Introduction
Maternal obesity [obesity prior to or during pregnancy, body mass index (BMI) ⩾30], gestational diabetes mellitus (GDM) and hypertensive disorders during pregnancy (HDP) are linked with a number of distinct adverse birth outcomes.Reference Catalano, McIntyre and Cruickshank 1 , Reference Sibai, Dekker and Kupferminc 2 In addition, animal and human studiesReference Davis, Lazdam and Lewandowski 3 – Reference Drake and Reynolds 5 have demonstrated the increased risk of obesity, cardiovascular disease and type 2 diabetes in offspring born to women with these conditions. Recent evidence has also implicated maternal obesity as a risk factor for impaired childhood cognitive development.Reference Alvarez-Bueno, Cavero-Redondo, Lucas-de la Cruz, Notario-Pacheco and Martinez-Vizcaino 6 However, the evidence is scarce for the association between maternal obesity and childhood physical development outcomes.Reference Adane, Mishra and Tooth 7 , Reference Yeung, Sundaram, Ghassabian, Xie and Buck Louis 8
Moreover, women might have different patterns of weight over their reproductive course of life and hence subsequent disease risks to women and to their children may not only be influenced by a discrete prenatal weight, but also by late adolescent and adult weight trajectories.Reference Thompson, Ananth, Jaddoe, Miller and Williams 9 Limited evidence has shown a link between early life as well as adulthood women’s weight trajectories and the risk of having a macrosomic babyReference Strutz, Richardson and Hussey 10 and obesity in offspring during childhood.Reference Li, Law, Lo Conte and Power 11 To date, however, whether pre-pregnancy weight trajectories are associated with childhood physical and cognitive development remains unknown.
A few studies, mainly in infants, have evaluated the association between maternal GDM and HDP and childhood development and of the studies that are available findings have been inconsistent, mainly because of methodological variations.Reference Adane, Mishra and Tooth 12 , Reference Tuovinen, Eriksson, Kajantie and Raikkonen 13 For instance, as compared to children of women without GDM, children born to women with GDM have been shown to have poorer,Reference Dionne, Boivin, Seguin, Perusse and Tremblay 14 , Reference Nomura, Marks and Grossman 15 betterReference Veena, Krishnaveni and Srinivasan 16 or equivalentReference Ornoy, Wolf, Ratzon, Greenbaum and Dulitzky 17 cognitive and language skills. Intrauterine exposure to pre-eclampsia has been also shown to have a negative impact on the motor development of adolescents,Reference Grace, Bulsara, Pennell and Hands 18 while in infants (⩽2 years) mixed,Reference Ghassabian, Sundaram, Wylie, Bell, Bello and Yeung 19 nullReference Schlapbach, Ersch, Adams, Bernet, Bucher and Latal 20 or contradictoryReference Silveira, Procianoy, Koch, Benjamin and Schlindwein 21 findings have been observed. Because most of these studies were small studies, which did not adjust for potential confounders such as pre-pregnancy obesity, authors of recent systematic reviews on this topicReference Adane, Mishra and Tooth 12 , Reference Tuovinen, Eriksson, Kajantie and Raikkonen 13 have called for further research.
In general, the increase in the prevalence of maternal obesity, GDM and HDPReference Poston, Caleyachetty and Cnattingius 22 – Reference Zhu and Zhang 24 means an increase in the number of children exposed to these maternal conditions during the intrauterine environment. As outlined above, however, there is limited evidence evaluating the independent effect of both pregnancy complications and maternal pre-pregnancy weight characteristics on the child outcomes in the first few years of life. We therefore aimed to examine the associations between maternal pre-pregnancy BMI trajectories, diabetes and HDP and offspring’s childhood physical and cognitive development.
Methods
Study design and participants
Data were from the Australian Longitudinal Study on Women’s Health (ALSWH) 1973–78 cohort and Mothers and their Children’s Health (MatCH) study. Full details of the ALSWH are available elsewhereReference Dobson, Hockey and Brown 25 but briefly, in 1996, 14,247 women born in 1973–1978 (aged 18–23 years, Survey 1) were randomly selected from the national health insurance database and surveyed about every 3 years until 2015 (aged 37–42 years, Survey 7).
MatCH is a sub-study of the ALSWH 1973–78 cohort in which 8929 women (63% of the original 1973–78 cohort) were invited to complete a survey about their children (up to three youngest children per woman). The 5318 women from the 1973–78 cohort who were not invited to participate in MatCH were those who had died, withdrawn from ALSWH, asked not to be contacted about sub-studies or reported infertility. During the MatCH study, conducted in 2016/2017, 3063 of the 8929 invited women provided a range of data about their children (n=5822). Although these 3063 women (and their 5822 children) were the total possible sample for the current study, two smaller sub-samples were used due to eligibility restrictions for the principal outcome measures. Sub-sample 1 consisted of 771 children (and their mothers) who were of an eligible age for the Ages and Stages Questionnaire (ASQ), that is, between 1 and 66 months of age. Sub-sample 2 were 708 children (and their mothers) who were eligible for having data from the Australian Early Development Census (AEDC).Reference Mishra, Moss and Loos 26 Full details of the eligibility and exclusion criteria for both these sub-samples are shown in Supplementary Fig. S1a and 1b, while characteristics of the mothers included in the ASQ and AEDC analyses versus those not included are presented in Supplementary Tables S2a and 2b.
The Human Research Ethics Committees at the University of Newcastle and the University of Queensland approved both studies. Informed consent was obtained from all participants at each survey.
Maternal exposure assessment
Diabetes during pregnancy, HDP (including pre-existing hypertension), maternal preconception BMI trajectory and pre-pregnancy BMI were the primary exposures of interest. Maternal height reported at Survey 1 (1996) and weight reported at Survey 1, Survey 2 (2000), Survey 3 (2003), Survey 4 (2006), Survey 5 (2009) and Survey 6 (2012) were used to calculate maternal BMI at each survey. Pre-pregnancy BMI was the BMI recorded at the survey immediately prior to the survey interval in which the child was born. For example, maternal BMI at Survey 5 (for children born between Survey 5 and 6) and maternal BMI at Survey 6 (for children born between Survey 6 and 7) were considered as pre-pregnancy BMI, categorized as normal (BMI <25), overweight (BMI 25.00–29.99) and obese (BMI ⩾30).
The diagnosis of GDM and HDP was self-reported and was not specific to each type of HDP. During the last three surveys (Surveys 5–7), women were asked whether they were diagnosed or treated for GDM and HDP for each live birth. If women did not complete either of these surveys or reported (during MatCH study) birth of a child after Survey 7, then these children were excluded during analysis since we do not have maternal GDM and HDP data. In addition, in each survey, self-reported physician diagnosed pre-existing diabetes (type 1 and type 2) and hypertension data were collected. Women with GDM were combined with women with pre-existing diabetes during the analysis.
In Australia over the study period, 2003–2015, according to the Australasian Diabetes during Pregnancy Society GDM management guidelines,Reference Hoffman, Nolan, Wilson, Oats and Simmons 27 a positive screening result for GDM should be made when a 1 h venous plasma glucose level was ⩾7.8 mmol/l after a 50 g glucose load, or ⩾8.0 mmol/l after a 75 g glucose load. Diagnosis is confirmed with a 75 g oral glucose tolerance test (fasting) with a venous plasma glucose level at 0 h of ⩾5.6 mmol/l and/or at 2 h of ⩾8.0 mmol/l. Similarly, as reported by the Society of Obstetric Medicine of Australia and New Zealand guidelines for the management of HDP,Reference Lowe, Brown and Dekker 28 each type of HDP is diagnosed as follows: chronic hypertension (blood pressure >140 mmHg systolic and/or >90 mmHg diastolic confirmed before pregnancy or before 20 completed weeks), gestational hypertension [new-onset hypertension (⩾140 mmHg systolic or ⩾90 mmHg diastolic blood pressure after 20 weeks of gestation)], pre-eclampsia (gestational hypertension with proteinuria ⩾300 mg/24-h or other maternal organ dysfunction) and pre-eclampsia superimposed on chronic hypertension. However, whether clinicians adhered to these guidelines is unknown.
Child outcome assessment
Cross-sectional data on child physical and cognitive developmental outcomes in this paper came from two sources: the gross motor and communication aspects of the ASQ were collected during the MatCH study and the AEDC (including gross and fine motor, cognitive and language outcomes) was obtained through linking mothers’ survey data to data collected through the AEDC as detailed below.
Gross motor development
Children’s (1–66 months) gross motor development was assessed using the ASQ, a parent-completed child development screening instrument.Reference Squires and Bricker 29 The ASQ assesses five domains of early childhood development (communication, gross and fine motor, problem-solving and personal–social domains) using 30 questions (six items per domain), but only the gross motor domain was included in this study. Each item in each domain has a choice of three responses: ‘Yes’, ‘Sometimes’ or ‘Not yet’, scored as 10, 5 or 0, respectively. Then, the sum of items in each domain, which ranges from 0 to 60, is compared with the cut-off values for that age group. Children who scored below the cut-off values of the gross motor aspects of the ASQ were considered to have a suspected gross motor delay (yes, no). The full ASQ was not included in the MatCH survey as it alone would have added 630 questionnaire items (21 age groups×5 subscales×6 questions), and the women were already required to complete a number of measures for up to three children.Reference Mishra, Moss and Loos 26 Thus, to minimize the burden of completing a bulky questionnaire, only the gross motor and communication domains of the ASQ were included in the MatCH study. The latter was not analysed in this study because of the small number of children with a suspected communication delay.
Gross and fine motor, language and cognitive skills and communication skills and general knowledge
Children’s gross and fine motor, cognitive and language development, and communication skills and general knowledge at the median age of 5 years were obtained from linked AEDC data. The AEDC uses a validated Australian version of the Canadian Early Development Instrument to measure five early childhood development areas; Physical health and Wellbeing (including gross and fine motor skills), Social competence, Emotional maturity, Language and Cognitive skills and Communication skills and General knowledge, assessed by school teachers when children commence their first year of full-time school.Reference Janus, Brinkman and Duku 30 In this study, we reported gross and fine motor skills, language and cognitive skills and communication skills and general knowledge. Children with scores in the lowest decile are considered ‘developmentally vulnerable’ on that domain, scores between 10 and 25% are ‘at-risk’ and those in the top 75% are ‘on track’. 31 During the analysis, the first two groups were collapsed to represent ‘vulnerable/at-risk children’.
Assessment of covariates and confounders
Maternal age (years), education (year 12/less, trade/apprenticeship/certificate/diploma, university degree and above), area of residence (major city, inner region, outer region/remote), 32 parity (nulliparous, primiparous, multiparous), physical activity (sedentary/low, moderate, high)Reference Brown, Ford, Burton, Marshall and Dobson 33 and smoking (never smoked, ex-smoker and current smoker) were reported at each ALSWH survey.
Statistical analyses
χ2 and t-tests were used to compare the distribution of maternal characteristics across childhood outcomes of offspring.
Group-based trajectory modelling
Proc Traj application,Reference Jones and Nagin 34 an independently designed SAS program, was used to identify maternal BMI trajectories before the conception of the index child (child included in the analysis). The preconception period was based on the child’s date of birth and the preconception BMI trajectories were estimated using five (for children with ASQ outcome) or three (for children with AEDC outcomes) data points before conception of the index child, making full use of all possible data before the conception of the index child. Children included in the ASQ assessment were younger [born between Surveys 5 (2009) and 7 (2015)] than those in the AEDC [born between Surveys 3 (2003) and 5 (2009)] and therefore had different maternal pre-pregnancy BMI data points. For instance, for the AEDC outcomes, for children born between Surveys 3 and 4, the preconception BMI trajectories were calculated using weight measurements from Surveys 1 to 3 (over 7 years); for children born between Surveys 4 and 5, the preconception BMI trajectories were from Surveys 2 to 4 (over 6 years) and so on; whereas for the ASQ gross motor outcome, for children born between Surveys 5 and 6, the preconception BMI trajectories were calculated using weight measurements from Surveys 1 to 5 (over 13 years); for children born between Surveys 6 and 7, the preconception BMI trajectories were from Surveys 2 to 6 (over 12 years). As the Proc Traj accommodates subjects with missing longitudinal data, women with at least two complete BMI data points were included in the trajectory modelling. Model fitness and number of trajectory groups were estimated via Bayesian information criteria (BIC). Further, the adequacies of the size of the trajectory groups (⩾5%), the meaningfulness of such clusters for subsequent analyses and the average posterior probabilities of group membership (>0.7) were considered.Reference Nagin and Odgers 35
A number of latent class models starting from one class to five class models with varying polynomial terms (up to a second degree) were fitted. The BIC value largely decreased from one up to five solution models, indicating increasing model fitness. However, the solution with three maternal BMI trajectories, named as normative (normal – stable BMI), chronically overweight (started overweight – remained overweight) and chronically obese (started obese – progressively severely obese), was chosen over the rest of the complex models as the latter included unstable group sizes (<5%). For the ASQ outcome, the corresponding group membership size was 57.5, 34.0 and 8.5%, respectively, whereas for the AEDC outcomes, 68.6, 26.5 and 4.9% of mothers had normative, chronically overweight and chronically obese BMI trajectories before the conception of the index child, respectively. In both cases, for each trajectory group, the average group membership probabilities exceeded 0.90, which shows a high degree of accuracy of individual assignment to groups. Further details are in the Supplementary Fig. S2a and 2b.
Generalized estimating equation models
A series of models based on the methods of generalized estimating equation analyses, which account for clustering of children in a family,Reference Hanley, Negassa, Edwardes and Forrester 36 was used to examine the associations between maternal exposures and child outcomes. Diabetes during pregnancy, HDP, maternal preconception BMI trajectory and pre-pregnancy BMI were the maternal key exposures and gross motor, and gross and fine motor skills, language and cognitive skills and communication skills and general knowledge were the main child outcomes. Log-binomial and log-Poisson (used if the former failed to converge) models were used to estimate risk ratios (RR) and 95% confidence intervals (CI).Reference McNutt, Wu, Xue and Hafner 37
All models were adjusted for maternal age, area of residence, parity, education, smoking and physical activity reported at the survey immediately prior to the survey interval in which the child was born. Diabetes during pregnancy and HDP models were further adjusted for maternal pre-pregnancy BMI. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata version 14 (StataCorp, College Station, TX, USA).
Sensitivity analyses restricted to women with at least three complete BMI data points and women who were not pregnant at the first three surveys of the ALSWH were performed to check consistency and robustness of the results of the main analyses, specifically the associations between maternal preconception BMI trajectories and child outcomes.
Results
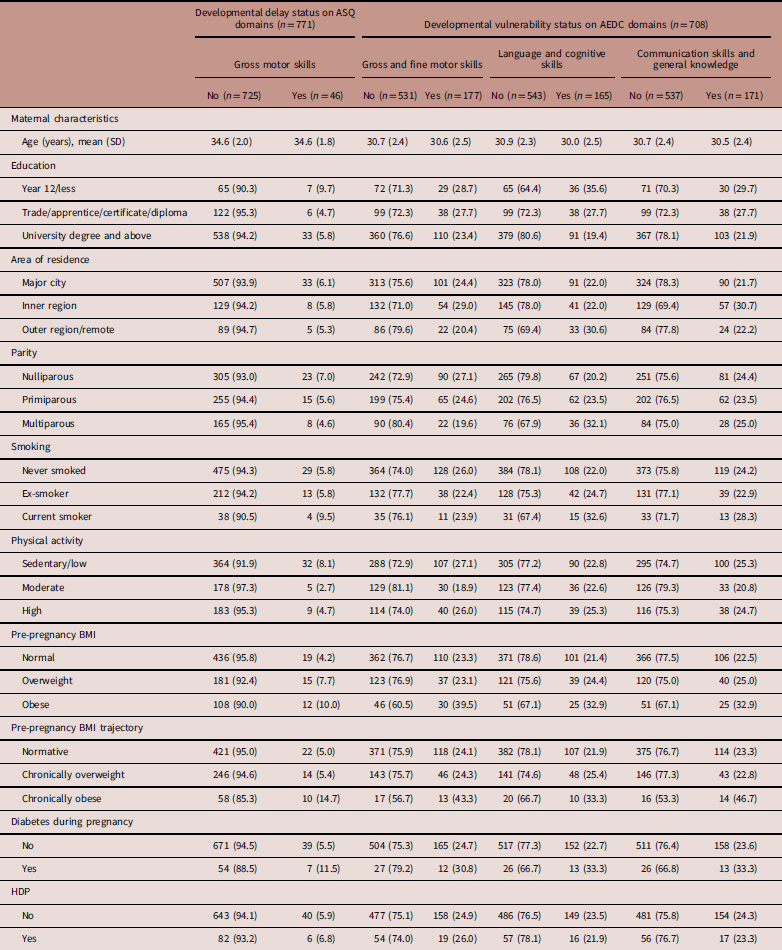
Table 1 presents the maternal characteristics by suspected gross motor delay (ASQ) or developmental vulnerability status on AEDC domains. A total of 771 children were included for the ASQ outcome of which 46 (6.0%) had suspected gross motor delay. Approximately 8 and 11% of women reported having physician-diagnosed diabetes during pregnancy and HDP, respectively. One-quarter (25.4%) and nearly one in six (15.6%) mothers of children were overweight and obese pre-pregnancy, respectively. Except maternal pre-pregnancy BMI and BMI trajectories, all maternal factors were not significantly associated with the suspected gross motor delay (P>0.05). Children with suspected gross motor delay were more likely to be born to obese or chronically obese women (P<0.01).
Table 1 Maternal characteristics by suspected gross motor delay [Ages and Stages Questionnaire (ASQ)] or developmental vulnerability status on Australian Early Development Census (AEDC) domainsFootnote a

ASQ, Ages and Stages Questionnaire; BMI, body mass index; HDP, hypertensive disorder of pregnancy; SD, standard deviation.
Unless indicated values are n (row %).
a Developmentally vulnerable group includes children at-risk on AEDC domains (whose score lies between 10 and 25%).
For the AEDC outcomes, 708 children were included; 177 (25.0%), 165 (23.3%) and 171 (24.2%) were classified as developmentally vulnerable/at-risk on the AEDC gross and fine motor skills, language and cognitive development and communication skills and general knowledge, respectively. While maternal education was significantly associated with all the AEDC outcomes, maternal preconception BMI trajectory was significantly associated with the AEDC gross and fine motor, and communication and general knowledge outcomes. Maternal pre-pregnancy BMI and parity were also significantly associated with the AEDC gross and fine motor, and communication and general knowledge outcomes, respectively.
Table 2 shows individual associations between diabetes during pregnancy, HDP, maternal pre-pregnancy BMI trajectories and pre-pregnancy BMI and childhood outcomes. In a fully adjusted model, children born to chronically obese women were significantly more likely to be classified as developmentally vulnerable/at-risk on gross and fine motor skills (RR=1.64, 95% CI: 1.04, 2.61) than children born to women with a normative BMI trajectory. Similarly, children of chronically obese mothers were more likely to be classified as developmentally vulnerable/at-risk on communication skills and general knowledge, but not language and cognitive development. Table 3 shows similar associations between maternal diabetes during pregnancy, HDP, maternal pre-pregnancy BMI trajectories and pre-pregnancy BMI, and offsprings’ childhood gross motor skill as measured by the ASQ. Children born to chronically obese women had more than twice the risk of suspected gross motor delay than children born to women with a normative BMI trajectory (RR=2.62, 95% CI: 1.26, 5.44).
Table 2 Associations between maternal pre-pregnancy body mass index (BMI) trajectories and pregnancy complications and risk of being classified as developmentally vulnerable/at-risk on the Australian Early Development Census (AEDC) [relative risk (RR) and 95% confidence interval (CI)] (n=708)Footnote a

HDP, hypertensive disorder of pregnancy.
Models were adjusted for maternal age, area of residence, parity, education, smoking and physical activity reported at the survey immediately prior to the survey interval in which the child was born.
a Maternal diabetes during pregnancy and HDP models were further adjusted for maternal pre-pregnancy BMI.
Table 3 Associations between maternal pre-pregnancy body mass index (BMI) trajectories and pregnancy complications and risk of suspected gross motor delay on the Ages and Stages Questionnaire (ASQ) [relative risk (RR) and 95% confidence interval (CI)] (n=771)Footnote a

HDP, hypertensive disorder of pregnancy.
Models were adjusted for maternal age, area of residence, parity, education, smoking and physical activity reported at the survey immediately prior to the survey interval in which the child was born.
a Maternal diabetes during in pregnancy and hypertensive disorder of pregnancy models were further adjusted for maternal pre-pregnancy BMI.
We also examined a single measure of maternal pre-pregnancy BMI and child outcomes and the results were similar to the maternal BMI trajectory analyses with some exceptions: the pre-pregnancy obesity effect size for the suspected gross motor delay was smaller than that of the chronically obese BMI trajectory and the association of maternal pre-pregnancy obesity and childhood communication skills and general knowledge did not reach statistical significance.
Children born to women with diabetes during pregnancy were at slightly greater risk of developmental delay, particularly gross motor, and language and cognitive skills, as compared with children born to women with no diabetes, although the 95% CI spanned the null value. HDP was not significantly associated with any of these child outcomes.
Results of the sensitivity analyses restricted to women with at least three complete BMI data points and women who were not pregnant at the first three surveys of the ALSWH were similar to the main analyses of maternal preconception BMI trajectory and child outcomes (Supplementary Tables S1a and 1b).
Discussion
Using a population-based cohort study of Australian women and their children, we have found that maternal BMI trajectories before conception have a significant impact on offspring’s childhood physical and cognitive development. Children born to women who had chronically obese BMI trajectory before conception were at higher risk of being classified as vulnerable or at-risk on the AEDC gross and fine motor skills, and communication skills and general knowledge domains. In addition, children born to women who showed a chronically obese BMI trajectory prior to pregnancy were at increased risk of suspected gross motor delay as measured by the ASQ when compared to children born to women with a normative BMI trajectory. However, our findings did not support the associations between maternal diabetes during pregnancy or HDP and any of these childhood outcomes.
The finding between maternal pre-pregnancy BMI trajectories and childhood physical and cognitive outcomes is in accordance with the general body of knowledge about maternal pre-pregnancy obesity and child developmentReference Adane, Mishra and Tooth 7 and it adds to that knowledge in a number of ways; maternal excessive body weight over the reproductive course of life and/ or being obese for a long duration may have an important cumulative impact on offspring’s childhood development. For instance, unlike the maternal pre-pregnancy BMI, maternal pre-pregnancy BMI trajectories were significantly associated with childhood communication skills and general knowledge in offspring. However, to our knowledge, the previous studiesReference Adane, Mishra and Tooth 7 in the association between maternal pre-pregnancy obesity and offspring’s physical and cognitive development were limited to one time point measure of maternal weight, thus leaving our study without comparison. Although not yet fully understood, the elevated level of nutrients and hormones and inflammatory factors associated with obesity has been frequently hypothesized as potential mechanisms linking maternal obesity with child neurodevelopment.Reference Godfrey, Reynolds and Prescott 38 For instance, during development, placental transfer of increased level of inflammatory factors such as cytokines has been suggested to impact the normal fetal brain development.Reference Bolton and Bilbo 39
We found no significant association between maternal diabetes during pregnancy or HDP and childhood physical and cognitive development of offspring. The existing evidence has also not shown a consistent picture about this possible link,Reference Adane, Mishra and Tooth 12 , Reference Tuovinen, Eriksson, Kajantie and Raikkonen 13 perhaps due to variations in the child outcomes measured or confounders adjusted for during analysis. For instance, a few previous studies have suggested that children born to diabetic or hypertensive mothers during pregnancy were more likely to have poorer physicalReference Grace, Bulsara, Pennell and Hands 18 , Reference Ghassabian, Sundaram, Wylie, Bell, Bello and Yeung 19 or cognitive development.Reference Dionne, Boivin, Seguin, Perusse and Tremblay 14 , Reference Leversen, Sommerfelt and Ronnestad 40 , Reference Morsing and Marsal 41 However, unlike the current study, most of these studies did not adjust for key confounders such as maternal pre-pregnancy obesity, and therefore, negative associations between maternal diabetes during pregnancy or HDP and childhood outcomes might have been due to such confounders or the effect might have been limited to younger infants.Reference Camprubi Robles, Campoy, Garcia Fernandez, Lopez-Pedrosa, Rueda and Martin 42
Conversely, our study might have been underpowered to detect differences in physical and cognitive outcomes, if any, particularly between children born to women with diabetes during pregnancy and children born to women without diabetes. However, in line with the current analysis, a recent studyReference Daraki, Roumeliotaki and Koutra 43 has found no association between maternal GDM and children’s cognitive and motor development at 4 years. Sufficiently powered prospective studies with detailed measures of child outcomes are needed to reach a definitive conclusion.
The most obvious limitation of this study is that all the data were self-reported. Obese women may underestimate their weight and they might have been categorized into chronically overweight or even to a normative trajectory group. Thus, this may decrease the effect of maternal weight trajectories, but self-reported weight has been demonstrated to be a reliable estimate.Reference Craig and Adams 44 Women could have also been misclassified if they did not correctly report their diabetes and HDP status. However, the reliability of the self-reported physician diagnosed GDM and HDP in our study population has shown high agreement with the medical records.Reference Gresham, Forder, Chojenta, Byles, Loxton and Hure 45 The lower response rate for the MatCH study as well as the lower proportion of women included in this paper may bias and compromise the generalizability of the results since mothers who are included in this study were considerably different from those who did not. For instance, based on the most recent ALSWH survey, the mothers completed, mothers who participated in this study were more likely to live in major cities (67.9 v. 57.8%), to have a university degree (73.8 v. 59.8%), to have three live births (31.3 v. 20.7%) and less likely to be current smoker (8.4 v. 4.9%) as compared with women not included (Supplementary Tables S2a and 2b). We also acknowledge the lack of data on gestational weight gain that has been found to be associated with child outcomes.Reference Pugh, Richardson and Hutcheon 46 The other limitation of this study is the lack of statistical power to separately assess the effect of pre-existing and gestational diabetes. Offspring of mothers with GDM might be at higher risk of developmental impairment than offspring of pre-existing diabetic mothers; the latter are more likely to have entered pregnancy with better controlled glucose levels following counselling and monitoring.
Despite these limitations, our study has a number of strengths. To our knowledge, this is the first population-based prospective cohort study to evaluate the associations between maternal pre-pregnancy BMI trajectories, diabetes during pregnancy and HDP and various offspring’s childhood outcomes. The longitudinal nature of the data and the group-based trajectory modelling provided us a unique opportunity to identify maternal BMI trajectories over relatively longer duration and to link this information to offspring’s physical and cognitive development. Unlike the previous studies, we were also able to adjust for time-varying lifestyle factors such as maternal pre-pregnancy BMI, smoking and physical activity.
This study has public health implications. Women, who are chronically obese for a period of 6–13 years before they conceive, have children at higher risk of developmental delay. Although largely similar effects were observed for a one point in time measure (pre-pregnancy obesity), use of a preconception BMI trajectory provides more opportunity for women to manage their weight before entering pregnancy: it expands the typical preconception period, usually the three months prior to pregnancy, which is too late a time point for women to lose a considerable amount of weight to reach a normal BMI.Reference Stephenson, Heslehurst and Hall 47 Therefore, during this period (6–13 years before conception) obese women should be encouraged to lose weight in order to minimize the impact of obesity to the women’s and their children’s health.
Although this study did not evaluate public health interventions, we can speculate on interventions that may be appropriate for women and their children. Dietary interventions combined with physical activity have been shown to be effective to lose weight.Reference Johns, Hartmann-Boyce, Jebb and Aveyard 48 Early childhood motor skills are fundamental for children to fully explore and learn their environment and have impact on later cognitive abilities.Reference Piek, Dawson, Smith and Gasson 49 Hence, children at risk of development delay may benefit from interventionsReference Squires and Bricker 29 targeted in early childhood – the period in development where environmental factors have an important impact. A stimulating home environment such as play activities/materials may help children to catch up in motor development.Reference Hua, Duan and Gu 50 , Reference Miquelote, Santos, Cacola, Montebelo and Gabbard 51 Moreover, children with motor delay have been found to be less physically active and are therefore at higher risk of obesity.Reference Williams, Pfeiffer and O'Neill 52 This may contribute to the burden of obesity and thereby perpetuate the vicious cycle of maternal obesity in future generations.
In summary, the results of this study suggest that maternal pre-pregnancy BMI trajectories before conception have significant impact on offspring’s early childhood physical and cognitive development. Although we did not find significant association between maternal HDP and offspring’s childhood outcomes, lack of statistical power precluded us from reaching a firm conclusion about this association with diabetes during pregnancy. Further sufficiently powered studies are clearly warranted.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174418000570
Financial Support
A.A.A. is supported by International Postgraduate Research Scholarship (IPRS) and UQ Centennial scholarship. MatCH is funded by National Health and Medical Research Council (APP1059550), G.D.M. is supported by National Health and Medical Research Council Principal Research Fellowship (APP1121822). This paper uses data from the AEDC. The AEDC is funded by the Australian Government Department of Education and Training. The findings and views reported are those of the author and should not be attributed to the Department or the Australian Government.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation [NHMRC National Statement on Ethical conduct in Human Research 2007 (updated May 2015), Australian code for the responsible conduct of research 2007, National Health and Medical Research Council Guidelines approved under Section 95 of the Privacy Act 1988 (November 2014) and Australian Privacy Principles guidelines (31 March 2015)] and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees (The University of Newcastle Human Research Ethics Committee reference H-2014-0246, The University of Queensland Human Behavioural and Social Sciences Ethical Review Committee reference 2014001213 and Australian Institute of Health and Welfare Ethics Committee reference EO2017/1/342).
Acknowledgments
The research on which this paper is based was conducted as part of the ALSWH by the University of Queensland and the University of Newcastle. The authors are grateful to the Australian Government Department of Health for funding and to the women who provided the survey data. The authors also acknowledge the assistance of the Data Linkage Unit at the Australian Institute of Health and Welfare (AIHW) for undertaking the data linkage to the AEDC.