Introduction
Soy is a food of high nutritional value, rich in proteins and unsaturated fats. It is also a source of phytochemicals, especially isoflavones. Isoflavones in the form of genistein and daidzein have gained research attention as phytoestrogens. These compounds share structural similarities with 17-β-estradiol, thus can interact with estrogen receptors (ERs), promoting selectivity for ER beta Reference Kostelac, Rechkemmer and Briviba1-Reference Chang, Charn and Park4 . The intake of soy isoflavones and protein has been associated with cardioprotective functions Reference Mann, Bonacasa, Ishii and Siow5,Reference Simmen, Mercado and Zavacki6 evidenced through increased lipid metabolism Reference Chi, Zhang and Zhang7,Reference Yan, Zhang, Li, Jiao and Dong8 and improved antioxidant profile Reference Qian, Guan and Huang9,Reference Yoon and Parque10 . However, the estrogenic activity of soy has raised concerns about its consumption during critical stages of development, such as gestation and lactation Reference Ruhlen, Howdeshell and Mao11 .
According to the Developmental Origins of Health and Disease theory, environmental conditions, nutrition, and/or hormones influence homeostatic regulation in the early stages of life and the development or protection against diseases throughout the stages of life Reference Rosenfeld12-Reference Dutra-Tavares, Silva and Nunes-Freitas15 . Several studies have shown that nutrition during lactation can increase Reference Rodrigues, Moura, Passos, Dutra and Lisboa16-Reference Desai, Jellyman, Han and Lane19 or decrease Reference Kaludjerovic and Ward20,Reference Peixoto, Moura and de Oliveira21 the risk of developing diseases in adulthood.
Recently, our group observed changes in the lipid profile of breast milk from lactating rats placed on a soybean diet, which programmed adult offspring for a phenotype of lower metabolic risk, low total cholesterol (TC) and LDL-cholesterol, and protection against changes in glycemic metabolism Reference Vieira, Brasiel and Ferreira22 . However, soy protein isolate consumption in the same model programmed the offspring to negative effects on body composition, lipid profile, and glycemic homeostasis, generating a profile of higher metabolic risk in adulthood Reference Brasiel, Ferreira and Vieira23 . Nonetheless, the factors involved in these changes are not known.
We are not aware of studies in animal models assessing the long-term consequences of exposure to soy or its isolated protein in lactation on adrenal medulla function and antioxidant activity, which are parameters closely related to cardiovascular protection, and which may be related to some changes previously observed in this model. The knowledge about the effects of exposure to soy and its protein at critical stages of life can be important to understand the epigenetic effects of food related to the protection and development of diseases. Thus, our objective was to evaluate the effects of consuming a soy-based diet and a diet based on isolated soy protein (SPI) during lactation on adrenal medulla function, antioxidant activity, and atherogenicity indexes in rats lactating and their offspring at weaning and adulthood.
Methods
Animals and diets
The design of the animal experiments was approved by the Ethics Committee of the Federal University of Juiz de Fora, Minas Gerais, Brazil (protocol 018/2014). The study was in compliance with local regulations (National Council for the Control of Animal Experimentation, CONCEA, Brazil).
Three-month-old Wistar rats (200–350 g) were maintained in a temperature-controlled room (22 ± 2°C) with 12:12 dark–light cycle. The animals were obtained from the Reproductive Biology Center of the Federal University of Juiz de Fora, Minas Gerais, Brazil. A day after the birth of the pups, all the litters were adjusted to six males pups per dam, to maximize lactation performance Reference Fishbeck and Rasmussen24 . In this period, six lactating rats with their offsprings were randomly assigned to each of the following groups: control (C) group received a casein-based diet (16.3% protein); soy protein isolate (SPI) group received a soy protein isolate-based diet (21.3% protein); soy (S) group received a soy-based diet (20.1% protein). The diets started at birth, defined as day 0 of lactation, and were stopped at weaning (21 days). All the rats had free access to their respective diets and water throughout the experiment. During pregnancy and after weaning until 150 days of age, all animals received the same diet, which is the standard laboratory diet for rodents (Nuvilab®, Paraná, Brazil), whose composition is 22% protein (mixture of bran soy, wheat, and corn, added with lysine and methionine), 66% carbohydrate, and 11% lipid.
The diets used during the lactation period were prepared manually in the Experimental Nutrition Laboratory of the Department of Nutrition, Federal University of Juiz de Fora, MG, Brazil, according to the American Institute of Nutrition (AIN-93G) Reference Reeves, Nielsen and Fahey25 . Commercial organic soy and soy protein isolates were processed to inactivate antinutritional factors. After, they were transformed into flour for the preparation of the soybean experimental diet, according to the methods of Soares et al. Reference Soares, Lucas and Boaventura26 and Vieira et al. Reference Vieira, Brasiel and Ferreira22 . The centesimal composition of the soy flour was analyzed according to reference methods 27 . Based on the data, the amount of protein, fat, and fiber in the S diet was adjusted. Samples of all the prepared diets were collected for chemical analysis 27 . The experimental diets were isoproteic and isoenergetic. The composition of the diets is shown in Table 1.
Table 1. Composition of diets.

Formulated based on recommendations of the AIN-93G for rodentdiets Reference Vieira, Brasiel and Ferreira22 ; aRhoster (Araçoiaba da Serra, SP, Brazil); bMundo Verde® with 36.9% protein, 26.3% carbohydrate, and 27% fat; cNutrisoy (Colombo, PR, Brazil); dMaisena, Unilever Best Foods Brasil LTDA (Mogi Guaçu, SP, Brazil); eUnião® (São Paulo, Brazil); fLiza® Cargil Agricultura LTDA (Mairinque, SP, Brazil); g Estimated quantity based on the isoflavone content of soy and SPI flours reported in the literature Reference Barbosa, Lajolo and Genovese28 .
The number of isoflavones in S and SPI diets was estimated based on the isoflavone content of soy and SPI flours reported in the literature. According to Barbosa et al. Reference Barbosa, Lajolo and Genovese28 , 100 g of soy flour has 200 mg of isoflavones, while 100 g of SPI has 124 mg of isoflavones. From the amount of added soy flour and soy protein isolate, the amount of isoflavones per 100 g of S diet and SPI diets were estimated to be 108 mg and 24.8 mg, respectively. Based on this information, maternal intake of isoflavones considering the average amount of food intake (FI) during lactation of the S and SPI animals were determined.
At 21 days, all lactating rats (6 rats/group) were euthanized, and three pups from each dam (18 pups/group) were randomly chosen and euthanized, while another three pups from each dam (total: 18 pups/group) were kept in cages until 150 days old, when they were euthanized. Offsprings from different litters per group were used to avoid litter effects (C: 6 litters; SPI: 6 litters; S: 6 litters). All the animals were euthanized with a lethal dose of Ketamine [(90 mg/Kg body weight (BW)] and Xylazine (10 mg/Kg BW). Blood samples were centrifuged (1000 g, 4ºC , 20 min). The following tissues were collected: liver, right adrenal, and left adrenal (maintained in 10% acetic acid). All tissues were stored in a freezer at −80°C until analysis.
Nutritional evaluation
FI and BW of the mothers and offsprings were monitored daily, during lactation and after weaning, and the same parameters were monitored in the offsprings every 4 days until the 150th day.
17-β-estradiol serum quantification
Blood samples from the dams at weaning were centrifuged for serum samples, which were stored at −80°C until time of assay. Serum 17-β-estradiol was determined by radioimmunoassay, using a commercial kit (ICN Pharmaceuticals, Inc, Diagnostics Division, Costa Mesa, CA) with an intra-assay coefficient of variance of 5.5% and 10 pg/ml sensitivity.
Atherogenicity indexes
Serum levels of TC, LDL-cholesterol, and HDL-cholesterol were measured in a previous study Reference Vieira, Brasiel and Ferreira22 . Atherogenic indices were calculated from TC/HDL (Index1), LDL/HDL (Index2), and (TC-HDL)/HDL ratios (Index3) Reference Choi, Suh, Jovem and Park29,Reference Yang, Shi, Hao, Li and Le30 .
Determination of hepatic triglyceride and cholesterol contents
Liver samples (50 mg) were homogenized in 1 mL of isopropanol and centrifuged (3219.8 g, 10 min, 4°C). Total triglyceride and cholesterol levels were measured by a colorimetric method using a commercial kit (Labtest, Lagoa Santa, Brazil). The data were expressed in mg/dL.
Antioxidant defense and oxidative stress biomarkers in liver
Determination of lipid peroxidation
Thiobarbituric acid reactive substances (TBARS) and metabolites were used as indicators of lipid peroxidation. They were measured following the method described by Buege and Aust Reference Buege and Aust31 . The results were expressed in nmol/mg protein.
Determination of oxidative stress index
Total glutathione content was determined by a method proposed by Griffith Reference Griffith32 . Absorbance was determined at 412 nm. The amount of GSSG was determined after the derivatization of total GSH with 2-vinyl pyridine. GSH was determined by subtracting GSSG from total glutathione. Oxidative stress index was calculated from the ratio of GSH/GSSG. The results were expressed in nmol/ml sample.
Determination of antioxidant enzyme activities
The enzymatic activity of glutathione reductase (GR) was determined according to the method proposed by Carlberg and Mannervik Reference Carlberg, Mannervik and Alton33 , based on the reduction of oxidized glutathione in the presence of a cofactor (NADPH). Glutathione peroxidase (GPx) activity was determined according to the method proposed by Paglia and Valentine Reference Paglia and Valentine34 . GPx activity was measured by the decrease in absorbance at 340 nm after 3 min. The results were expressed in U/mg protein.
Liver superoxide dismutase (SOD) activity was measured according to Marklund and Marklund Reference Marklund and Marklund35 . One unit of SOD activity was defined as the amount of enzyme that inhibited the rate of autoxidation of pyrogallol by 50%, determined at 570 nm. The results were expressed in U/mg protein.
Catalase (CAT) activity was determined by the method of Aebi Reference Aebi36 , based on the decomposition of H2O2 at 240 nm for 3 min. And 10 µL of homogenate supernatant was added to a cuvette containing 100 mM of phosphate buffer (pH 7.2), and the reaction was initiated by the addition of 10 mM of H2O2. The results were expressed in µmoL/mg protein.
Analysis of adrenal medulla function
The adrenals underwent different treatments according to protocol. The left adrenal samples were maintained in 10% acetic acid at −80ºC for the quantification of total catecholamine content. For Western blot analysis, the right adrenal glands were immediately frozen in −80ºC. For catecholamine release analysis, the medulla of different adrenal glands was removed and stimulated.
Catecholamine release assay
The experiment was performed as previously described by Martins et al. (2004) Reference Martins, Souza and Shio37 with some modifications: catecholamines medullae were incubated for 15 min in six different wells containing 200 µl of Krebs solution (5 min well−1) for basal secretion analysis. Then, the medullae were transferred to a new well and stimulated with 25 mm of caffeine (200 µl well−1) or 30 mM of potassium (200 µl well−1) or 50 mM of carbamylcholine (200 µl well−1) for 5 min.
Catecholamine quantification
The total level of catecholamines was quantified using the trihydroxyindole method Reference Kelner, Levine, Morita and Pollard38,Reference Trevenzoli, Valle and Machado39 . Adrenaline standards were used for quantification. The parameters used in the fluorometer were 420-nm excitation and 510-nm emission wavelengths.
Expression of the enzyme tyrosine hydroxylase (TH)
The expression of tyrosine hydroxylase (TH) was performed by Western blotting, following protocol already described in the literature Reference Trevenzoli, Valle and Machado39 . The bands were revealed by chemiluminescence (ECL, Amersham Biosciences) accompanied by exposure to autoradiography film. The bands were quantified by densitometry, using Scion Image software. Tubulin was used as a standard.
Statistical analysis
The calculation of sample size was performed by power analysis, using statistical software available online (http://www.3rs-reduction.co.uk/html/6__power_and_sample_size.html), considered statistical power of 0.9 and type 1 error rate Reference Charan and Kantharia40 . The data were analyzed by the statistical program GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) and expressed as mean ± S.E.M. The differences between the groups were determined by one-way ANOVA followed by Newman–Keuls posttest. The results were considered significant when p < 0.05.
Results
Mothers
The mothers fed SPI or soy diets did not present changes in FI and BW during lactation (Fig. 1a). The estimated average intake of isoflavones in the lactating rats was 41.3 ± 1.785 and 9.87 ± 2.039 for the S and SPI group, respectively. 7-β-estradiol serum concentrations in the lactating rats were not altered by SPI consumption; however, it levels decreased with soybean consumption compared to the C group (Table 2).

Fig. 1. Food intake and body weight. Maternal body weight during lactation (a); offspring food intake after weaning until 150 days old (b); body weight of pups during lactation (c) and until 150 days old (d), whose dams were fed casein control (![]() ), soy protein isolate (
), soy protein isolate (![]() ) or soy (
) or soy (![]() ) diet. Values are means for 6 dams/group, 36 pups/group during lactation, and 18 rats/group after weaning. Mean values were significantly different to those of controls: *p < 0.05 compared with a C; #p < 0.05 compared with S or SPI.
) diet. Values are means for 6 dams/group, 36 pups/group during lactation, and 18 rats/group after weaning. Mean values were significantly different to those of controls: *p < 0.05 compared with a C; #p < 0.05 compared with S or SPI.
Table 2. Maternal intake of soy or soy protein isolate and its effects on nutritional, hormonal, atherogenic, and oxidative markers at the end of lactation.

C: control; SPI: soy protein isolate; S: soy. TC: total cholesterol, LDL: LDL-cholesteol; HDL: HDL-cholesterol, TBARS: metabolites thiobarbituric acid reactive substances, GSSG: glutathione oxidized, GSH: glutathione reduced, GPx: glutathione peroxidase, GR: glutathione reductase, SOD: superoxide dismutase, CAT: catalase. The experimental data were obtained using six animals/group. Mean values were significantly different to those of controls: *p < 0.05 compared with a C; #p < 0.05 compared with SPI or S. Values in bold when p-value <0.05.
The intake of SPI and soy diet in lactation did not change atherogenic index; the activity of the enzymes GPx and GR, total glutathione content, GSH, GSSG, as well as the GSH/GSSG ratio of the mothers at the end of lactation. On the other hand, the activity of the antioxidant enzyme SOD was lower in the S mothers compared to the C and SPI groups, whereas CAT activity was higher when compared to the SPI group. TBARS was lower only in the SPI mothers compared to the C group. Also, adrenal medulla function was not altered in the lactating rats (Table 2).
Pups: nutritional evaluation
A lower FI among the S offspring compared to SPI offsprings was only observed on day 25 (p = 0.0389; Fig. 1b). BW was lower (p < 0.05) between days 7 and 11 of lactation in the S offspring compared to the C offspring (Fig. 1c, 1d).
Pups: Atherogenicity indexes and hepatic triglyceride and cholesterol
At weaning, both S and SPI offspring showed lower atherogenicity indices (1, 2, and 3) compared to C. In adulthood, the SPI offspring presented higher indices (1 and 3) compared to control. All the atherogenic indices of the S offspring were similar to those of the control group, thus having lower levels than the SPI offspring (Table 3).
Table 3. Atherogenicity indexes and hepatic triglyceride and cholesterol at 21 days (weaning) and 150 days (adult) offspring.
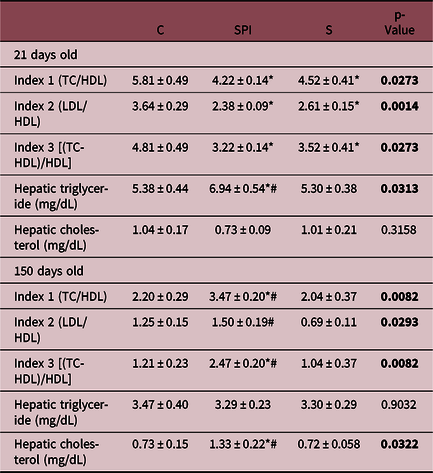
C: control; SPI: soy protein isolate; S: soy; TC: total cholesterol, LDL: LDL-cholesterol; HDL: HDL-cholesterol. The experimental data were obtained using six animals/group. Mean values were significantly different to those of controls: *p < 0.05 compared with a C; #p < 0.05 compared with SPI or S. Values in bold when p-value <0.05.
At weaning, SPI pups showed increased hepatic triglyceride content compared to the C offspring, while S pups presented a decrease in this parameter compared to SPI, at this phase, there was no change in hepatic cholesterol content. At 150 days, the SPI offspring presented high hepatic cholesterol compared to the C group, and the S groups did not differ from the control. Hepatic TG was not changed at this age (Table 3).
Pups: Antioxidant defense and oxidative stress biomarkers in liver
When the activity of the enzymes responsible for antioxidant defense was evaluated in the S offspring at 21 days of age (Fig. 2a, 2b, 2c, 2d), we observed an increase in the levels of SOD (vs. C: +10.87%, vs. SPI: +9.18%, p = 0.039), GPx (vs. C: +137.69%, p = 0.0125), and GR (vs. C: +33.01%, p = 0.0231). The SPI offspring presented a higher activity of GR enzyme compared to the C group (+53.68%, p = 0.0231). These results indicate that the consumption of S or SPI diet during lactation generally improves antioxidant activity of offspring for weaning. At 150 days old, the S offspring showed high levels of CAT (vs. C: +16.3%, vs. SPI: +17.55%, p = 0.0061) which can be cardioprotective. Regarding oxidative stress biomarkers at 21 days old (Fig. 2e, 2f, 2g), the S offspring had high levels of TBARS (vs. C: +127.27%, vs. SPI: +143.9%, p = 0.0151), indicating higher oxidative stress; however, the SPI offspring presented increased oxidative stress index GSH/GSSG (vs. C: +39.44%, vs. S: +47.22%, p = 0.0053), indicating lower oxidative stress. Offsprings from the S and SPI groups had low levels of GSSG in relation to the control group (−22.09% and −33.97%; p = 0.0017, respectively). At 150 days old, the SPI offspring had lower levels of TBARS compared to control (−39.82%, p = 0.0103).

Fig. 2. Antioxidant enzyme activities and oxidative stress biomarkers in the liver. Activities of antioxidant hepatic enzymes superoxide dismutase (a), catalase (b), glutathione peroxidase (c), glutathione reductase (d), levels of thiobarbituric acid reactive substances (e), total glutathione content, reduced glutathione (GSH), oxidized glutathione (GSSG), and stress index (GSH/GSSG) of the offspring at 21 (f) and 150 days old (g) whose mothers were fed casein control (C – black bar), soy protein isolate (SPI – gray bar), or soy (S – white bar) diet. The experimental data were obtained using six animals/group. Mean values were significantly different to those of controls: *p < 0.05 compared with a C; #p < 0.05 compared with SPI or S.
Pups: Analysis of adrenal medulla function
Catecholamines in the adrenal glands (Fig. 3a, 3b) were not altered in the experimental groups at 21 days. At 150 days of age, the S offspring showed no change in absolute and relative adrenal catecholamine content; however, the SPI group presented lower absolute (vs. C: −15.82%, vs. S: −14.68%, p = 0.0436) and relative (vs. C: −17.96%, vs. S:-29,45%, p = 0.0081) catecholamines in the adrenal glands. To assess whether this reduction is related to lower synthesis and higher catecholamine secretion, we evaluated the expression of TH (enzyme responsible for catecholamine biosynthesis) and in vitro secretion by the adrenal gland. The treatment during lactation did not affect the weight of the adrenal glands for both periods of 21 days (C: 10.03 ± 0.77, SPI: 11.73 ± 0.54, S: 11.74 ± 1.33) and 150 days (C: 27.92 ± 0.60, SPI: 27.68 ± 0.78, S: 26.08 ± 1.93). There was no change in TH expression among the offsprings in adulthood (Fig. 3c, 3d), indicating no alteration of catecholamine synthesis by the adrenal glands. In vitro secretion of catecholamines in the adrenal medulla showed that maternal intake of soy or SPI during lactation did not alter basal secretion of this hormone in the adult offsprings (Fig. 3e). As regards response to caffeine, group SPI showed increased secretion of carbamylcholine (vs. C: +44.45%, vs. S: +79.18%, p = 0.0070), but the secretion of the same among the groups remained unchanged when stimulated with carbamylcholine or potassium (Fig. 3f). Thus, the lower catecholamine content in the adrenal gland of the adult SPI offspring may be related to its higher secretion, indicating an increased cardiovascular risk.
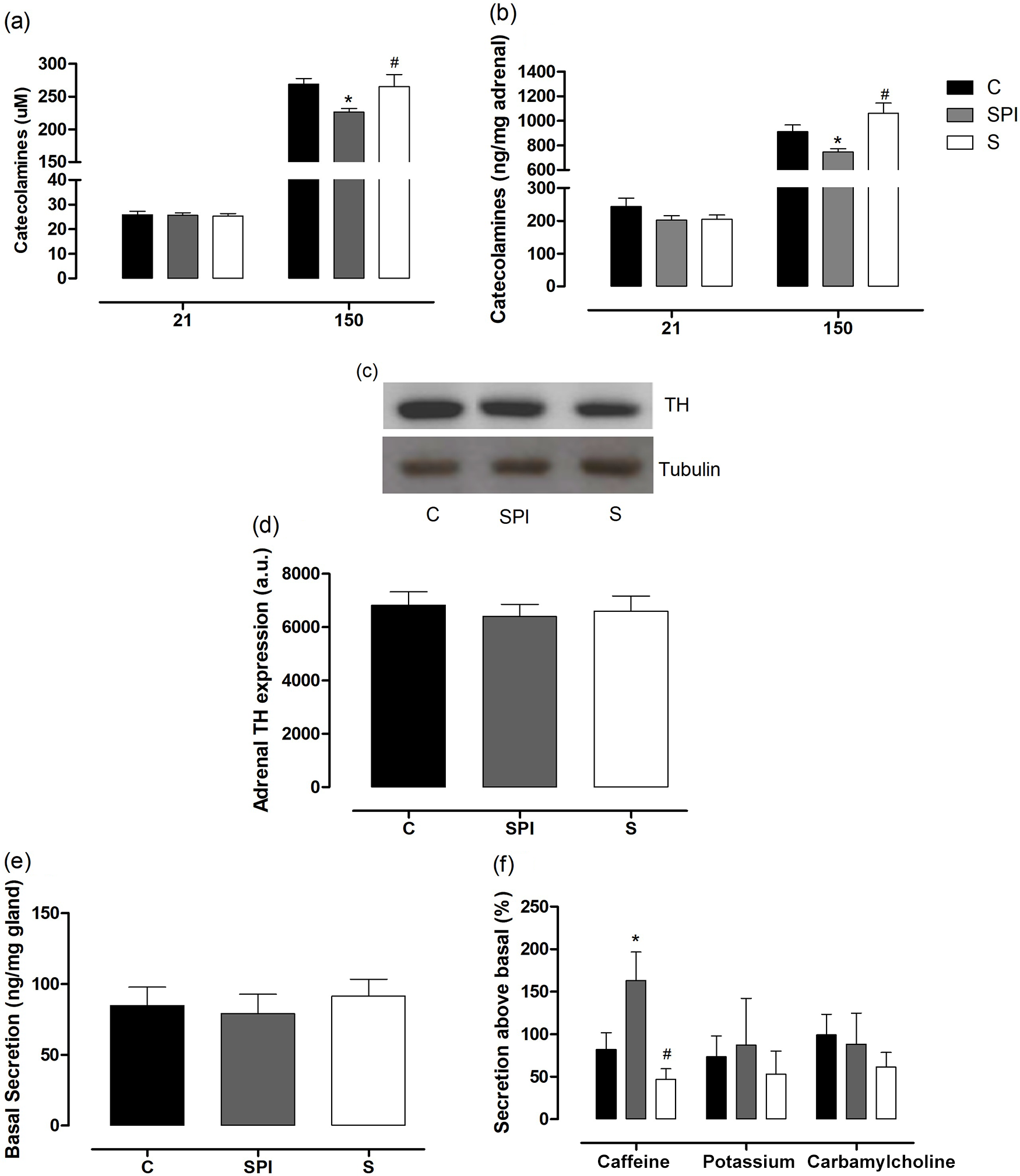
Fig. 3. Adrenal medulla function of the offspring whose mothers were fed casein control (C – black bar), soy protein isolate (SPI – gray bar), or soy (S – white bar) diet. Catecholamine entire content (a) and amount relative to gland weight (b) in adrenal glands of the offspring at 21 and 150 days old; the experimental data were obtained using eight animals/group. Enzyme tyrosine hydroxylase expression per representative blots (c) and optical density (d) evaluated by Western blotting in the adrenal medullae of the offspring at 150 days; the experimental data were obtained using six animals/group. Catecholamine release assay: catecholamines basal (e) and secretion by 25 mM of caffeine, 30 mM of potassium or 50 mM of carbamylcholine (f) of the offspring at 150 days old; the experimental data were obtained using 10 animals/group. Mean values were significantly different from those of controls: *p < 0.05 compared with a C; #p < 0.05 compared with SPI or S.
PS: The graphics program used to create the figure was GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Discussion
The results from this study demonstrate that the consumption of soy or its protein isolate during lactation promotes different endocrine-metabolic alterations and antioxidant response in offsprings. In general, negative effects were observed with the consumption of soy protein isolate. This reinforces the idea that foods have different effects when consumed as a nutrient or bioactive component alone, especially during a critical period in life Reference Vieira, Brasiel and Ferreira22 . It is known that exposure to different factors in critical phases of life, such as lactation, can promote metabolic changes in the progeny, programming it for health or disease Reference Rosenfeld12-Reference Desai, Jellyman, Han and Lane19 .
In this study, it was observed that the consumption of soybeans and its protein did not alter maternal nutritional status nor that of the offsprings at weaning and adulthood. Only a punctual reduction of body mass among the S offspring was observed from the 7th to 11th day of lactation. Thus, reinforcing the data of Vieira et al. Reference Vieira, Brasiel and Ferreira22 , this change is possibly related to the more significant transfer of isoflavones and/or estradiol via breast milk to the offspring in this period, as both offsprings reduce adipogenesis and the accumulation of body fat Reference Yang, Lia and Xionga41,Reference Palacios-González, Zarain-Herzberg and Flores-Galiza42 . Indeed, the estimated amount of isoflavones in diet S is more significant than SPI, contributing to a higher intake of these flavonoids by the S dams (41.3 ± 2.785 mg/day) compared to the SPI dams (9.87 ± 2.039 mg/day), since both groups presented similar FI.
Regarding atherogenic indices, no changes in these indices were observed among the S or SPI dams at the end of lactation. On the other hand, both offsprings presented significantly lower atherogenic indexes at weaning. This protection against cardiovascular diseases observed at weaning was not reflected equally in adulthood for the SPI offspring, since higher atherogenicity indices 1 (TC/HDL) and 3 [(TC-HDL)/HDL] were observed in relation to the offsprings whose mothers consumed casein-based diets or soy-based diets. So far, these results indicate that maternal SPI consumption in lactation seems to promote a higher metabolic risk profile in adulthood. On the other hand, there are studies that demonstrate a beneficial effect of soy protein on the lipid profile and prevention of cardiovascular diseases in adult animals receiving soy protein Reference Simmen, Mercado and Zavacki6,Reference Torres, Torre-Villalvazo and Tovar43,Reference Tovar, Murguía and Cruz44 . However, we cannot compare our results with these studies, as we offer soy protein to the mother and evaluate the long-term effect on the offspring.
The hepatic levels of cholesterol and TG are in accordance with the trend seen for the atherogenic indexes, and lipid profile changes demonstrated in a recent group study Reference Vieira, Brasiel and Ferreira22 . It was observed that the SPI offspring presented an increase in hepatic TG at 21 days and 150 days, while the S offspring maintained the levels of these parameters similar to those of the control. It is not clear which mechanisms are related to these changes, but there is evidence that epigenetic changes are associated with the regulation of progeny gene expression. In fact, Han et al. Reference Han, Won and Kwon45 demonstrated that maternal consumption of SPI before mating, during pregnancy, and lactation increased the expression of genes involved in lipid metabolism and liver development, such as peroxisome proliferator-activated receptors (PPARα) and mTOR modulation pathway. These authors associate these effects with the amino acid composition of soy protein alone, since the results were less expressive when isoflavone genistein was consumed. In addition to amino acid composition, bioactive peptides originating from soy protein isolate may exert effects on lipid metabolism and antioxidant activity Reference Rizzello, Tagluazucchi and Badini46 .
The parameters of oxidative stress and antioxidant response were evaluated in liver tissue given that soybean has antioxidant properties which may confer cardioprotective effects Reference Yan, Zhang, Li, Jiao and Dong8-Reference Yoon and Parque10,Reference Erba, Casiraghi, Martinez-Conesa, Goi and Massaccesi47,Reference Javanbakht, Sadria and Djalali48 . Studies show that estradiol increases the levels of antioxidant enzymes in the body Reference Mann, Bonacasa, Ishii and Siow5,Reference Lima, Belló-Klein and Flues49 . The reduction of SOD levels in S mothers may be directly related to the decrease of this hormone, although this does not apply to CAT since its levels were increased. It can not be ruled out that the higher isoflavones content in the soybean diet counterbalance the effects of estradiol.
At weaning, the S offspring showed a higher TBARS content, associated with higher activity of the antioxidant enzymes SOD, GPx and GR, and reduction of GSSG. These data allow us to infer that the S offspring responds to higher lipid peroxidation, increasing the activity of the antioxidant defense enzymes. A recent study by our group showed that animals whose mothers consumed soybean in lactation showed increased TG at weaning Reference Vieira, Brasiel and Ferreira22 , and as already described in the literature lipid peroxidation has a strong positive correlation with hypertriglyceridemia Reference Giacomini, Hahn and Siqueira50 , thus it is plausible that hypertriglyceridemia contributes to the increase in lipid peroxidation in S offspring. The level of TBARS did not change in the SPI offspring. Also, an increase and decrease in GR and GSSG, respectively, contributes to a higher GSH/GSSG ratio, indicating lower oxidative stress in the SPI offsprings (Supplementary Figure S1). The improvement in the antioxidant profile at weaning has already been observed in other programming models Reference Yoon, Won and Kwon51 . An analysis of glutathione cycle showed an increase in GSH/GSSG ratio due to the higher activity of GR and reduction of oxidized glutathione, which in turn reduces GSSG concentration. Although the concentration of GSSG is low in the S offspring associated with increase in GR, it also presents a high GPx activity, maintaining the GSH/GSSG ratio constant (Supplementary Figure S2).
Maternal consumption of S and SPI in lactation did not promote consistent changes in the antioxidant response of adult offspring. We observed only increased CAT activity in the S offspring, which may indicate a response to increased oxidative stress. The adult SPI offspring presented a reduction in TBARS, without altering antioxidant enzymes and glutathione concentrations, suggesting the presence of other mechanisms of nonenzymatic-free radical neutralization. The physicochemical properties, composition of amino acid, bioactive peptides, and the kinetics of digestion of SPI may be factors associated with the efficiency of nonenzymatic mechanisms, affecting neutralization of free radicals, lipid peroxide inhibition, and metal-ion chelating Reference Smaranayaka and Li-Chan52 . Additionally, soy protein has a higher concentration of amino acid histidine compared to casein, contributing to its antioxidant activity Reference Singh, Vij and Hati53 .
Catecholamines are also related to the pathophysiology of cardiovascular diseases, but, until the present moment, the effects of S and SPI on catecholamine concentrations had not yet been evaluated in in vivo studies. It was observed that the consumption of S or SPI during lactation did not alter the content of catecholamines in the adrenal medulla of lactating rats and their offsprings at weaning. In vitro studies have shown that the synthesis and secretion of catecholamines by the adrenal gland are affected by isoflavone concentration Reference Liu, Yanagihara and Toyohira54,Reference Yanagihara, Toyohira and Shinohara55 . So, it is possible that the amount of bioavailable isoflavone in S and SPI dams was not able to alter the synthesis of catecholamines in the adrenal gland. Another critical issue is that in vitro studies evaluate the effect of daidzein in isolation Reference Liu, Yanagihara and Toyohira54 . In the present study, we used soybean and SPI, which contains all forms of isoflavones, guaranteeing a higher concentration of genistein than daidzein.
In adulthood, we observed that the SPI offspring presented lower absolute and relative catecholamine content compared to the control and S groups. To test if this alteration was related to lower synthesis or higher secretion of catecholamines by the adrenal, we evaluated the expression of TH and in vitro secretion of catecholamines by the adrenal medulla. The expression of TH, enzyme-limiting catecholamine in biosynthesis, was not altered in the adult offsprings. On the other hand, we evidenced a higher secretion of catecholamines in SPI offspring when stimulated by caffeine. Thus, the lower catecholamine content in SPI group appears not to be related to increased biosynthesis but to its higher secretion.
This result demonstrates that SPI affects the intracellular pools of calcium Reference Garcia, García-De-Diego, Gandía, Borges and García-Sancho56 , mainly resulting in the programming of catecholamine metabolism. It is known that estradiol increases the levels of intracellular calcium by mobilization of the endoplasmic reticulum Reference Revankar, Cimino, Sklar, Arterburn and Prossnitz57,Reference Nilsson, Olde and Leeb-Lundberg58 . Therefore, a higher estradiol content may lead to an increase in cytosolic Ca+2 and thus, increased secretion of catecholamines. The SPI dams did not present alteration in serum estradiol and, in addition, the consumption of isoflavones was 1/4 lower than the S dams. Thus, the SPI diet seems to be more estrogenic when compared to casein, since with similar levels of estradiol, the presence of isoflavones in this diet exerts estrogenic activity, intensifying calcium mobilization of the endoplasmic reticulum, and programming increased catecholamine secretion. However, the S mothers presented estradiol reduction and received high isoflavones during lactation, which may have interfered with the action of this hormone by competing for similar binding sites, thus exerting an antagonistic effect. The interaction between estradiol concentration and high isoflavone content in the S diet may have programmed the adult offspring for normalization of the cytosolic Ca+2, not interfering with the secretion of catecholamines.
Given the above, it can be concluded that maternal consumption of soy protein isolate during lactation programmed the adult offspring the worsening of atherogenic indices, associated with higher liver cholesterol and decreased catecholamine in the adrenal medulla. The data suggest that the consumption of SPI during lactation should be done with caution. On the other hand, in spite of the soy-based diet being rich in lipids, its consumption during lactation did not program consistent deleterious effects in adult offspring on these parameters in relation to casein consumption.
As limitation of the study, estimation of isoflavone content of the experimental diets using data from the literature and information from product manufacturers. In relation to atherogenic indexes, despite having confirmation validated only with a study in humans, several studies have been using it as a parameter related to the alteration of the metabolic response in rodents Reference Hegazy, Abdel-Azeem, Zeidan and Ibrahim59-Reference Halima, Sonia and Sarra61 . It is also important to mention the difficulty of the lactational programming models, in totally isolating what interferes more in the programming, whether the mother’s milk that suffered the intervention or the consumption of the maternal diet by the offspring in the last days of lactation. However, other lactational programming models have shown that the initial lactation period (mainly the first 12 postnatal days) seems to be essential for programming Reference Passos, Ramos, Mouço and Moura62 .
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174421000180
Acknowledgments
The authors would like to thank Silvioney A. Silva, Franciane Toledo, Juliana M. M. Lopes and Maria Lucia Pedrosa for their support during analyzes and Raúl M.G. Garcia (in memorial) for the support, teachings, and dedication to this research.
Financial support
This research was funded by the National Council for Scientific and Technological Development (CNPq-nº476867/2011-8), Coordination for the Improvement of Higher Education Personnel (CAPES), and Pro-rectory of Research and Postgraduate studies of the Federal University of Juiz de Fora (ProPesq-UFJF).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (Wistar rats) and have been approved by the institutional committee [Ethics Committee of the Federal University of Juiz de Fora at Minas Gerais, Brazil (protocol 018/2014)].









