Introduction
Malnutrition comes in many forms, including undernutrition (not enough protein or carbohydrate or food to eat), micronutrient deficiencies (not enough vitamins and minerals) and overweight (linked to an unbalanced or unhealthy diet). Approximately 462 million adults worldwide are underweight, and around 45% of deaths among children under 5 years of age are linked to undernutrition.Reference Black, Allen and Bhutta 1 , 2 Indeed, adverse exposures in early life, particularly relating to nutrition, are linked to susceptibility to chronic non-communicable diseases in adulthood.Reference Manolio, Collins and Cox 3 , Reference Tain, Huang and Hsu 4 Insight from animal models indicates that maternal malnutrition negatively impacts the intrauterine environment, altering fetal programming and development.
The notion that the intrauterine environment influences the development of the fetus is not new. Past studies of intrauterine undernutrition identified changes in the structure of key fetal organs such as the heart, kidney and brain.Reference Barker 5 – Reference Habib, Zhang and Baum 9 What is new, however, is the concept that early life exposure to undernutrition affects adult disease susceptibility. Anthropometry (i.e. weight) at birth was identified two decades ago as predictive of adult coronary heart disease, type 2 diabetes and metabolic syndrome; this finding prompted the ‘Barker hypothesis’ that a suboptimal intrauterine environment induces compensatory responses in the fetus that may permanently affect the adult phenotype.Reference Barker 5 , Reference Barker 8 , Reference Nijland, Ford and Nathanielsz 10 For example, a cross-sectional study has shown that low birth weight is associated with decreased overall adult health status as well as reduced reproductive capability.Reference Boeri, Ventimiglia and Capogrosso 11 Although birth weight is a poor proxy for nutritional events during gestation, the Barker hypothesis has been confirmed in many independent cohorts across the developed and developing world.Reference Langley-Evans and Sculley 12 – Reference Kalhan and Wilson-Costello 14
Similarly, the ‘thrifty phenotype’ hypothesis proposes that poor fetal nutrition imposes mechanisms of nutritional economy upon the growing individual. Follow-up studies of babies born during the 1944 Dutch famine implicate a role for nutrition in programming disease risk.Reference Roseboom, Van Der Meulen and Ravelli 15 In conditions of severe intrauterine deprivation, the developing fetus may lose structural units that program physiological function and determine risk of disease in adult life;Reference McMillen and Robinson 16 this phenomenon is termed nutritional programming. Programming occurs because developmental plasticity allows the fetus to adapt its tissue structure in response to environmental changes. In this sense, the same genotype can produce different phenotypic outcomes depending upon inputs during development.Reference Vangen, Nordhagen and Lie 17 The conditions of early life, when added to adult lifestyle – for example, diet, physical activity, smoking habits and alcohol consumption – are the main determinants of our long-term health and well-being.
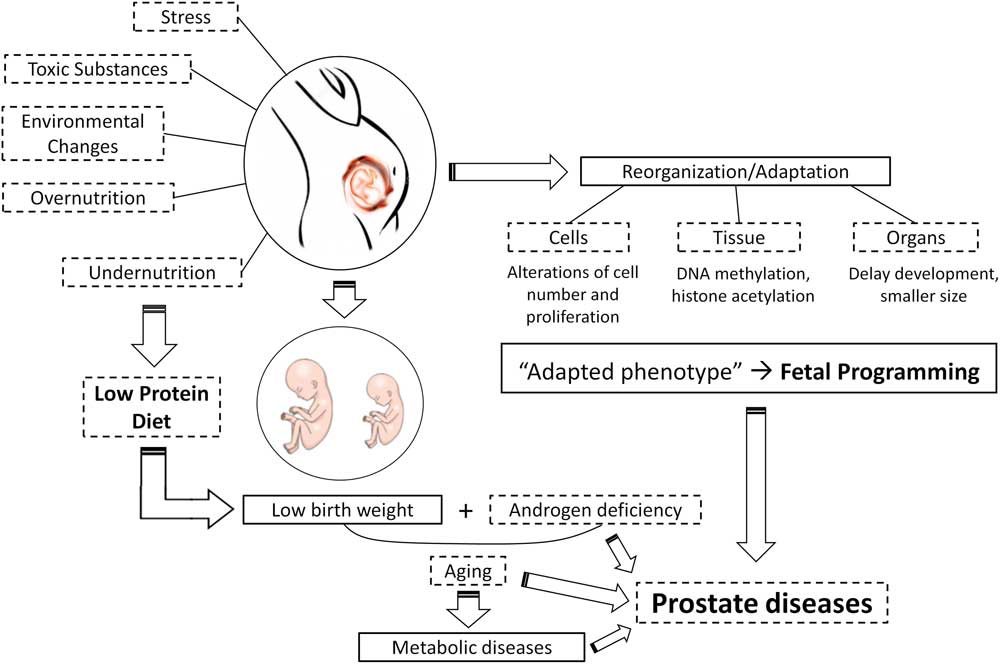
Together, these hypotheses have become known as the fetal origins or the developmental origins of health and disease (DOHaD), and produced a new branch of scientific pursuit (Fig. 1). DOHaD establishes that events occurring in critical periods of development are memorized, leading to the formation of an organism with an ‘adapted phenotype.’ Environmental factors in early life, such as nutrition, stress, endocrine disruption and pollution are some of the insults that trigger developmental programming.Reference Langley-Evans and McMullen 18
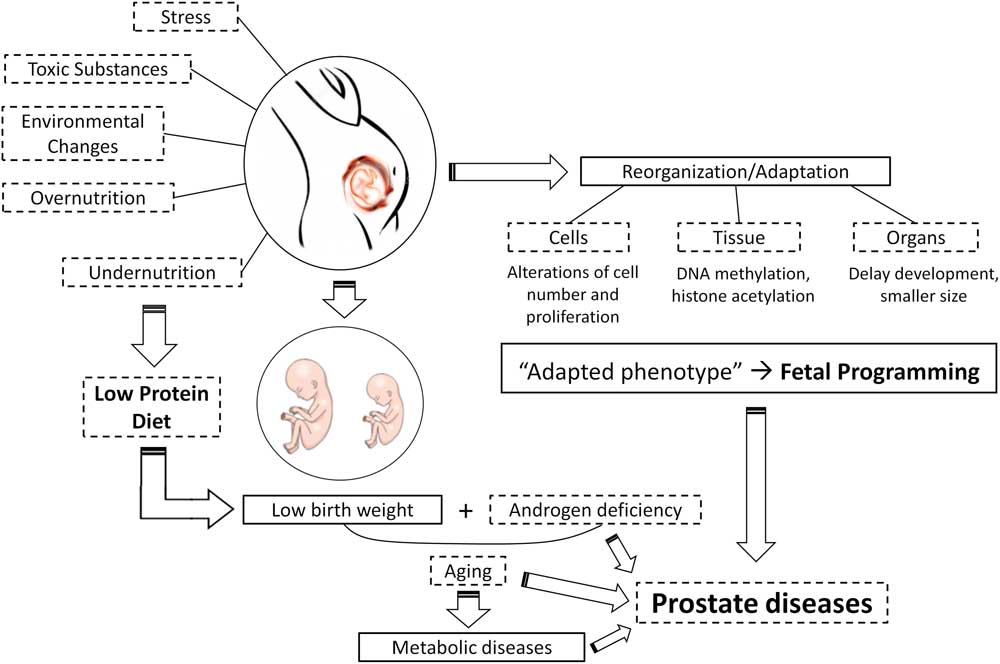
Fig. 1 A schematic example of developmental origins of health and disease hypothesis illustrating prostate reprogramming as a function of maternal protein malnutrition.
Nutrient requirements for an organism depend on developmental state, reproductive activity and age.Reference Langley-Evans 19 In general, during early life, malnutrition could be a result of intrauterine undernutrition, inadequate breastfeeding, the late introduction of complementary foods or the introduction of inadequate complementary foods.Reference Barker, Gluckman and Godfrey 20 Nutritional programming resulting from poor nutrition status during pregnancy can lead to irreversible consequences in tissue formation and differentiation. These consequences stem from the physiological adaptations that occur to ensure nutrient supply to the most vital organs at the expense of others. This programming in response to variations in the quality or quantity of nutrients consumed during pregnancy is geared toward increasing the fetal survival rate.Reference Fowden and Forhead 21 However, the processes that underlie the disordered organ development are poorly defined.
Animal models are used to provide some mechanistic insight for the link between maternal diet and adult disease. In animal studies, the major challenge is to capture life course exposures and identify ‘windows of susceptibility,’ or those time points during which nutritional exposures have the greatest impact on development and disease. Approaches to studying nutritional programming in animal models range from limiting the total food intake to more specific manipulations such as overfeeding or restriction of macro- and micronutrients.Reference Langley-Evans 19 Most animal studies investigating early nutritional programming have centered on the use of rodent models; however, other animal models are also important, including porcine, ovine and primate models.Reference Vuguin 22
Such animal studies have indicated that the mechanisms underlying the effects of malnutrition on development are related to alterations in placental function, including control of maternal-fetal endocrine exchanges, modified transcription factor expression and the epigenetic regulation of gene expression, via non-coding RNAs, DNA methylation or histone methylation.Reference Vaiserman 23 Such modifications triggered during developmentally sensitive stages of early life play a central role in regulating long-term health and disease outcomes. One of the most extensively studied models, the maternal low-protein rat model, established by Snoeck et al. in the early 1990s,Reference Jiang, Sun and Xiong 24 – Reference Jahan-Mihan, Rodriguez, Christie, Sadeghi and Zerbe 27 has demonstrated that protein content and sources in maternal diets are capable of producing shifts in the fetal environment, driving the tissue remodeling response and altering future disease risk.Reference Jahan-Mihan, Rodriguez, Christie, Sadeghi and Zerbe 27 , Reference Dai, Wu, Hang, Zhu and Wu 28 Evidence also exists that adverse outcomes extend beyond first generation to induce transgenerational effects.Reference Chadio and Kotsampasi 29 In this sense, finding strategies to improve maternal nutritional status will benefit the present as well as the future generation.
Given the high prevalence of nutritional deficiencies globally and the growing epidemic of chronic disease, it is increasingly important to understand the impacts of fetal malnutrition on the life course. As our group has been studying prostate development and aging under the umbrella of different models of fetal programming, the main purpose of our study is to discuss the role of maternal low intake of protein in influencing organ development and aging. This review will use prostate reprogramming as a function of maternal nutrition as a specific example. Such insights into the complex molecular context on developmental trajectories of adult-onset disease, specifically focusing on prostate biology, may help future studies on this field.
Proposed mechanisms of fetal programming by low-protein diet
Studies of nutritional programming using animal models have been ongoing since the early 1990s. The most used and best characterized model employs maternal protein restriction during rat pregnancy and/or lactation periods.Reference Langley-Evans and Sculley 12 The protein requirement for gestation and lactation as a percentage of the diet is similar to that for growth of weanling rats – about 12% – when highly digestible protein of a balanced amino acid pattern is used.Reference Benevenga, Calvert and Eckhert 30 It is important to understand the difference in metabolic and physiologic responses to isocaloric protein malnutrition and protein energy malnutrition. Although protein malnutrition is more related to cultural constraints, protein energy malnutrition is related to poverty and economic adversity. The latter results in responses that are similar to those of starvation. In contrast, isocaloric protein malnutrition leads to suppression of proteolysis and maintenance of lean body mass and may lead to gain in fat mass.Reference Cahill 31
Studies using rodent models have shown that maternal malnutrition by isocaloric low-protein diet during pregnancy or during early postnatal life can lead to metabolic and physiological changes in adult life even when the animals have free access to a normal diet after weaning.Reference Zambrano, Rodríguez-González and Guzmán 32 , Reference Zambrano, Bautista and Deás 33 Studies investigating the impact of fetal protein restriction upon longevity have also demonstrated that rats undernourished in utero have a shorter lifespan.Reference Aihie Sayer, Dunn, Langley-Evans and Cooper 34
Nutrient deprivation can act as a strong programming stimulus and promotes several structural changes in fetal tissue. Among them are altered cell number, an imbalanced distribution of cell types or hormone receptor numbers in an organ, and altered blood supply.Reference Jahan-Mihan, Rodriguez, Christie, Sadeghi and Zerbe 27 , Reference Brameld, Buttery, Dawson and Harper 35 – Reference Colombelli, Santos and Camargo 38 Dietary protein provides an important source of amino acids that are essential precursors in the synthesis of hormones, neurotransmitters and nitric oxide. In addition, they act as important regulators in the metabolic pathways related to development and reproduction.Reference Hou, Yin and Wu 39
Physiological responses to dietary proteins are also determined by the proteins’ characteristics arising from their amino acid composition, bioactive peptides (BAPs) and digestion kinetics. Thus, nutritional adequacy of amino acids may not be the only characteristic of the maternal diet to impact the offspring.Reference Jahan-Mihan, Szeto, Luhovyy, Huot and Anderson 40 The timing of fetal programming is also important. For example, the source of protein (casein- v. soy protein-based diets) in the maternal diet exerts an effect on body weight and glucose metabolism that is magnified when maternal diet is extended from gestation alone to gestation and lactation in male offspring of rats. This finding highlights an important consideration: study results should be compared cautiously because of wide variation in study design (e.g. protein restriction v. protein-free diets, duration of dietary protocol).
A lack of protein in the maternal diet causes characteristic changes in one-carbon metabolism. Certain amino acids, such as methionine, serine and glycine, not only contribute to protein mass, but also play a unique role in the regulation of cellular metabolism and proliferation and may impact fetal growth. Methionine, an essential or indispensable amino acid and a component of all proteins, is the immediate source of the methyl (one carbon) groups required for the methylation of nucleic acids, proteins, biogenic amines and phospholipids.Reference Kalhan 41 Even low-protein diets with similar protein content may affect programming differently because lower protein intake in humans and other animals has been shown to cause hyper-homocysteinemia and perturbations in one-carbon metabolism. For example, the low-protein Southampton diet results in higher systolic blood pressure in offspring,Reference Langley-Evans 42 whereas the Hope Farm diet has no effect on blood pressure.Reference Ozanne, Wang, Coleman and Smith 43 Adding glycine, which reduces plasma homocysteine, to the Southampton diet normalizes blood pressure.Reference Kalhan, Uppal and Moorman 44 This suggests that the methionine load is a contributor to the phenotype of the Southampton diet.Reference Jackson, Dunn, Marchand and Langley-Evans 45 Hyper-homocysteinemia can be related to hypomethylation of DNA that consequently alters organogenesis and embryonic vasculogenesis by influencing its major events, increasing metabolic syndrome risk.Reference Wu, Imhoff-Kunsch and Girard 25 , Reference Steegers-Theunissen and Steegers 46 Further, supplementation of a low-protein gestational diet with taurine (2.5%) restores normal insulin secretion. Taurine participates in homocysteine metabolism and reduces the demand for cysteine.Reference Cherif, Reusens, Ahn, Hoet and Remacle 47 In addition, the essential nutrient choline is required for the maintenance of structural integrity and signaling function of cell membranes, for neurotransmission, for transport of lipids, and as a source of methyl groups for methylation and epigenetic programming.Reference Sanders and Zeisel 48
BAPs, or protein fragments that can influence health, have been detected in the plasma of pregnant and lactating women. For example, casokinins originating from all major subunits of casein are much higher than those from soy protein.Reference Pupovac and Anderson 49 BAPs abundant in casein, called β-casomorphins, can affect food intake regulation, gastrointestinal motility and plasma insulin concentration.Reference Teschemacher 50 These peptides directly influence numerous biological processes evoking behavioral, gastrointestinal, hormonal, immunological, neurological and nutritional responses. However, it remains unknown if BAPs cross the placenta or have a role in the development of regulatory systems.Reference Clare and Swaisgood 51 , Reference Nielsen, Beverly, Qu and Dallas 52 Moreover, the rate of protein digestion and the resulting hormonal responses in dams and peak amino acid concentrations in fetuses may also influence the development of regulatory systems. In this sense, proteins can be classified as either ‘fast’ or ‘slow.’Reference Bos, Metges and Gaudichon 53 Casein is considered a slow protein; whey and soy are fast proteins. Plasma concentrations of serine, tyrosine, valine, isoleucine, branched-chain amino acids, lysine and total amino acids are higher, and arginine and tryptophan are lower after a casein meal compared with after a soy protein meal in humans. Hormonal responses to these proteins are also markedly different.Reference Tang, Moore, Kujbida, Tarnopolsky and Phillips 54 For example, a higher concentration of plasma insulin is noted after whey protein consumption as compared with casein at 60 min after ingestion. However, to our knowledge, there are no studies examining the role of protein digestion kinetics on fetal programming.
Another potential mediator of the effects of maternal undernutrition on fetal programming is the placenta. The relationship between placental function and fetal nutrition is complex because amino acids can be synthesized de novo within the placenta.Reference Cleal and Lewis 55 Moreover, the ability of the placenta to provide the fetus with substrates depends upon the quality of placentation. Placentation differs between species, with rodents and humans having a discoid, hemochorial placenta, whereas in sheep the placentas are cotyledonary synepitheliochorial, which may represent an evolutionary development and can limit the transport of some molecules from the mother to fetus.Reference Carter and Mess 56 , Reference Poston 57 In addition, the placenta is a major source of endocrine signals that play a role in maintaining the pregnancy, is involved in modulating fetal growth rate and organ maturation.Reference Langley-Evans 19 ,58 Despite these putative effects, the role of the placenta in programming has not been investigated in depth.
Fetal programming and developmental plasticity
In mammals, intrauterine development is a critical period of plasticity for most organs and systems. Fetal development occurs through sequential events including morulation, gastrulation and organogenesis. Each step is dependent upon meticulous orchestration of cell differentiation, migration, proliferation and apoptosis. These processes can be perturbed by inadequate environmental stimuli during sensitive periods, producing effects that persist across the life course and lead to pathological conditions in adulthood.Reference Koopman 59
Maternal nutritional and metabolic statuses are critical in determining not only reproduction, but also long-term health and viability of offspring. During the periconceptual and preimplantation periods, nutrient, oxygen and hormone levels affect development of the oocyte and blastocyst, with consequences for the distribution of cells between the trophoblast and inner cell mass. If developmental plasticity leads to a change or adaptation that is permanent, it is considered a ‘programming’ change and is associated with persistent effects in structure and/or function. The problem starts when individuals developmentally adapted to one environment are exposed to another.Reference Bateson, Barker and Clutton-Brock 60 Accordingly, mice exposed to a low- (6%), medium- (18%) or high- (36%) protein diet in utero or through lactation have lower survival rates at 2 years if weaned onto a diet that differed from that of their mother.Reference Bateson, Barker and Clutton-Brock 60
Poor intrauterine nutrition results in the growth of vital organs, specifically the brain, at the expense of other organs.Reference Cohen, Baerts and Bel 61 Such adaptations may increase the chance of fetal survival by means of ‘brain sparing,’ but result in difficulty coping with nutritional abundance as an adult.Reference Roseboom, Van Der Meulen and Ravelli 15 Further, some children return to their genetic trajectory through compensatory growth, recovering size after a period of growth delay or arrest through catch-up growth. This phenomenon often results in overcompensation, whereby the offspring exceeds normal weight and often has excessive fat deposition.Reference Jee, Baron, Phillip and Bhutta 62 This rapid and excessive growth has been associated with the development of adult obesity, insulin resistance, metabolic syndrome and type 2 diabetes.Reference Ong, Ahmed, Emmett, Preece and Dunger 63 It happens especially with people living in countries that are undergoing swift economic and nutritional transitions, exposing individuals to conditions that promote weight gain.Reference Norris, Osmond and Gigante 64
Maternal protein malnutrition (MPM) during developmental programming can also alter the balance of reactive oxygen species. Proteins such as glutathione and albumin provide amino acids needed for antioxidant synthesis to combat reactive oxygen species.Reference Luo, Wang and Feng 65 MPM can lead directly to a pro-oxidative state by creating protein deficiencies. Increasing oxidative stress leads to macromolecular damage, including to DNA, specifically telomeres, that can contribute to permanent effects on the regulation of cellular aging.Reference Tarry-Adkins and Ozanne 66 In addition, pancreatic β cells are sensitive to reactive oxygen species, and oxidative stress can blunt insulin secretion.Reference Luo, Fraser and Julien 67 In this sense, early and ongoing exposures to oxidative insults could result in eventual manifestations of metabolic syndrome and related disorders.Reference Lenzen 68
MPM can promote changes in both the pregnant rat and the offspring. During organogenesis, environmental insults may cause discrete structural defects that permanently reduce the functional capacity of an organ. The effects of MPM on offspring organ structure alterations include: less kidney microvascular development,Reference Dunford, Sinclair and Kwong 69 fewer nephrons in the kidney,Reference Habib, Zhang and Baum 70 fewer pancreatic β cells,Reference Dahri, Snoeck, Reusens-Billen, Remacle and Hoet 71 fewer brain capillaries,Reference Bennis-Taleb, Remacle, Hoet and Reusens 72 fewer neurons that control appetite in the hypothalamus,Reference Plagemann, Harder and Rake 73 delay in lung developmentReference Farid, Mahmoud, Salem, Abdel-Alrahman and Hafez 74 and altered cellular ratio of liver cell types.Reference Burns, Desai and Cohen 75 Further, MPM affects offspring organ function, including by inducing impaired glucose tolerance,Reference Simmons, Gounis, Bangalore and Ogata 76 peripheral insulin resistance,Reference Simmons, Flozak and Ogata 77 coronary diseaseReference Barker, Osmond, Forsén, Kajantie and Eriksson 78 and hypertension.Reference Gluckman and Hanson 79
In addition, there is evidence that fetal growth and development depend primarily on nutrient and oxygen supply. As nutrient and oxygen availability invariably affect the endocrine environment, the role of hormones as programming signals has also been examined in humans and experimental animals.Reference Fowden and Forhead 21 Hormones regulate normal growth and development in utero, and their concentrations and bioactivity change in response to many of the environmental challenges known to cause intrauterine programming.Reference Fowden and Forhead 21 MPM can modify hormone production as well as the capacity of cells to respond to hormone signals.Reference Langley-Evans and McMullen 18 , Reference Bertram and Hanson 80 , Reference Qasem, Yablonski and Li 81 As some of these hormones cross the placenta, the fetal endocrine response to adverse conditions reflects the activity of both maternal and fetal endocrine glands, and depends on the type, duration, severity and gestational age at onset of the insult. Subsequently, fetal programming caused by MPM can impair the development of several organs, including the reproductive system. For example, MPM increases maternal estradiol, corticosterone and testosterone levels at gestational day 19 in albino Wistar rats, which is a critical period of plasticity for male reproductive system development, particularly the prostate.Reference Zambrano, Rodríguez-González and Guzmán 32 Taken together, these nutritional programming effects may be either direct or mediated by endocrine changes in the mother to alter organ development and function.
Fetal programming and the male reproductive system
Development was historically believed to be gene-led, a matter of activating and switching off the expression of genes. However, the observation that the developing mammal has the ability to respond to environmental insults changes the way in which we think about the developmental process. Adaptive responses triggered by any kind of insult may promote profound and irreversible effects upon the physiology of the fetus. As fetal organs grow at different rates, the timing of the insult is important in determining the tissue specificity of the programmed effects. In altricial species that are immature at birth (e.g. rodents and rabbits), the period of developmental plasticity extends after birth; in contrast, precocial species (e.g. human, sheep, pig) are more physiologically mature at birth.Reference Vuguin 22 , Reference Ozanne and Hales 82 If the insult occurs at the time of organogenesis, the changes may be severe and lead to a permanent developmental deficit. In some systems, a specific trigger or a second challenge may be required postnatally to unmask the intrauterine programming. In several physiological systems, sex-linked differences in intrauterine programming do not appear until puberty, when the onset of gonadal steroidogenesis uncovers physiological abnormalities in peripheral tissues or in the hypothalamic–pituitary–gonadal axis itself. In the majority of physiological systems studied, the adverse consequences of intrauterine compromise become more evident with increasing age, as compensatory adaptations in other tissues and organ systems fail.Reference McMillen and Robinson 16 Yet, while the literature on protein restriction and disease risk is extensive, it is largely based on rodent models; few studies have assessed the specific role of protein restriction in human risk of disease. The effect of this programming in adulthood and during aging is unclear, in part because of the possibility of reversion or the compensation of these adverse effects observed earlier in life.Reference Langley-Evans and McMullen 18
In the last few decades, approximately one in six couples suffer from involuntary subfertility; generally defined as being unable to conceive after 1 year of unprotected intercourseReference Rowe, Comhaire, Hargreave and Mellows 83 and male factors contribute to ~33–50% of these cases.Reference Chow and Cheung 84 Given the difficulties in retrospectively assessing in utero nutrition in humans, birth weight is commonly used as a proxy for nutritional conditions during fetal life. In this sense, emerging data from clinical studies have associated low birth weight with male subfertility.Reference Francois, de Zegher, Spiessens, d’Hooghe and Vanderschueren 85 Other endocrine pathways, such as the hypothalamic–pituitary–gonadal axis, have also been implicated. For example, men born small for gestational age have higher gonadotropin with lower testosterone and inhibin B levels, suggesting poor testicular responsiveness potentially influencing fertility.Reference Cicognani, Alessandroni and Pasini 86 Recently, a cross-sectional study observed reduced sperm motility and higher rates of abnormal sperm morphology, including asthenozoospermia and teratozoospermia in adult infertile individuals born with low birth weight.Reference Boeri, Ventimiglia and Capogrosso 11 Other findings were higher follicle stimulating hormone (FSH) values, lower mean testicular volume, as well as lower testosterone levels in adulthood.Reference Vanbillemont, Lapauw and Bogaert 87 Although there are controversies regarding the relationship between the developmental environment and postnatal reproductive function in men, this evidence suggests that early life events may contribute to male infertility.
MPM can directly or indirectly affect hormone release, hormone receptor expression/distribution, cellular function and tissue organization, growth, differentiation and maturation.Reference Zambrano, Rodríguez-González and Guzmán 32 , Reference Colombelli, Santos and Camargo 38 , Reference Qasem, Yablonski and Li 81 , Reference Léonhardt, Lesage and Croix 88 – Reference Ramos, Babinski, Costa and Sampaio 91 Further, hormone level alterations in utero appear to have long-term consequences for reproductive function.Reference Rhind, Rae and Brooks 92 , Reference Fowden, Giussani and Forhead 93 These changes may be isolated or widespread events, depending on the nature and timing of the programming stimulus.Reference McMillen and Robinson 16 , Reference Fowden, Giussani and Forhead 93
Most of what we know regarding fetal programming and reproductive outcomes comes from experimental studies. A few reports in sheep and rats indicate that male sexual development and the normal ontogeny of gonadal development and function can be disrupted by maternal malnutrition.Reference Rae, Kyle and Miller 94 – Reference Gardner, Ozanne and Sinclair 96 MPM also delays the time of puberty in ratsReference Van Weissenbruch, Engelbregt, Veening and Delemarre-van de Waal 97 , Reference Noriega, Howdeshell and Furr 98 and can lead to reduced sperm count (at postnatal day or PND-270) and reduced capacity to impregnate female rats.Reference Zambrano, Rodríguez-González and Guzmán 32 The authors attribute those results to lower number of Sertoli cells in the testicles, which leads to a disorganization of the seminiferous tubules. Alteration in germ cell proliferation and maturation is also related to increased oxidative stress and loss of antioxidant defense in the testis.Reference Rodríguez-González, Vigueras-Villaseñor and Millán 99 , Reference Asadi, Bahmani, Kheradmand and Rafieian-Kopaei 100 These findings highlight the negative impact of nutritional programming on the male reproductive system.
Another parameter evaluated is the ano-genital distance (AGD). AGD is a biomarker for proper prenatal androgen exposure, especially testosterone synthesized by fetal testes.Reference Graham and Gandelman 101 , Reference Swan, Main and Liu 102 Androgens, acting through the androgen receptor (AR), regulate male sexual differentiation during development, sperm production beginning from puberty and maintenance of prostate homeostasis.Reference Zambrano, Rodríguez-González and Guzmán 32 , Reference Cunha 103 , Reference Verze, Cai and Lorenzetti 104 MPM alters AGD in male offspringReference Zambrano, Rodríguez-González and Guzmán 32 suggesting an impairment of hypothalamic–hypophyseal–gonadal axis and the development and homeostasis of organs that are under androgenic control.Reference Cunha 103 A study from our group found that MPM decreases AGD in male offspring at birth and reduces serum testosterone levels at PND-30.Reference Rinaldi, Justulin and Lacorte 105 Other studies showed MPM-associated reductions in the serum concentration of luteinizing hormone (LH) and FSH; primary hormones involved in the functioning of the male reproductive system. Those alterations are accompanied by the reduction of testis, epididymis and prostate weights.Reference Zambrano, Rodríguez-González and Guzmán 32 , Reference Zambrano, Bautista and Deás 33 , Reference Guzmán, Cabrera and Cárdenas 89 , Reference Ramos, Babinski, Costa and Sampaio 91 , Reference Fernandez-Twinn, Ozanne and Ekizoglou 106 – Reference Fernandez-Twinn, Ekizoglou, Gusterson, Luan and Ozanne 108 MPM also induces an alteration in serum estradiolReference Teixeira, Silandre and de Souza Santos 109 , Reference Ibrahim, Bayomy and Elbakry 110 reduction of AR expression in the testis,Reference Rodríguez-González, Vigueras-Villaseñor and Millán 111 reduction of leptin receptor expression in the testis and prostate,Reference Gombar and Ramos 112 reduction of aquaporin-9 expression in the epididymis,Reference Arrighi, Aralla, Genovese, Picabea and Bielli 113 and smaller acini in the dorsolateral prostate (DLP)Reference Ramos, Babinski, Costa and Sampaio 91 , Reference Ibrahim, Bayomy and Elbakry 110 and ventral prostate (VP).Reference Rinaldi, Justulin and Lacorte 105 These results suggest an impaired intrauterine androgenic signaling.Reference Page, Sottas and Hardy 114 However, the mechanisms involved in this process are still under investigation.
Fetal programming and prostate development
The seminal plasma contains a biochemically complex mixture of glandular secretions that is transferred to the female sexual tract as part of the ejaculate.Reference Untergasser, Madersbacher and Berger 115 Ejaculation, liquefaction and clotting of seminal fluid create a synchronized cascade that enables sperm to perform all the biological processes necessary to reach and fertilize the egg. The seminal fluid also contains nutrients as well as Zn2+, citrate and kallikreins, which are essential for sperm motility and nutrition; these factors are mainly secreted by the prostate gland.Reference Verze, Cai and Lorenzetti 104 In this sense, the prostate morphophysiology has received increasing scientific interest, once seminal plasma composition is determinant of male fertility/infertility and reproductive success. Some studies investigated the effects of MPM on prostate development and aging (Table 1). Independently of the diet protocol, the common findings were alterations of serum androgen levels and glandular weight. Thus, early insults during prostate development may permanently alter morphology and/or function. Moreover, these early life exposures appear to influence the onset of late life diseases, such as prostatitis, benign prostatic hyperplasia (BPH) and prostate cancer.Reference Rinaldi, Justulin and Lacorte 105 , Reference Risbridger, Almahbobi and Taylor 116 – Reference Cowin, Foster and Pedersen 118 These diseases are also potentially linked with impaired fertility status at different ages.Reference Verze, Cai and Lorenzetti 104 As the mechanisms underlying these changes are not completely understood, we attempt to clarify some of these changes from the perspective of prostate development.
Table 1 Effects of maternal protein malnutrition on rat prostate lobe development
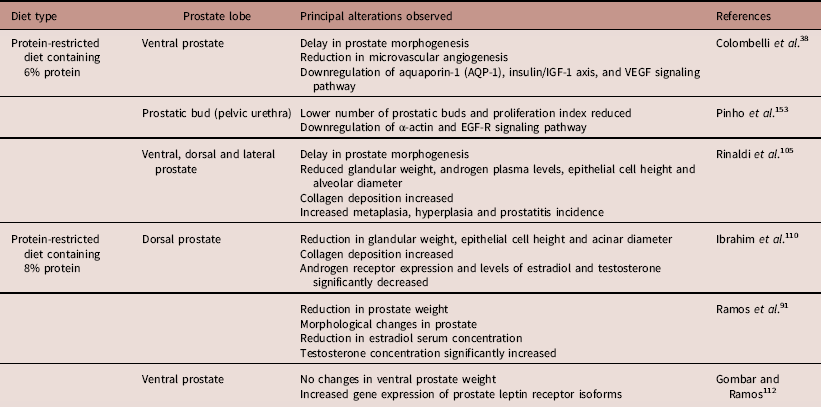
IGF, insulin-like growth factor; VEGF, vascular epithelial growth factor; EGF-R, epidermal growth factor receptor.
The prostate gland develops embryologically from the endodermal urogenital sinus (UGS) under the influence of androgens produced by fetal Leydig cells.Reference Prins, Cooke and Birch 119 The developmental process is continuous, and can be categorized into five distinct stages involving determination, initiation or budding, branching morphogenesis, differentiation and pubertal maturation.Reference Prins and Putz 120 Briefly, the process starts when epithelial stem/progenitor cells form outgrowths or buds that penetrate into the surrounding UGS mesenchyme in the ventral, dorsal and lateral directions caudal to the bladder.Reference Cunha 103 At birth, the rodent prostate lobes primarily consist of unbranched, solid, elongating buds or ducts that sprout lengthening and morphogenesis start with the formation of ducts.Reference Prins, Cooke and Birch 119 Branching morphogenesis begins when the elongating UGS epithelial buds contact the mesenchymal pads that are peripheral to the periurethral smooth muscle.Reference Timms, Mohs and Didio 121 The branching pattern is complex and lobe-specific, and starts in the VP at PND3-5 in the rat and in the DLP 2 days later. The epithelial stem/progenitor cells differentiate in basal and luminal cells. They have differential expression of cytokeratins and AR, accompanied by the onset of lumen formation that reaches the distal ends of the ducts at PND-12.Reference Prins and Birch 122 Epithelial and mesenchymal cell differentiation is temporally coordinated with branching morphogenesis. At the same time, prostatic mesenchymal cells condense around the elongating buds or branching ducts, a periductal layer of smooth muscle cells forms while the interduct cells differentiate into fibroblasts.Reference Hayward, Baskin and Haughney 123 Lumenization of the solid epithelial cords begins in the proximal ducts and spreads to the distal tips,Reference Marker, Donjacour, Dahiya and Cunha 124 occurring concomitantly with functional differentiation of luminal epithelial cells.Reference Prins and Birch 122 Prostatic secretory proteins are detectable from PND-20 in rodents, and become more abundant as testosterone serum levels increase.Reference Lukacs, Goldstein, Lawson, Cheng and Witte 125 , Reference Oliveira, Dzinic and Bonfil 126 Morphogenesis of the rodent prostate is complete by PND-20, and the final maturation process is reached shortly thereafter with the onset of puberty.Reference Marker, Donjacour, Dahiya and Cunha 124
The events of branching morphogenesis are common to all branching organs including kidney, lung and salivary glands. The branching process is regulated by a genetic code specific for particular cell types and organs, but environmental conditions also determine the dynamics of the development, growth, symmetry and function of the branched organ. The pattern of morphogenesis in developing organs, including the prostate, is laid down during the fetal/neonatal period and is a major determinant of the size, function and disease of the gland in adulthood.Reference Risbridger, Almahbobi and Taylor 116 , Reference Marker, Donjacour, Dahiya and Cunha 124 , Reference Sugimura, Cunha and Donjacour 127
During development, organogenesis and tissue differentiation occur through a continuous series of tightly regulated and precisely timed molecular, biochemical and cellular events. The temporal programming of rat prostate development is between fetal days 18.5 and 19.5, but can extend after birth.Reference Corbier, Edwards and Roffi 128 , Reference Welsh, Saunders and Fisken 129 This period coincides with the onset of testosterone production by the fetal testes. Thus, if the synthesis or action of testosterone is insufficient, the masculinization process is impaired, resulting in hypospadias, cryptorchidism, underdeveloped prostate and reduction of AGD.Reference Welsh, Saunders and Fisken 129 Studies also described mutations of the AR and alteration in the expression and activity of the 5-α-reductase enzyme.Reference Imperato-McGinley and Zhu 130 However, if androgen action continues to occur after the masculinization process, when both LH and its receptors are expressed, it may also affect the size and/or structure of the testes,Reference Welsh, Saunders and Fisken 129 leading to a permanent reduction in testosterone synthesis.
Prostate organogenesis is a complex process that is primarily mediated by the presence of androgens and subsequent mesenchyme–epithelial interactions. The precise mechanism of how androgens mediate prostate epithelial induction and budding is unknown. At present, there are two major hypotheses: the andromedin model and the smooth muscle model.Reference Toivanen and Shen 131 Fibroblast growth factor (FGF) 7 and FGF10 were among the first molecules to be suggested as candidate andromedins.Reference Lu, Luo, Kan and McKeehan 132 , Reference Yan, Fukabori, Nikolaropoulos, Wang and McKeehan 133 Although FGF7 can stimulate epithelial budding and ductal branching in the absence of dihyrotestosterone (DHT) in neonatal prostate organ culture,Reference Sugimura, Cunha and Donjacour 127 FGF10 is unable to perform this function. The smooth muscle hypothesis proposes that androgen signaling has indirect effects on epithelial growth by regulating the differentiation of smooth muscle. During organogenesis, the two processes are coordinated by reciprocal epithelial–stromal signaling.Reference Timms 134 Mechanisms of epithelial specification involves the winged-helix transcription factor FOXA1, the homeodomain transcription factor NKX3.1, the homeobox gene HOXB13 and the sex determining transcription factor SOX9.Reference Huang, Pu, Birch and Prins 135 – Reference Dutta, Le Magnen and Mitrofanova 138 Following the formation of prostatic buds, the epithelium undergoes extensive proximal-distal outgrowth and branching morphogenesis. The activities of Sonic hedgehog (SHH) and bone morphogenic protein (BMP)4/7 pathways appear to coordinate epithelial–mesenchymal interactions during prostate branching morphogenesis.Reference Prins and Putz 120 , Reference Yu and Bushman 139 , Reference Pu, Huang and Prins 140 Notch, Activin signaling pathways, glial cell-derived neurotrophic factor and Ephrin signaling are involved as well.Reference Toivanen and Shen 131 However, the precise mechanisms involved and their interactions with other relevant signaling pathways are still largely unresolved.
The interplay between genes and the early environment shapes development and leads to both normal or abnormal structure and function of prostate. For example, SHH play crucial roles in cell survival, proliferation, cell-fate determination and differentiation.Reference McMahon, Ingham and Tabin 141 SHH is expressed differently across prostate lobes or over time and is responsive to a diverse set of intrauterine perturbations. SHH-signaling disruption at later stages of VP development (in vitro) resulted in reduced organ size and proliferation of ductal tip epithelia,Reference Freestone, Marker and Grace 142 decreased FGF10 transcript and increased BMP4 expression in the adjacent mesenchyme.Reference Pu, Huang and Prins 140 In this sense, SHH and FGF are strong candidates for mediating the effects of MPM, as well as WNTs and BMPs. Elucidating how these signaling pathways and transcriptional regulators are integrated to mediate prostate specification/differentiation and whether MPM influences them will be relevant for understanding their roles in prostate development, prostatic hypertrophy and prostate cancer.
Androgens have been described as essential for prostate gland development and maintenance throughout life. In addition to androgens, other hormones regulate prostate growth/function and influence growth/progression of prostate cancer, including estrogens,Reference Prins and Korach 143 retinoids,Reference Schenk, Riboli and Chatterjee 144 prolactin,Reference Dagvadorj, Collins and Jomain 145 growth hormone (GH), and insulin-like growth factor (IGF)-1.Reference Wang, Prins and Coschigano 146 Undernutrition can alter maternal as well as fetal concentrations of many hormones, including estrogen, GH, IGFs, insulin, glucocorticoids, catecholamines, leptin, thyroid hormones and placental hormones such as the eicosanoids, sex steroids and placental lactogen.Reference Fowden and Forhead 21 , Reference Zambrano, Rodríguez-González and Guzmán 32 , Reference Fowden and Forhead 58 , Reference Powolny, Wang, Carlton, Hoot and Clinton 147 These endocrine changes can affect fetal growth and development either directly or indirectly by altering the delivery, uptake and metabolic fate of nutrients in the fetoplacental tissues.Reference Fowden 148 However, these hormones and the mechanisms underlying their roles in developmental plasticity, particularly in the prostate gland, remain to be identified.
There is also some evidence that MPM interferes in cell proliferation/differentiation processesReference Brameld, Buttery, Dawson and Harper 35 and triggers the inappropriate activation of certain genes by epigenetic mechanisms.Reference Barker, Osmond, Forsén, Kajantie and Eriksson 78 , Reference Zheng, Xiao, Zhang, Wang, Yu and Xu 149 These changes may compromise the physiology, function and longevity of different organs.Reference Langley-Evans and McMullen 18 , Reference Walker and Ho 150 A few studies demonstrated that MPM during gestation or gestation/lactation delayed VP,Reference Colombelli, Santos and Camargo 38 , Reference Rinaldi, Justulin and Lacorte 105 dorsal prostateReference Ramos, Babinski, Costa and Sampaio 91 , Reference Rinaldi, Justulin and Lacorte 105 and lateral prostate development and maturationReference Rinaldi, Justulin and Lacorte 105 based on the epithelial proliferation rate and glandular morphology.
The transition of undifferentiated epithelial cords of the embryonic prostate into fully differentiated basal and luminal cells in the adult prostate has been an active area of investigation. More recently, lineage-tracing studies using specific Cre drivers have suggested that basal progenitors give rise to the mature prostate epithelium during organogenesis.Reference Wuidart, Ousset and Rulands 151 For example, lineage-tracing studies of basal cells using ΔNp63cre mice have shown that p63-expressing basal cells in the UGS can give rise to all three prostate epithelial cell typesReference Pignon, Grisanzio and Geng 152 . However, there is significant co-expression of basal and luminal markers during early organogenesis,Reference Prins and Birch 122 and basally located cells continue to express luminal markers. Therefore, it is unclear whether the progenitors of luminal cells are exclusively basal at this stage.
Considering the cellular complexity of the prostate epithelium, our group initiated investigations on the impact of MPM on binomial cell proliferation/differentiation, as well as the maintenance of cellular phenotypes. We found an imbalance between epithelial basal and luminal phenotypes in VP at PND-10 and 21.Reference Colombelli, Santos and Camargo 38 This phenomenon may be linked to the lower levels of testosterone and DHT in male offspring from protein-restricted dams.Reference Rinaldi, Justulin and Lacorte 105 , Reference Pinho, Ribeiro and Rinaldi 153 MPM also compromised angiogenesis during prostate development, leading to fewer blood vessels.Reference Colombelli, Santos and Camargo 38 Future studies are aimed at elucidating in more detail the mechanisms involved in this developmental programming of prostate.
Another study from our group described that MPM increased the incidence and aggressiveness of prostatitis.Reference Rinaldi, Justulin and Lacorte 105 Among all prostatic diseases, prostatitis has the greatest potential to affect fertility.Reference Verze, Cai and Lorenzetti 104 Prostatic inflammation is linked with fertility alteration, a pertinent finding for men in their prime reproductive years.Reference Wagenlehner, Pilatz and Linn 154 Furthermore, recent data support the role of prostatic inflammation as a predisposing factor for development of BPH and prostate cancer.Reference Verze, Cai and Lorenzetti 104 , Reference Ficarra, Rossanese and Zazzara 155 Taken together, these data reinforce the importance of a better understanding of prostate fetal programming and the characterization of the mechanisms involved in this process. Such knowledge could provide much-needed direction for strategies to avoid or treat these diseases.
Fetal programming and prostate aging
In the last decade, a number of animal models have been established to study developmental programming. Most of the long-term health outcomes in offspring exposed to severe nutritional deprivation in early life included cardiovascular disease and metabolic syndromes such as type 2 diabetics, obesity, insulin resistance, dyslipidemia and hypertension in the adult life, especially in aging.Reference Petry, Dorling, Pawlak, Ozanne and Hales 156 – Reference Wang, Wang and Li 160 However, few studies have evaluated the effects of MPM on the prostate during aging. Aging alone is directly linked to a decrease in sex hormone levels, including testosterone production, reduced testis weight and impaired testis function, thus leading to loss of reproductive potential.Reference Veldhuis 161 In humans above the age of 50, with each successive decade (until age 79) the prevalence of hypogonadism increases; indeed, 55% of individuals in the 70–79 age group have hypogonadism, as compared with 24% in the 50–59 age group.Reference Dhindsa, Prabhakar and Sethi 162 Interestingly, low testosterone levels are linked with insulin resistance implicated in hyperglycemia, hypertension, dyslipidemia and increased risk of vascular disease.Reference Moretti, Lanzolla, Moretti, Gnessi and Carmina 163 – Reference Schianca, Fra and Brustia 165 Low testosterone also mediates an increase of serum markers of inflammation.Reference Burney, Hayes and Smiechowska 166 , Reference Wickramatilake, Mohideen and Pathirana 167 This is an important consideration as MPM leads to increased inflammation in a systemic way, starting in the post-breastfeeding period until adulthood.Reference Zheng, Xiao, Zhang, Wang, Yu and Xu 149 , Reference Reis, Feres and Ignacio-Souza 168 , Reference Tarry-Adkins, Fernandez-Twinn and Hargreaves 169
An imbalance between the sex hormones testosterone and estradiol leads to increased incidence in important age-related diseases like Alzheimer’s disease,Reference Barron and Pike 170 cardiovascular diseases,Reference Magnani, Moser and Murabito 171 sarcopeniaReference Sipilä, Narici and Kjaer 172 and prostate cancer.Reference Gann, Hennekens, Ma, Longcope and Stampfer 173 MPM promotes alterations in serum androgenReference Noriega, Howdeshell and Furr 98 and estradiol levels.Reference Zambrano, Rodríguez-González and Guzmán 32 Androgens can cause an increase in oxidative stress and alterations in intracellular glutathione levels and the activity of other detoxification enzymes required for the maintenance of the cellular prooxidant–antioxidant balance such as gamma-glutamyl transpeptidase.Reference Udensi and Tchounwou 174 Chronic increases in oxidative stress over time are known to induce somatic mutations and neoplastic transformation that contributes to prostate cancer initiation, promotion and progression.Reference Khandrika, Kumar, Koul, Maroni and Koul 175 Even though testosterone is the predominant hormone, estradiol can be synthesized from testosterone in the prostate stromal cells which in turn can trigger expression of pro-inflammatory cytokines within the prostate. This highlights the fact that estrogens play important roles in normal, healthy adult males.Reference Nelles, Hu and Prins 176
Systemic inflammation is related to both MPM and aging. A low-protein diet during pregnancy has been identified as a risk factor for prostate diseases as it induces prostate gland inflammation in adulthood.Reference Rinaldi, Justulin and Lacorte 105 Chronic inflammation can occur in part through oxidative stress, and in turn can mediate most chronic diseases including cancer. The development of prostate cancer may be triggered by signaling of reactive oxygen species. Increased reactive oxygen species occur either through an increase in reactive oxygen species production or from a loss of antioxidant defense mechanisms. The imbalance results in significant damage to cell structures.Reference Khandrika, Kumar, Koul, Maroni and Koul 175 More studies are needed to explore and understand better the mechanisms involved in such cases.
The pathogenesis of prostate cancer is unclear, although the hypothesis that male infertility may be a harbinger of certain types of malignancy is gaining clinical acceptance.Reference Walsh, Schembri and Turek 177 – Reference Hanson, Eisenberg and Hotaling 179 This connection is likely multifactorial, with a combination of hormonal, genetic, in utero and environmental factors.Reference Skakkebaek, Rajpert-De and Buck 180 Possible mechanistic links between male infertility and testicular/prostate cancer includes fetal reprogramming by high estrogen levels in utero and hormonal disruptions during embryologic development, leading to later problems related to steroidogenesis and spermatogenesis. With abnormal gonadal function, the prostate may receive aberrant signals during key phases of development, which could result in an elevated risk of malignancy.Reference Hanson, Eisenberg and Hotaling 179 , Reference Skakkebaek, Rajpert-De and Buck 180 Maternal nutrition is known to affect fetal growth and birth weight, which interferes in fertility rates and prostate cancer risk.Reference Boeri, Ventimiglia and Capogrosso 11 , Reference Hanson, Eisenberg and Hotaling 179 However, mechanisms linking prostate cancer to male infertility remain largely hypothetical, and, given the somewhat conflicting nature of current data, identifying a causal relationship between these two disease processes remains a challenge for future studies.
Final considerations
The role of maternal low-protein diets in the development of phenotypes in offspring has been studied extensively. Progress in this field has benefited from integrated analyses combining knowledge gained from studies of human cohorts, animal models and cell systems. Moreover, strong evidence has shown that improvements in healthy aging require better nutrition of girls and young women. Today in the Western world, many fetuses are malnourished because their mothers are chronically malnourished.Reference Barker, Osmond, Thornburg, Kajantie and Eriksson 181 Maternal undernutrition has been described as one of the most neglected aspects of nutrition in public health globally.Reference Bhutta and Haider 182 Ensuring a healthy nutritional status and lifestyle before and during pregnancy is one of the best ways to help support the healthy growth and development of the unborn child. Protecting maternal nutrition and health will not only prevent chronic disease, but will also produce new generations with better health and well-being through their lives.
In addition, attention must be given to the effect of maternal obesity on offspring health. To our knowledge, no study has examined the effect of quantity and source of protein consumed during pregnancy in obese mothers on both mothers’ health and their children. Moreover, cohort studies covering the whole life course, focusing on critical windows of exposure and the time course of exposure to disease (birth cohorts, adolescent cohorts and young adult cohorts), should be considered. It is particularly important because there is consistent evidence that overweight is associated with increased risks of several types of cancer.
Studies presented in this review provide evidence that a mother’s diet during pregnancy can exert major effects on the short- and long-term health of their children. It is important to remember that diet is shaped by many factors such as traditions, knowledge about diet, food availability, food prices, cultural acceptance and health conditions. It is also critically relevant to consider the fact that any change in protein content or source of maternal diet will influence embryonic/fetal development in multiple ways. In this sense, adequate nutritional status is crucial for both prostate morphogenesis during early life as well as homeostasis of the gland throughout the lifespan.
Acknowledgments
This review represents part of the PhD thesis developed by J.C.R. to the Institute of Biosciences, Sao Paulo State University (UNESP), Botucatu/Brazil. This review was previously edited by Fresh Eyes Editing service. The authors declare that they are entirely responsible for the scientific content of the paper.
Financial Support
This study was supported by São Paulo Research Foundation (FAPESP: grant number 2009/50204-6; 2013/09649-0).
Conflicts of Interest
None.