outcomes of people conceived via donated oocytes met the criteria for inclusion, 23 of which were included in the meta-analysis.Reference Gibbons, Cedars and Ness 36 – 59 The remaining five studies were used in a qualitative analysis.Reference Nelson and Lawlor 60 – Reference Pados, Camus, Van Steirteghem, Bonduelle and Devroey 64 Two studies reported on comparisons to spontaneous conceptions but the data did not allow for meta-analysis. Subsequently all meta-analysis were comparisons against neonates conceived with autologous oocytes. From these studies a total of 201,628 neonatal health outcomes from donor oocytes and 432,361 from autologous oocytes have been analysed.
The actual number of offspring is overestimated due to the overlap of some studiesReference Gibbons, Cedars and Ness 36 , Reference Sunderam, Chang and Flowers 43 – Reference Wright, Chang, Jeng, Chen and Macaluso 45 in relation to the data they obtained from the Society for Assisted Reproductive Technology databases covering the same years. We did not have access to the raw data and therefore the exact number cannot be ascertained, however, a sensitivity analysis was conducted to determine if it affected the meta-analysis outcomes (described later). The majority of the studies represented national data obtained from multicentres (71.4%), of which most were from the United States of America [data from Society for Assisted Reproductive Technology (SART) and Centers for Disease Control and Prevention (CDC)], while the single largest study was from a national study in the United Kingdom with data collected by the HFEA (Human Fertilisation and Embryology Authority). There is no consensus in the types of outcomes data these studies collected.
Cryopreservation data were recorded in some studies, however, the data were either poorly stratified, or in some instances was cryopreserved embryos created from donated oocytes and therefore could not be used in a meta-analysis. Consequently we do not report on the effects of cryopreservation on donated oocyte outcomes, however, we have left references to which studies noted cryopreservation in Table 1. Subsequently data will include both fresh and cryopreserved outcomes unless otherwise specified.
Oocyte donation birth weights
One of the most common outcomes reported was birth weights, which also included the categories of LBW<2500 g, VLBW<1500 g and IUGR or small for gestation age.
Ten studies reported a significant reduction in either the birth weights (P=0.02),Reference Krieg, Henne and Westphal 38 or increased incidences of low birth weight category births ((P<0.001),Reference Nelson and Lawlor 60 (P<0.01)Reference Sunderam, Chang and Flowers 43 – Reference Wright, Schieve, Reynolds and Jeng 47 ), IUGR (P<0.011),Reference Corradetti, Talebi Chahvar, Biondini, Giannubilo and Tranquilli 63 or increased odds ratios for low (1.21 v. 1)Reference Gibbons, Cedars and Ness 36 or very low birth weights (1.28 v. 1)Reference Gibbons, Cedars and Ness 36 of children conceived from donated oocytes when compared to those conceived from autologous oocytes. A further study found a similar significance but only in multiples and not singletons (P<0.05).Reference Söderström-Anttila, Tiitinen, Foudila and Hovatta 39 Another two studies reported a higher incidence of LBW for donor oocyte neonates, but did not conduct statistical analysis on the outcome.Reference Kalra, Ratcliffe, Coutifaris, Molinaro and Barnhart 42 , Reference Thapar, Harold and Rice 61 The four studies reporting no significant difference in birth weights or higher birth weights in donor oocyte neonates were associated with the smallest sample sizes,Reference Zegers-Hochschild, Masoli and Schwarze 37 , Reference Söderström-Anttila, Tiitinen, Foudila and Hovatta 39 – Reference Friedman, Copperman and Brodman 41 while studies reporting significant differences were associated with large multicentre national cohorts.Reference Gibbons, Cedars and Ness 36 , Reference Kalra, Ratcliffe, Coutifaris, Molinaro and Barnhart 42 – Reference Wright, Schieve, Reynolds and Jeng 47 , Reference Nelson and Lawlor 60
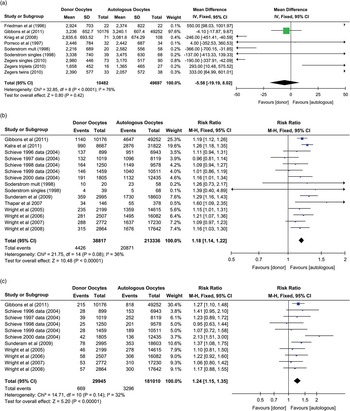
Meta-analysis of mean birth weights showed that donor oocyte neonates (singleton and multiple deliveries) had a lower mean difference but was not statistically different to control cohorts (autologous oocytes) (mean difference −5.58 g, CI: −19.19 to –8.02 g, P=0.42, I 2=76%) (Fig. 2a). When multiple deliveries were excluded, the outcome was relatively unchanged (mean difference −4.91 g, CI: −18.63 to –8.81 g, P=0.48, I 2=26%), even though heterogeneity was improved. These data are extensively influenced by the publication of Gibbons et al. who used a 3-year data set from U.S. national data obtained by the SART.Reference Gibbons, Cedars and Ness 36 Donor oocyte neonates were at an increased risk ratio (RR: 1.18, CI: 1.14–1.22, P<0.00001, I 2=36%) observed for being born <2500 g (LBW) when compared to autologous oocytes (Fig. 2b). Schieve et al. stratified the data into years and subsequently each year was treated as a separate study to match the data from other studies that also reported on annual CDC and SART data (the Gibbons et al. data from the continuous data analysis of mean birth weights could not be similarly stratified as it was pooled data). Similarly to LBW, the risk of being born below 1500 g (VLBW) was increased for those conceived from donor oocytes compared to autologous oocytes (RR: 1.24, CI: 1.15–1.35, P<0.00001, I 2=32%) (Fig. 2c). VLBW was the only outcome measure that was of fresh donor oocytes only. These birth weight outcomes were for all neonates irrespective of whether they were born preterm (<37 weeks), at term (37–42 weeks), or post term (>42 weeks).

Fig. 2 Forest plots of birth weight outcomes of neonates from donor oocytes v. autologous oocytes; (a) mean differences of birth weights, (b) risk ratio for being born of low birth weight<2500 g, (c) risk ratio for being born of very low birth weight <1500 g.
The data reported by Gibbons et al.Reference Gibbons, Cedars and Ness 36 and Kalra et al.Reference Kalra, Ratcliffe, Coutifaris, Molinaro and Barnhart 42 covered SART data from 2004 to 2006, which overlapped with the SART data (same years) reported by Wright et al.Reference Wright, Chang, Jeng and Macaluso 44 , Reference Wright, Chang, Jeng, Chen and Macaluso 45 and Sunderam et al.,Reference Sunderam, Chang and Flowers 43 however, outcome measures and numbers differed. Subsequently, they were included in the review and meta-analysis. To determine if the inclusion of Gibbons et al. and Kalra et al. adversely affected the meta-analysis, a sensitivity analysis was performed whereby they were removed from the meta-analysis. The outcomes for donor oocyte neonates was similar irrespective of the exclusion or inclusion of Gibbons et al. and Kalra et al. with LBW (excluded RR: 1.14, CI: 1.09–1.19, P<0.00001, I 2=31%), (included RR: 1.18, CI: 1.14–1.22, P<0.00001, I 2=36%), and VLBW (excluded RR: 1.23, CI: 1.12–1.36, P<0.0001, I 2=39%), (included RR: 1.24, CI: 1.15–1.35, P<0.00001, I 2=32%).
Oocyte donation PD
A total of 10 studies reported on the incidences of being born prematurely (PD<37 weeks).Reference Porreco, Schoolcraft and Schoolcraft 40 , Reference Kalra, Ratcliffe, Coutifaris, Molinaro and Barnhart 42 – Reference Schieve, Ferre and Peterson 48 , Reference Nelson and Lawlor 60 , Reference Sheffer-Mimouni, Mashiach, Dor, Levran and Seidman 62 Of these ten studies, six reported that neonates conceived of donor oocytes were significantly more likely to be born PD (P<0.001),Reference Nelson and Lawlor 60 (P<0.01).Reference Sunderam, Chang and Flowers 43 – Reference Wright, Schieve, Reynolds and Jeng 47 Kalra et al. and Schieve et al. did not statistically analyse the donor v. autologous oocyte results.Reference Kalra, Ratcliffe, Coutifaris, Molinaro and Barnhart 42 , Reference Schieve, Ferre and Peterson 48 Porreco et al. reported a higher percentage of donor oocyte neonates born PD, but it was not statistically significant (P value not reported).Reference Porreco, Schoolcraft and Schoolcraft 40 Meta-analysis showed a significant increased risk of being born PD as a result of using donor rather than autologous oocytes (RR: 1.26, CI: 1.23–1.30, P<0.00001, I 2=0%) (Fig. 3a). Five studies investigated gestational age in absolute terms (age in weeks) with two finding that donor oocytes were more likely to be born at a lower gestational age (P<0.01),Reference Krieg, Henne and Westphal 38 , Reference Friedman, Copperman and Brodman 41 while the other three (P=0.563),Reference Gibbons, Cedars and Ness 36 (P value not reported),Reference Zegers-Hochschild, Masoli and Schwarze 37 , Reference Porreco, Schoolcraft and Schoolcraft 40 found no difference when compared with autologous oocyte gestations. The Krieg et al. gestational age data were also associated with a lower birth weight. By contrast, Zegers-Hochschild et al. found no significant difference in singleton or twin gestational ages (P value not reported).Reference Zegers-Hochschild, Masoli and Schwarze 37 Additionally, Porreco et al. found no difference between groups in respect to birth weights, gestational age (including singleton v. multiple delivery analysis) and PD.Reference Porreco, Schoolcraft and Schoolcraft 40 Meta-analysis of the continuous data on gestational age showed that donor oocytes were born significantly earlier by 0.3 weeks when compared to autologous oocytes (mean difference −0.3 weeks, CI: −0.35 to −0.25 weeks, P<0.00001, I 2=40%) (Zegers-Hochschild et al.Reference Zegers-Hochschild, Masoli and Schwarze 37 was excluded due to lack of standard deviation data).

Fig. 3 Forest plots of preterm and term delivery outcomes of neonates from donor oocytes v. autologous oocytes; (a) risk ratio for being born preterm (<37 weeks), (b) risk ratio for being born preterm and of low birth weight, (c) risk ratio of being born term and low birth weight.
Oocyte donation birth weight with PD
Seven studies correlated the birth weight results with the incidences of the neonates being born prematurely.Reference Kalra, Ratcliffe, Coutifaris, Molinaro and Barnhart 42 – Reference Schieve, Ferre and Peterson 48 Of these, three found a significant correlation in donor oocyte neonates when compared with autologous oocyte neonates being born PD and LBW (P<0.01).Reference Sunderam, Chang and Flowers 43 , Reference Wright, Chang, Jeng and Macaluso 46 , Reference Wright, Schieve, Reynolds and Jeng 47 One study also showed an association of PD with being born VLBW (P<0.01).Reference Sunderam, Chang and Flowers 43 Schieve et al. did not perform statistical analysis of donor v. autologous oocytes; however, in every year of data there was a higher percentage of donor oocyte neonates born PD with LBW (8.8 v. 6.7% (1996), 8.1 v. 6.5% (1997), 8.9 v. 6.7% (1998), 7.5 v. 6.2% (1999), 8.2 v. 6.4% (2000)).Reference Schieve, Ferre and Peterson 48 The other two studies found no significant difference. Meta-analysis of the incidence of PD with LBW in donor oocytes showed a significant increased risk compared with autologous oocytes (RR: 1.24, CI: 1.19–1.29, P<0.00001, I 2=46%) (Fig. 3b).
Seven studies correlated the birth weight results with the incidences of the neonates being born at term.Reference Kalra, Ratcliffe, Coutifaris, Molinaro and Barnhart 42 – Reference Schieve, Ferre and Peterson 48 For those born at term with donated oocytes there was a decreased risk of LBW (RR: 0.86, CI: 0.8–0.93, P=0.0003, I 2=0%) (Fig. 3c).
Oocyte donation BD
The reporting of BD was absent from recent analysis of donor oocyte outcomes but were presented in earlier studies by Medical Research International (MRI) et al. 56 – 58 Additionally, data collected by the SART from clinics in the United States from 1990 to 1997 reported on congenital malformations and BD, 49 – 53 and Sheffer-Mimouni et al.Reference Sheffer-Mimouni, Mashiach, Dor, Levran and Seidman 62 reported on congenital malformations.Reference Sheffer-Mimouni, Mashiach, Dor, Levran and Seidman 62 The MRI and SART data did not include statistical analysis but rather reported percentages of incidences in which a higher percentage of BD was observed in 3 of the 10 years (see Table 1). Sheffer-Mimouni et al. found no significant difference to the general population in Israel (statistics not reported).Reference Sheffer-Mimouni, Mashiach, Dor, Levran and Seidman 62 Meta-analysis of BD showed no increased risk ratio for fresh donor v. fresh autologous oocytes, although a non-significant tendency to a lower risk was observed (RR: 0.89, CI: 0.75–1.05, P=0.15, I 2=48%) (Fig. 4). The data set from the BD meta-analysis contained the incidences of twin and multiple (triplets or higher order) birth occurrences. Donor oocyte neonates were more likely to be born as a twin or from higher order deliveries (RR: 1.1, CI: 1.07–1.13, P<0.00001, I 2=31%), in the cohorts included in the BD meta-analysis.

Fig. 4 Forest plots of birth defect risk ratios of neonates from donor oocytes v. autologous oocytes.
Oocyte donation other outcomes
Söderström-Anttila et al. also investigated the length of time neonates stayed in hospital before going home and the incidences of them being admitted to the intensive care unit (ICU).Reference Söderström-Anttila, Tiitinen, Foudila and Hovatta 39 The authors found a significant increase in the length of stay in the hospital of donor oocyte singleton neonates (P<0.01), as well as an increase in the incidence of admissions to the ICU for donor oocyte multiple birth neonates (P<0.01), when compared to autologous multiple birth oocytes.Reference Söderström-Anttila, Tiitinen, Foudila and Hovatta 39
Effect of multiplicity on outcomes
Multiple births is a well known confounder for negative neonatal outcomes. Subsequently we assessed the risk ratios for the occurrences of multiple deliveries for the studies included in this review. As shown above, there was an increased risk of multiple deliveries as a result of using donor rather than autologous oocytes (RR: 1.1, CI: 1.07–1.13, P<0.00001, I 2=31%), in the studies used for BD meta-analysis. The studies showing increased multiplicity were typically those published before 2000 and since that time there has been a greater awareness of the need to reduce the number of embryos implanted. For example the ASRM guidelines which described the maximum number of embryos to be implanted were introduced in 1998 but have been adjusted down over the course of the period since with resultant multiple pregnancies decreasing dramatically during that time. 59 Therefore it was important to determine whether the increased risk ratios observed in the outcomes reported above would still hold in an analysis of singleton outcomes.
Mean birth weights remained lower but still not significant for donor oocyte neonates when compared to autologous oocyte neonates (Singleton mean difference; −4.91 g, CI: −18.63 to –8.81 g, P=0.48, I 2=26% v. All births mean difference −5.58 g, CI: −19.19 to –8.02 g, P=0.42, I 2=76%). The risk of being born of LBW as a result of using donor oocytes compared with autologous oocytes remained significant (Singleton RR: 1.17, CI: 1.12–1.23, P<0.00001, I 2=59% v. All births RR: 1.18, CI: 1.14–1.22, P<0.00001, I 2=36%), as did being born of VLBW (Singleton RR: 1.31, CI: 1.11–1.54, P=0.001, I 2=65% v. All births RR: 1.24, CI: 1.15–1.35, P<0.00001, I 2=32%), PD (Singleton RR: 1.27, CI: 1.22–1.32, P<0.00001, I 2=5% v. All births RR: 1.26, CI: 1.23–1.30, P<0.00001, I 2=0%), as well as PD and LBW (Singleton RR: 1.17, CI: 1.10–1.24, P<0.00001, I 2=42% v. All births RR: 1.24, CI: 1.19–1.29, P<0.00001, I 2=46%). Additionally the association of decreased risk of donor oocyte neonates being born at term of LBW also remained in the meta-analysis of singletons (Singleton RR: 0.86, CI: 0.77–0.96, P=0.007, I 2=2% v. All births RR: 0.86, CI: 0.8–0.93, P=0.0003, I 2=0%). Controlling for multiple births in the meta-analysis of outcomes did not alter the increased risk of poor neonatal outcomes for donor oocyte neonate singletons when compared with autologous oocyte singletons.
Risk of bias
There was variation in the methodological quality of the studies included (Table 2). In general, the reporting of the length of time the woman had been receiving treatment (number of previous attempts) was poorly documented and even when such data were presented it was not stratified into donor v. autologous treatments. This lack of stratification also occurred frequently in the reporting of other confounders such as maternal age, parity, BMI, reasons for infertility and other demographic data. Singleton v. multiple deliveries was poorly stratified into donor v. autologous treatments in many of the studies.
Table 2 Critical appraisal and risk of bias of included studies

a Data presented but not stratified donor v. autologous, or not used in an analysis.
b Statistics used appropriately but did not analyse donor v. autologous.
c Comparison group was used but was published data not comparison cohort.
Maternal age and parity data where reported are presented in Table 3. Mean birth weight meta-analysis incorporated 97.6% of weighted data adjusted and controlled for maternal ages and parity.Reference Gibbons, Cedars and Ness 36 Of those studies reporting maternal ages and low birth weight, the associations were conflicting. Gibbons et al. adjusted for maternal age and found no correlation with maternal age,Reference Gibbons, Cedars and Ness 36 whereas the higher maternal ages of the donor oocyte cohort reported by Sunderam et al. were associated with increased risk of low birth weight and very low birth weight.Reference Sunderam, Chang and Flowers 43 The single study reporting maternal age data in relation to PD, found donor oocyte cohort maternal ages to be proportionally higher compared to the autologous oocyte cohort and was associated with PD and PD with low birth weight.Reference Sunderam, Chang and Flowers 43 Maternal age and parity data of included studies investigating BD were not reported.
Table 3 Maternal age and parity reporting of included studies

Only those studies reporting maternal age and parity are listed.
a A function of live birth delivery rates.
Studies reporting outcomes such as birth weights, LBW, VLBW, GA and PD were deemed to be reporting objective criteria. However, those only reporting BD (or what was classified as congenital malformations) were more subjective in nature as BD can potentially be missed in the neonatal period. Five of the studies stated that the reporting of BD outcomes was poor and therefore the data set did not contain all BD data. 51 – 54 The study by Pados et al. did not have a comparison cohort but only referred to previously published work, which was limited,Reference Pados, Camus, Van Steirteghem, Bonduelle and Devroey 64 while Corradetti et al. did not adequately describe the selection of comparators.Reference Corradetti, Talebi Chahvar, Biondini, Giannubilo and Tranquilli 63
Funnel plot analysis showed publication bias in oocyte donation health outcome meta-analysis for fresh oocyte BD, while birth weights were too heterogeneous. Outcome measures LBW, VLBW, PD, PD with LBW, and term with LBW were symmetrical. Funnel plot analysis of singleton outcomes showed that birth weight data again was too heterogeneous, and that LBW, PD, and term with LBW were symmetrical, while VLBW and PD with LBW were asymmetrical showing the presence of reporting bias.
Limitations of study
This review was limited by the restriction to three databases, limited hand-searching and the restriction to the English language. Methodological quality and reporting details in earlier publications was generally lower than later studies, which prevented the inclusion of some studies in meta-analysis.
From the data retrieved via this review, the lack of statistical analysis in some of these studies is a concern as is the ability for the studies to capture accurate data when self-reporting is involved. The AFS and SART 54 , and SART and ASRM 51 – 53 , reported that the incidence of BD were low but that ‘more stringent requirements for follow-up and reporting’ (P1126, P19, P703, P395, respectively) were to be implemented in subsequent years. 51 – 54 This suggests that the ability to accurately capture this data could be improved. Furthermore, in 2000 they suggested that there were still limitations on the data for birth outcomes, 49 thereby suggesting that the incidences of BD in all groups may be underreported.
The reporting of multiple deliveries was inconsistent and not all data were appropriately stratified for analysis of singleton v. multiple birth outcomes. While rates of multiples were broken down in some studies, in some of these instances the outcome data were pooled rather than stratified and therefore meta-analysis could not be performed in a stratified manner for those studies, thereby reducing the number of studies available for meta-analysis of singleton data. However, when meta-analysis of the singleton data were performed, similar risk ratios for negative outcomes were observed suggesting that multiple birth confounding data did not significantly influence the analysis on outcomes.
Methodological quality and reporting of outcomes in a systematic way generally improved over time, with later studies better addressing the donor v. autologous outcomes question. Consistency in reporting, with increased analysis of confounders such as multiple deliveries, maternal age, parity and cryopreservation, can be improved.
Discussion
This review has demonstrated that donor oocyte neonates, when compared with autologous oocyte neonates, are at increased risk of being born of low birth weight, very low birth weight, preterm, preterm with low birth weight, and have a lower gestational age. The incidences of low birth weight were not increased, rather they were decreased when donor oocyte neonates were born at normal gestation. These correlations also occurred when controlling for multiple deliveries. However, singleton very low birth weight and PD with low birth weight analysis showed the presence of some reporting bias and more data is required to clarify these outcomes in singletons. The majority of comparison group data and all of meta-analysis comparison data were of autologous oocytes, of which only two studies of small sample size reported spontaneous conception data as a comparison.Reference Sheffer-Mimouni, Mashiach, Dor, Levran and Seidman 62 , Reference Pados, Camus, Van Steirteghem, Bonduelle and Devroey 64 The use of autologous oocyte neonates as the comparison cohort rather than spontaneous conceptions is a more appropriate comparison as the manipulation of oocytes may influence neonatal outcomes and therefore strengthens the review findings.
Meta-analysis showed no increase in the incidences of BD occurring as a result of using donor v. autologous oocytes, but rather a non-significant decrease was observed. While several studies (particularly those involving SART data) reported on the incidences of multiple deliveries, the incidences of BD was not appropriately stratified by plurality to determine if the BD were occurring in the multiple deliveries, and subsequently could only be used to determine the risk ratio for all births as a result of using donated or autologous oocytes. The use of donor oocytes was correlated with multiple deliveries in the studies used for BD meta-analysis. While this is a known confounder, a lower risk of BD was observed. This would be consistent with oocytes being donated by women younger than the recipients and in whom lower incidences occur of poor quality oocytes such as those with aneuploidy.Reference Obradors, Rius and Daina 65 , Reference Kuliev, Cieslak and Verlinsky 66 In comparison to ART outcomes in general, the incidences of BD in donor oocyte neonates are less (ART birth defects RR: 1.32, CI: 1.24–1.42, P=0.000, I 2=47%).Reference Hansen, Kurinczuk, Milne, de Klerk and Bower 3
The results of this review suggest that the incidences of BD and or congenital malformations are not adversely affected by the use of fresh donor oocytes and that data should be collected on the BD incidences from neonates conceived with cryopreserved donor oocytes. This is pertinent considering that oocyte cryopreservation often involves the use of genotoxic cryoprotectants,Reference Aye, Di Giorgio and De 67 with some researchers reporting increased incidences of DNA damage.Reference Men, Monson, Parrish and Rutledge 68 , Reference Stachowiak, Papis and Kruszewski 69
There was only one study investigating length of hospital stays and admissions to neonatal intensive care units (NICU) for those neonates conceived with donor rather than autologous oocytes. The increased length of hospital stay and NICU admissions found by Söderström-Anttila et al. (including in singletons), has been corroborated since the review census date. Malchau et al. reported a significant increase in the percentage of donor oocyte singleton neonates entering NICU (24.2 v. 7.6%, P<0.0001), and an increase in their length of stay (2.5±7.5 v. 0.9±5.8 days, P=0.002).Reference Malchau, Loft and Larsen 70 Further investigation in this area is therefore warranted.
Additionally, others have reported increased negative neonatal outcomes following oocyte donation when compared to autologous oocytes of low birth weight, very low birth weight, PD, very PD, small for gestational age, and very small for gestational age;Reference Marino, Moore and Willson 2 and PD, and low birth weightReference Malchau, Loft and Larsen 70 since the literature search was completed and support the findings of this review.
Patients undergoing treatment for infertility require accurate information, not only about the expected pregnancy and take home baby rates of the treatment, but also about the expected health of their infant and how this might be affected by the procedure. Clinicians involved in interventions for the treatment of infertility have an obligation to provide this information, to the best of their ability, in counselling couples about treatment choices.
Increased incidences of low birth weight, very low birth weight, lower gestational age, PD, PD with low birth weight, and preeclampsia that are correlated with the use of donor oocytes constitute obstetric risks that will pose a challenge for obstetricians in their provision of care. Preliminary evidence suggestive of increased NICU admissions associated with donor oocytes are also of concern. While low birth weight can be avoided in donor oocyte conceptions when the fetus reaches normal gestation, it is unclear if this is clinically possible or advisable, considering the correlation with preeclampsia. It could possibly be viewed that preeclampsia represents a far greater risk to mother and fetus than low birth weight and PD outcomes. Therefore preeclampsia would warrant the primary consideration of the obstetrician. Patients should be counselled on the gestational/perinatal risks so that they are fully informed of potential outcomes and therefore maintain full autonomy over their reproductive choices.
The results of this review also highlight other areas that require further investigation. Studies that specifically stratify and isolate negative neonatal outcomes as a direct consequence of preeclampsia rather than just as a correlation will help ascertain if the increased incidences are primarily the result of a significant immunological challenge or if a more minor immunological response can also produce these outcomes. Finally, studies are required that investigate the longitudinal outcomes into childhood and adulthood to determine if these negative neonatal outcomes resulting from their conception with donated oocytes also negatively impact their long term health as currently there are no such studies.
Due to poor neonatal outcomes of low birth weights and preterm deliveries being associated with increased incidences of morbidity and mortality in later life,Reference Class, Rickert, Lichtenstein and D’Onofrio 8 – 10 the donor oocyte cohort represent a significant health care burden for the individual and society. This is irrespective of any reasons as to why this cohort fares worse than autologous oocyte neonates or spontaneously conceived neonates.
Acknowledgements
None.
Financial Support
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
None.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi./org/10.1017/S2040174415007898