Introduction
Vitamin D deficiency is surprisingly common. In a recent review Lips suggested that the prevalence of vitamin D insufficiency in many countries across each continent was as high as 50%.Reference Lips1 Of concern is the prevalence of vitamin D deficiency in women of child bearing age. In a study of more than 900 pregnant women in Sydney, 15% of the women, 11% of their neonates and 71% of veiled women were classified as vitamin D deficient, with a further high proportion classed as insufficient,Reference Bowyer, Catling-Paul and Daimond2 and studies of women who are of fertile age, pregnant or breast feeding in many other countries report similar findings.Reference Dawodu and Wagner3 The fetus cannot produce vitamin D; the principal source of vitamin D in humans is manufacture in the skin through the action of sunlight, with the remainder obtained from supplements and food sources. Vitamin D status in the fetus is therefore determined by the vitamin D status of the mother.
Vitamin D acts at a single nuclear receptor, the vitamin D receptor (VDR), which can be found in the rat kidney from E15 onwards.Reference Johnson, Grande and Roche4 When bound by the active form 1,25-dihydroxyvitamin D3 (1,25(OH)2D) the VDR regulates the transcription of a large number of genes in addition to those involved in calcium and bone metabolism. The VDR interacts with a cyclic AMP-dependent response element in the renin gene promoter region to suppress renin gene transcription.Reference Yuan, Pan and Kong5 In adult studies, treatment with 1,25(OH)2D reduced renal renin gene expression in vitamin D replete mice and decreased it to control levels in vitamin D deficient animals.Reference Li, Kong and Wei6 Mice lacking either the VDR or 1α-hydroxylase [the renal enzyme which makes 1,25(OH)2D] have very high kidney renin mRNA and systemic renin and Ang II levels, leading to hypertension, cardiac hypertrophy and proteinuria.Reference Li, Kong and Wei6, Reference Zhou, Lu and Cao7 Renin mRNA levels are elevated in young VDR (−/−) mice, before any changes in plasma calcium and PTH occur, and dietary calcium replacement in older knockouts does not normalize expression.Reference Li, Kong and Wei6, Reference Zhou, Lu and Cao7 The first aim of this study was to examine the impact of maternal vitamin D deficiency on renin expression in the fetal kidney. We hypothesized that maternal vitamin D deficiency would result in upregulation of fetal renal renin expression, as occurs in the adult kidney.
Along with renin we examined expression of the recently described (pro)renin receptor [(P)RR]. When prorenin binds to the (P)RR it undergoes conformational change which exposes the active site, so that it can generate angiotensin I (Ang I) from angiotensinogen.Reference Nguyen, Delarue and Burcklé8 Renin or prorenin binding to the (P)RR also have effects unrelated to Ang II, activating intracellular pathways such as transforming growth factor β 1 (TGF-β1) and hence possibly contributing to renal fibrosis.Reference Nguyen, Delarue and Burcklé8, Reference Huang, Wongamorntham and Kasting9 We also measured renal expression of nephrin, a key component of the slit diaphragm in the glomerular filtration barrier.Reference Welsh and Saleem10 Nephrin loss commonly occurs in proteinuric kidney disease, and one of the mechanisms by which vitamin D is thought to be renoprotective in the adult kidney is by increasing nephrin expression.Reference Welsh and Saleem10, Reference Deb, Wang and Zhang11 We wanted to see whether vitamin D deficiency would alter the expression of nephrin in the fetal kidney during the important period of nephron formation and development.
Our second aim was to determine the impact of maternal vitamin D deficiency on renal renin gene expression and renal function in adulthood. The intrarenal renin–angiotensin system (RAS) is essential for normal kidney development,Reference Guron and Friberg12 and changes in the RAS have been implicated in a number of programming models of hypertension.Reference Moritz, Wintour, Black, Bertram and Caruna13, Reference Vehaskari and Woods14 Therefore if vitamin D deficiency causes upregulation of the RAS during development, this has the potential to have long-lasting effects. We studied adults at 22–38 weeks because the effects of programming are accentuated with time.
Others have reported that in rats maternal Vitamin D deficiency altered renalReference Maka, Makrakis and Parkington15 and cardiac development,Reference Gezmish, Tare and Parkington16 and 7-week-old offspring had elevated arterial pressure and heart rate and increased myogenic tone.Reference Tare, Emmett and Coleman17 However, in these studies, the offspring were weaned onto a vitamin D deficient diet, so the possibility that coexisting vitamin D deficiency was responsible for the cardiovascular alterations in these young offspring cannot be excluded. Hence, in our study, like in the recently reported study in mice by Nascimento et al.,Reference Nascimento, Ceciliano, Aguila and Mandarim-de-Lacerda18 offspring were weaned onto normal chow so that the effects of maternal vitamin D deficiency could be differentiated from the effects of continued vitamin D deficiency in postnatal life.
We were particularly interested to examine the effects of maternal vitamin D deficiency on renal function during adulthood. Although there is evidence in rats and mice that vitamin D deficiency in early development stimulated nephrogenesis,Reference Maka, Makrakis and Parkington15, Reference Nascimento, Ceciliano, Aguila and Mandarim-de-Lacerda18 and delayed glomerular maturationReference Nascimento, Ceciliano, Aguila and Mandarim-de-Lacerda18 this finding is in contrast to other studies in which incubation of metanephroi with vitamin D before transplantation increased glomerular number.Reference Rogers, Droege, Dusso and Hammerman19 Furthermore, it is unknown whether any additional nephrons formed in the vitamin D deficient environment are structurally and functionally normal and therefore what the implications are for future renal function. In addition to examining renal function in adult offspring, we determined whether there were long-term effects on the renal expression of genes for the (P)RR, TGF-β1 and nephrin, all of which may be altered in renal disease.
Methods
Animals
Four-week-old virgin female Sprague–Dawley rats were randomly assigned to two maternal groups, control and vitamin D deficient. Controls (n = 13) were fed standard rodent chow containing 1 IU vitamin D3 (cholecalciferol) per gram, 0.45% Ca2+ and 0.5% phosphate (AIN93G; Gordon's Specialty Feeds, Yanderra, NSW, Australia). Vitamin D deficient dams (DEF, n = 16) were fed a modified version of this chow, with no vitamin D3 and with added Ca2+ (2.5%) and phosphate (1.5%).Reference Weishaar and Simpson20 Rats were communally housed at 20 ± 2°C under a 12 h UVB-free light/dark cycle, with ad libitum access to food and water. Consistent with other studies,Reference Maka, Makrakis and Parkington15–Reference Nascimento, Ceciliano, Aguila and Mandarim-de-Lacerda18 dams were maintained on their respective diets for at least 6 weeks before mating to ensure that those in the DEF group entered pregnancy in a state of vitamin D deficiency; plasma 25(OH)D levels (methods described below) on day 20 of pregnancy were 6 ± 1 (mean ± s.e.m.) nmol/l (n = 5).
Females were time-mated with vitamin D replete males from 10 weeks of age, and the appearance of a vaginal mucus plug was taken as day 1 of pregnancy. Dams were housed individually close to term. A small subset were euthanised at E20 for fetal tissue collection (details below). For all other dams, litter size was adjusted to 12 pups within 24 h of delivery, with a similar number of male and female pups where possible. Offspring were taken for postmortem from each litter on postnatal days (PNDs) 3 and 20. Remaining offspring (male and female) from each litter were weaned onto standard laboratory rodent chow on PND 21 and studied as adults (22–38 weeks). They were weighed weekly and handled regularly. Dams were euthanised after weaning.
Urine collection
At 33 weeks of age male and female offspring were placed individually into metabolic cages (Harvard, SDR Clinical Technology, Middle cove, NSW, Australia) and acclimatised for 24 h before a 24 h urine collection was made. The volume of collected urine was measured and it was stored at −20°C. Water and ground standard rodent chow were given ad libitum, and food and water intake measured. Water balance was calculated as the difference between water intake and urine output.
Postmortem analysis
Postmortem dissections to assess offspring growth were carried out on PNDs 3 and 20, and at 22 and 38 weeks of age. Kidneys were collected from fetuses (E20) and from 38-week-old male offspring to measure gene expression. At all ages, body weights were recorded and animals were killed by decapitation, with dams and offspring aged 20 days and older deeply anaesthetised beforehand (80 mg/kg ketamine and 10 mg/kg xylazine i.p.). In these anaesthetised animals, blood was collected by cardiac puncture into heparinized tubes and plasma separated by centrifugation for 10 min at 1560 g and 4°C. Plasma was stored at −20°C. Kidneys were removed, weighed, and snap-frozen in liquid nitrogen then stored at −80°C. Hearts were removed and weighed, then dissected and left and right ventricular weights recorded. In adult offspring the right retroperitoneal white adipose tissue depot and the inguinal (females) and testicular (males) white adipose tissue depots were removed and weighed.
Biochemistry
Plasma and urinary osmolality was measured by freezing point depression using a Fiske One-Ten Osmometer (model 3D11; Needham Heights, MA, USA), and sodium and potassium levels were determined by flame photometry (Radiometer Flame Photometer model FLM3 and Hyperion Diluter, model A6241, Radiometer Copenhagen, Denmark). Plasma and urinary creatinines were measured using the method of HaeckelReference Haeckel21 using a microplate reader (model 680 XR, Bio-Rad Laboratories Pty Ltd., Regents Park, NSW, Australia) at 510 nm. Urinary protein concentrations were determined by Lowry's method,Reference Lowry, Rosebrough, Farr and Randall22 and urinary protein was expressed as μg/mg creatinine. Plasma 25-hydroxyvitamin D3 (25(OH)D) measurements were made using chromatography-dual mass spectrometry by Pathology Queensland (Royal Brisbane and Women's Hospitals, Herston, QLD, Australia) following deproteination and solid phase extraction.
Real-time PCR
Renal mRNA levels for renin, the (pro)renin receptor, TGF-β1 and nephrin were measured in E20 fetuses and in 38-week-old male offspring. For the fetal studies, kidneys from three to five fetuses of the same litter were pooled. A total of nine pooled samples from three control dams, and seven pooled samples from five DEF dams were studied. Total RNA was extracted from homogenized whole kidney using spin columns (PureLink RNA Mini Kit, Invitrogen, Mulgrave, Australia), and treated on-column with PureLink DNase (Invitrogen). RNA quality and quantity were assessed using a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and agarose gel electrophoresis. Total RNA was reverse transcribed using a kit (High Capacity RNA to cDNA, Applied Biosystems) and thermal sequencer (FTS-960 Thermal Sequencer; Corbett Research, Sydney, Australia), with control reactions containing no reverse transcriptase.
Real time PCR reactions were carried out in duplicate on 10 ng cDNA using an Eppendorf Mastercycler ep realplexReference Bowyer, Catling-Paul and Daimond2 and Applied Biosystems TaqMan Gene Expression Assays (Assay IDs: Renin, Rn00688582-m1; (pro)renin receptor (Atp6ap2), Rn01430718-m1; TGF-β1, Rn01475963-m1; nephrin, Rn00575235-m1), with 18S rRNA as housekeeper (Applied Biosystems, Scoresby, VIC, Australia). Five replicates of a calibrator sample consisting of an equal mixture of cDNA from all control animals were included in each assay. The mRNA level for each target gene, relative to the calibrator, was determined using the comparative threshold cycle ![]() $$--><$>({\rm{ - }}{{{\rm{2}}}^{\rDelta \rDelta {\rm{C}_{\rm{T}}}}} )$$$
method. Preliminary experiments were carried out for each target gene to ensure that the efficiencies of the target and housekeeper reactions were approximately equal.
$$--><$>({\rm{ - }}{{{\rm{2}}}^{\rDelta \rDelta {\rm{C}_{\rm{T}}}}} )$$$
method. Preliminary experiments were carried out for each target gene to ensure that the efficiencies of the target and housekeeper reactions were approximately equal.
Statistical analysis
Separate analyses were carried out for male and female offspring, and data are reported as means ± standard error of the mean (s.e.m.) unless otherwise stated. For all offspring data both number of animals (n) and number of litters (m) are reported. Litter means were calculated for fetal genes before statistical analysis. SPSS Statistics 20 (SPSS Inc., Chicago, IL, USA) was used to compare means between groups by unpaired t-tests. A Mann–Whitney U-test was used where required. Significance was set at α = 0.05.
Results
Dams, fetuses and litters
At E20, maternal weights were not different between control (386 ± 21 g, n = 3) and DEF (440 ± 13 g, n = 5). Heart weight was similar (control: 1.04 ± 0.04 g, n = 3; DEF: 0.96 ± 0.05 g, n = 5) but kidney weight was reduced in the DEF group (control: 2.5 ± 0.2 g, n = 3; DEF: 1.9 ± 0.1 g, n = 5; P = 0.02). The total number of fetuses (control: 11 ± 2; DEF: 15 ± 1) and their average weight (control: 3.9 ± 0.1 g; DEF: 4.1 ± 0.1 g) were not different.
Dams in both groups spontaneously delivered on day 22 of pregnancy. Maternal body weights were similar in the two groups after birth (control: 326 ± 10 g, n = 10; DEF: 330 ± 6 g, n = 9). Litter weights (control: 91.5 ± 4.6 g; DEF: 93.4 ± 3.6 g) and the number of pups per litter (13 ± 1 v. 14 ± 1) were also similar (n = 10 per group). When dams were killed at 23–24 days after delivery, body weight (control: 311 ± 7 g, n = 10; DEF: 323 ± 6 g, n = 11) and heart weight (control: 1.07 ± 0.03 g, n = 10; DEF: 1.10 ± 0.03 g, n = 11) were not different between groups. However, kidney weight was reduced in DEF dams (control: 2.7 ± 0.1 g, n = 10; DEF: 2.3 ± 0.1 g, n = 11; P = 0.001).
Offspring growth
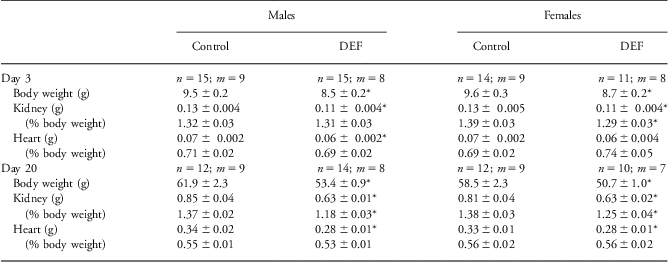
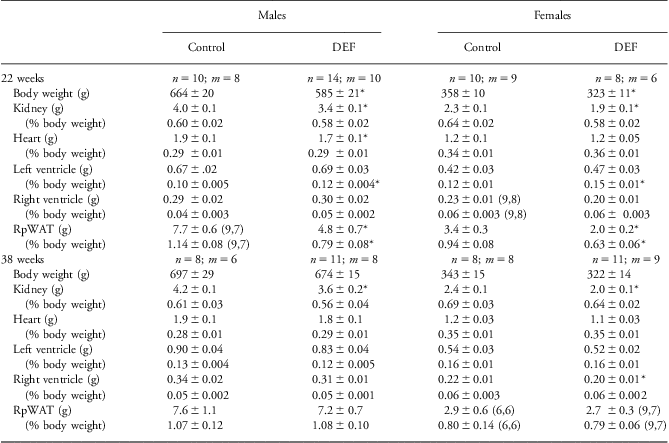
Offspring of DEF dams were growth restricted and their kidneys were smaller. Male and female offspring weighed ∼10% less than control offspring by PND 3, at weaning and as young adults at 22 weeks (P < 0.05, Tables 1 and 2); body weights in the older adults (38 weeks) were similar between groups. Kidney weights were less at every age studied, and in females on PND 3 and 20 and in males on PND 20 the kidney-to-body weight ratio was lower (Table 1). In females at 22 weeks the kidney-to-body weight ratio also tended to be smaller (P < 0.06; Table 2).
Table 1 Overall body growth and renal and cardiac weights in young male and female offspring of control and vitamin D-deficient (DEF) dams

Values are means ± s.e.m.n = number of animals; m = number of litters.
*P < 0.05 compared with controls of the same age and sex.
Table 2 Growth of male and female adult offspring of control and vitamin D-deficient (DEF) dams assessed at 22 and 38 weeks of age

Values are means ± s.e.m.n = number of animals; m = number of litters.
Parentheses indicate n, m when they differ from the values at the top of the column.
*P < 0.05 compared with controls of the same age and sex.
Total heart weight was smaller in male DEF offspring at PND 3, 20 and 22 weeks, and in female DEF offspring at weaning on PND 20. In both sexes there was evidence of left ventricular hypertrophy at 22 weeks of age, with an increased left ventricle-to-body weight ratio (P < 0.05; Table 2), but by 38 weeks of age this was no longer the case.
At 22 weeks in DEF offspring of both sexes, mean weights for the right retroperitoneal white adipose tissue depots were 35–40% less, and relative weights were lower as well (Table 2). Similarly in DEF male offspring, testicular fat pad weight was reduced (control: 11.7 ± 0.9 g, n = 8, m = 7; DEF: 6.5 ± 0.8 g, n = 14, m = 10; P = 0.001) and reduced relative to body weight (control: 1.75 ± 0.11%, n = 8, m = 7; DEF: 1.08 ± 0.09%, n = 14, m = 10; P = 0.001). In female offspring there was no significant difference in inguinal fat pad weights between the groups (control 2.9 ± 0.3 g and 0.81 ± 0.09%, n = 10, m = 9; 2.1 ± 0.5 g and 0.65 ± 0.10%, n = 6, m = 5). At 38 weeks there no differences in any fat pad depots examined between the two groups (retroperitoneal, Table 2; testicular: control 10.9 ± 2.4 g and 1.56 ± 0.27% body weight, n = 3, m = 3; DEF 8.7 ± 0.7 g and 1.30 ± 0.10%, n = 11, m = 8; inguinal: control 1.7 ± 0.4 g and 0.48 ± 0.10%, n = 3, m = 3; DEF 1.8 ± 0.2 g and 0.56 ± 0.06%, n = 9, m = 7).
Renal gene expression
Fetuses carried by vitamin D deficient mothers overexpressed renin (P < 0.05, Fig. 1a), but renal mRNA levels for the (pro)renin receptor, TGF-β1 and nephrin were similar to fetuses of control dams. In the 38-week-old adult male DEF offspring renal mRNA levels for renin were still high, almost double that of controls (P < 0.05, Fig. 1b), while expression of the other genes remained similar to controls. Expression of the endogenous control 18S rRNA was similar between groups at both ages

Fig. 1 Effects of maternal vitamin D deficiency on renal gene expression of renin and related genes in E20 fetuses (a) and 38-week-old adult male offspring (b). Values are mean ± s.e.m. for offspring of control (open bars) and DEF mothers (closed bars). (a) Control n = 3 litters, DEF n = 5 litters. (b) Control n = 8, m = 6; DEF n = 8, m = 8. mRNA levels were normalized to 18S rRNA and expressed relative to the control group at each age as calibrator. *P < 0.05 relative to controls, unpaired t-test.
24 h food and water intake, renal function and plasma vitamin D levels
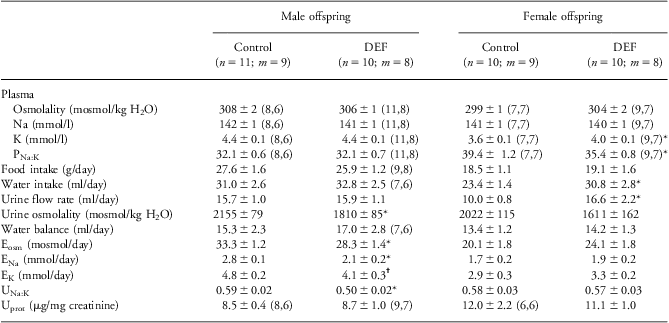
Effects of maternal vitamin D deficiency on plasma composition and renal function in older adult offspring were strikingly sex specific (Table 3, Fig. 2). In males, plasma composition was not affected by maternal vitamin D deficiency, and they ate and drank similar amounts to control offspring. While their urine output was similar to controls, the urine was more dilute (Table 3). Creatinine clearance was reduced in DEF males, and plasma creatinine levels were elevated (Fig. 2). Solute excretion rates were lower (for sodium, potassium and total osmoles, P < 0.05), with a greater reduction in sodium than potassium excretion meaning that the urinary sodium-to-potassium ratio was also less than in control offspring (P < 0.05). The urinary protein-to-creatinine ratio was similar to controls.
Table 3 Food and water intake and renal function in 33 week old adult offspring of control and vitamin D-deficient (DEF) dams

Values are means ± s.e.m.n = number of animals; m = number of litters.
Parentheses indicate n, m when they differ from the values at the top of the column. Ex excretion rate of x, PNa:K, UNa:K plasma and urinary sodium-to-potassium ratios, Uprot urinary protein.
*P < 0.05 compared with same sex control group; †P < 0.05 compared with same sex control group, Mann–Whitney U-test.

Fig. 2 Effects of maternal vitamin D deficiency on creatinine clearance (a) and plasma creatinine (b) in 33-week-old adult offspring. Values are mean ± s.e.m. for offspring of control (open bars; males n = 8, m = 6; females n = 6-7, m = 6–7) and DEF mothers (closed bars; males n = 10–11, m = 8; females n = 9, m = 7). *P < 0.05 relative to controls, unpaired t-test.
Female DEF offspring were affected in a different manner. Plasma potassium levels were elevated so that the plasma sodium-to-potassium ratio was reduced (Table 3). They drank more than controls and produced more urine, which tended to be of a lower osmolality (P = 0.053). Unlike DEF males, plasma creatinine and creatinine clearance were similar to controls (Fig. 2), as were excretion rates and the urinary sodium-to-potassium ratio.
Plasma 25(OH)D levels in 38-week-old offspring were similar between groups for both males (control: 22 ± 2 nmol/l, n = 8, m = 6; DEF: 18 ± 1, n = 8, m = 8) and females (control: 16 ± 2, n = 7, m = 7; DEF: 15 ± 1 nmol/l, n = 7, m = 5).
Discussion
We carried out this study to examine the long-term impact of maternal vitamin D deficiency in offspring that had been weaned onto a normal diet. We found that maternal vitamin D deficiency had long lasting effects on the growth and function of the kidney and on cardiac growth, and that overexpression of renin occurred in the fetal kidney and was sustained into late adulthood. Where both sexes were examined there was distinct sex specificity in the effects of maternal vitamin D deficiency. Water handling was altered in females only, and a deterioration of renal function, as evidenced by elevated plasma creatinine concentrations, reduced creatinine clearance and a global reduction in renal excretion rates, was observed only in male offspring.
An important finding from this study is the upregulation of renin gene expression in offspring of vitamin D deficient mothers. Studies in the adult have found that 1,25(OH)2 vitamin D3 and its analogues directly suppress renin gene expression, while vitamin D deficiency is associated with an increase in renin transcription and translation and an upregulation of the intrarenal RAS.Reference Li, Kong and Wei6, Reference Zhang, Zhang and Ning23, Reference Weng, Sprague and Jisu24 In the current study, E20 fetuses of vitamin D deficient mothers also overexpressed renin, which presents the intriguing possibility that a pregnant woman's vitamin D status modulates the intrarenal RAS of her fetus.
Our measurements of fetal gene expression and early life renal growth were during the nephrogenic period, which in the rat extends from E12 to approximately PND 10.Reference Moritz, Wintour, Black, Bertram and Caruna13 Newborn kidney renin levels are ∼20 times those of the adult,Reference Gomez, Lynch and Sturgill25 and a highly active intrarenal RAS is necessary for normal renal development,Reference Guron and Friberg12 however, the implications for the developing kidney of excessive expression of renin is not known. In the fetal rat the cells producing renin extend along the intrarenal arterial tree, far beyond the classical juxtaglomerular localization seen in the adult under basal conditions.Reference Gomez, Lynch and Sturgill25 Others have reported that young offspring of rats and mice fed a similar vitamin D deficient diet to the current study throughout gestation and lactation have increased nephron number and reduced glomerular volume.Reference Maka, Makrakis and Parkington15, Reference Nascimento, Ceciliano, Aguila and Mandarim-de-Lacerda18 However, in contrast to those studies in which offspring kidney weight was either unaffectedReference Maka, Makrakis and Parkington15 or increasedReference Nascimento, Ceciliano, Aguila and Mandarim-de-Lacerda18 following maternal vitamin D deficiency, in the current study the kidneys were smaller both during the nephrogenic period (mean renal mass 15% less than controls on PND 3) and shortly after its completion on day 20 (22–26% less).
Renal renin mRNA levels were almost two-fold higher in 38-week-old males that had been exposed to maternal vitamin D deficiency but had been on a normal diet since weaning. As adults, these offspring had similar 25(OH)D levels to the control group. This suggests that vitamin D deficiency during gestation and early postnatal life programmes for overexpression of renin by the adult kidney. We would expect that total renin levels both in the kidney and circulation would be raised given the sustained stimulation of renin gene expression in DEF offspring.Reference Castrop, Hocherl and Kurtz26 Nascimento et al.Reference Nascimento, Ceciliano, Aguila and Mandarim-de-Lacerda18 reported increased renin immunoreactivity in kidneys of 6-month-old offspring of vitamin D deficient mice, using an antibody that did not distinguish between inactive and active renin. Interestingly, in that study kidney renin levels were not increased at weaning.
High intrarenal levels of renin and Ang II increase glomerular capillary pressure and cause fibrosis, inflammation and podocyte damage, and so play a major role in the initiation and progression of kidney disease.Reference Kobori, Nangaku, Navar and Nishiyama27 Although the adult males in the present study were not proteinuric (nor was renal nephrin expression reduced), there was good evidence of impaired renal function. Plasma creatinine concentrations were high and creatinine clearance was reduced, indicating markedly reduced functional capacity. In clinical practice, as much as 50% of glomerular filtration rate may be lost before an increase in plasma creatinine levels is seen.Reference Stevens, Coresh, Greene and Levey28 In keeping with a reduction in glomerular filtration rate, excretion rates for osmoles and all electrolytes studied were reduced, despite no difference between groups in food intake and hence solute load. As well, the lower urinary sodium-to-potassium ratio in vitamin D deficient males is consistent with Ang II-mediated increases in aldosterone secretion, and together with the reduced sodium excretion suggests that these animals were retaining salt. None of these differences were seen in female offspring. Based on these findings we postulate that in males, exposure to vitamin D deficiency during gestation and early postnatal life predisposes to kidney disease in adulthood.
Female offspring of vitamin D deficient mothers drank more water and their urine output was correspondingly higher, so that water balance was similar to control animals. The mechanism underlying this is unknown. Interestingly, VDR knockout mice had polyuria, driven by polydipsia which was in turn mediated by central increases in Ang II.Reference Kong, Zhang and Li29 Programming of central mechanisms controlling thirst and drinking may have occurred during the period of vitamin D deprivation. In other models of developmental vitamin D deficiency where rats are deprived of vitamin D during gestation, but have a normal vitamin D environment thereafter, substantial alterations in many other areas of brain development and function have been described.Reference Eyles, Feron and Cui30
We did not weigh separate cardiac chambers in young offspring, but found that during the time that they were exposed to maternal vitamin D deficiency, both male and female offspring had smaller hearts than controls. Sprague–Dawley rats that had been subjected to maternal vitamin D deficiency tended to have a smaller heart volume at 3 days, but at weaning (28 days) had a greater left ventricular wall volume, cardiac myocyte number and volume, and the percentage of mononucleated cardiac myocytes were greater than controls in both males and females.Reference Gezmish, Tare and Parkington16 There is evidence from the current study that this left ventricular hypertrophy extended into adult life, far beyond exposure to maternal vitamin D deficiency, as indicated by an increased left ventricle-to-body weight ratio in the younger adult offspring (22 weeks). These findings suggest that maternal vitamin D status may have long-term effects on cardiac structure, regardless of vitamin D status later in life.
VDR-null mice are hypertensive, and vitamin D deficiency has been associated with high blood pressure both in animals and humans.Reference Li, Kong and Wei6, Reference Weng, Sprague and Jisu24, Reference Boldo, Campbell, Luthra and White31 As well, 7–8-week-old offspring of vitamin D deficient mothers that were weaned onto a vitamin D deficient diet were hypertensive,Reference Tare, Emmett and Coleman17 as were the F1 and F2 generation male offspring of vitamin D deficient mice, despite being weaned onto a normal chow diet.Reference Nascimento, Ceciliano, Aguila and Mandarim-de-Lacerda18 The greater left ventricle-to-body weight ratio at 22 weeks in the current study suggests that DEF offspring of both sexes may have been hypertensive at this age, however, by late adulthood (38 weeks) no difference was apparent.
Both Gezmish et al. Reference Gezmish, Tare and Parkington16 and Tare et al. Reference Tare, Emmett and Coleman17 reported reduced body weights in their vitamin D deficient offspring by 4 and 7 weeks of age respectively, and body growth was slowed in our DEF offspring despite being weaned onto a vitamin D replete diet. Interestingly, the retroperitoneal and testicular fat depots were also smaller in DEF offspring at 22 weeks of age, but this effect had disappeared by 38 weeks, as had the effect on body weight. The VDR is expressed in adipose tissueReference Narvaez, Matthews, Broun, Chan and Welsh32 and there is emerging evidence that vitamin D may play a role in energy homoeostasis and the maintenance of adiposity. In mice, life long absence (through gene ablation) of either the VDR or 1α-hydroxylase, the enzyme which catalyses the second hydroxylation step to produce 1,25(OH)2D, resulted in a smaller body weight and reduced subcutaneous and visceral adiposity at 2, 4 and 6 months of age, and reduced serum leptin levels.Reference Narvaez, Matthews, Broun, Chan and Welsh32 Our finding that exposure to vitamin D deficiency only during gestation and lactation was associated with changes in adiposity in adulthood warrants further investigation.
Vitamin D status in humans is significantly influenced by behaviour and geography, both of which are modifiable. Conditions that contribute to inadequate cutaneous vitamin D production in a pregnant woman (e.g. lack of sun exposure, use of sunscreen, modest dress, latitude) do not necessarily apply to her children, especially in their later life. This study provides evidence that exposure to maternal vitamin D deficiency alone has important implications for renal renin expression and renal disease risk, in offspring studied well into adulthood. It gives further support to the need for programmes promoting maternal vitamin D sufficiency during pregnancy and lactation.
Acknowledgements
The authors thank Ms Julie Marten for assistance in animal care and handling and Hasnah Bahari for assistance with tissue collection.
Financial Support
This work was supported by the Faculty of Medicine, UNSW (Early Researcher Grant).
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (New South Wales Animal Research Act 1985 and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes) and have been approved by the institutional committee (Animal Care and Ethics Committee of the University of New South Wales).