Introduction
Maternal deficiencies in either macronutrients or micronutrients during pregnancy can adversely affect nutrient transport to the developing embryo, leading to fetal growth restriction and subsequent low birth weight.Reference Wu, Bazer, Cudd, Meininger and Spencer 1 There is overwhelming evidence to suggest that infants born small for gestational age have a significantly increased risk of developing cardiovascular and renal disease in later life.Reference Brenner, Garcia and Anderson 2 – Reference Vikse, Irgens, Leivestad, Hallan and Iversen 4 This is often due to adverse intrauterine events that place the developing embryo under stress and restrict growth during gestation.Reference Ojeda, Grigore and Alexander 5 As seen in animal models, this ultimately leads to altered organogenesis and places the offspring at increased risk for developing hypertensionReference Gambling, Dunford and Wallace 6 – Reference Goyal and Longo 8 and impaired renal functionReference Alwasel and Ashton 9 , Reference Lelièvre-Pégorier, Vilar and Ferrier 10 in adulthood.
A reduction in glomerular number can be a major contributor to the development of cardiovascular disease during later adult life, as suggested by numerous clinical studies.Reference Keller, Zimmer, Mall, Ritz and Amann 11 – Reference Hoy, Hughson, Singh, Douglas-Denton and Bertram 14 A nephron deficit reduces the renal capacity for sodium excretion, leading to sodium retention and increased plasma volume. These changes in renal physiology increase mean arterial pressure (MAP), leading to glomerular hypertension and scarring. This creates a positive feedback loop to further increase blood pressure (BP) through progressive glomerular damage. Although maternal calorie/protein restriction is known to reduce glomerular number and increase adult BP,Reference Woods, Weeks and Rasch 15 – Reference Hoppe, Evans, Bertram and Moritz 17 few studies have investigated the role of gestational micronutrient deficiencies in programming renal and cardiovascular function in adult offspring.
Micronutrient deficiencies during pregnancy are well known to adversely impact both maternal and fetal health and lead to poor pregnancy outcomes.Reference Allen 18 Many women of reproductive age do not meet the recommended levels for dietary Mg2+ intake, so a significant proportion of women may be Mg2+-deficient either before conception or during pregnancy. One study examining dietary Mg2+ intake in a French population reported that over 20% of women (aged 35–60) consumed less than two-third of the recommended daily allowance (320 mg).Reference Durlach, Pages, Bac, Bara and Guiet-Bara 19 Low dietary Mg2+ intake is also common in younger women, as a recent Australian study of over 450 17-year-old girls found that only 15% met the recommended daily intake, and <50% met the estimated average requirement.Reference Parker, Vivian, Oddy, Beilin, Mori and O’Sullivan 20 These studies suggest that low Mg2+ intake in women of reproductive age may be highly prevalent. Although Mg2+ levels during pregnancy have not been comprehensively studied, one study in an Indian population reported that~45% of pregnant women had low serum Mg2+ levels (<0.75 mmol/l).Reference Pathak, Kapoor, Kapil and Dwivedi 21 We have recently investigated the effect of maternal Mg2+ deficiency on fetal and early postnatal outcomes using an animal model.Reference Schlegel, Cuffe, Moritz and Paravicini 22 Our results demonstrated that maternal hypomagnesemia caused fetal growth restriction, embryonic loss and placental abnormalities. These findings clearly emphasize the important role of Mg2+ in placental health and normal embryonic development, however, the long-term consequences for offspring born to Mg2+-deficient mothers have not yet been determined. In the present study, we used this recently described mouse model of maternal Mg2+ deficiencyReference Schlegel, Cuffe, Moritz and Paravicini 22 to investigate how maternal hypomagnesemia affects cardiovascular and renal outcomes in adult offspring.
Methods
Ethics
All studies were approved by The University of Queensland Anatomical Biosciences Animal Ethics Committee (SBMS/154/12/NHMRC/NHF) and conducted according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (NHMRC).
Animal treatment
A total of 24 dams and their offspring were treated as per Schlegel et al.Reference Schlegel, Cuffe, Moritz and Paravicini 22 Briefly, female CD1 mice (7 weeks old) were fed either a control Mg2+ diet (0.2%, w/w Mg2+), or a Mg2+-deficient diet (0.02%, w/w, Glen Forrest Stockfeeders, Specialty Feeds, Western Australia) for 4 weeks before mating and throughout gestation (n=6 dams/treatment group). This Mg2+-deficient diet significantly reduces maternal plasma Mg2+ concentrations by~40% compared with controls.Reference Schlegel, Cuffe, Moritz and Paravicini 22 Female mice were then mated with CD1 males (fed a standard laboratory chow diet) and allowed to litter naturally. The Mg2+-deficient diet was continued until weaning at postnatal day 21 (PN21), when a subset of offspring was euthanized for determination of glomerular number. All other animals were maintained on a 12:12 h light:dark cycle with food (standard rodent chow) and water ad libitum, and were studied at 6 months of age.
Stereological estimation of glomerular number
One to two pups of each sex from each litter were euthanized at PN21 and the right kidney from each pup was collected, weighed, fixed in 4% paraformaldehyde and processed to paraffin. Total glomerular number was estimated using unbiased stereological analysisReference Cullen-McEwen, Armitage, Nyengaard, Moritz and Bertram 23 (n=6/group).
Renal function and electrolyte analysis
A cohort of mice was habituated to metabolic cages on 2 consecutive days for 3 h/day. On the 3rd day, mice were placed in metabolic cages for 24 h with food and water available ad libitum. Animal weights, food and water consumption were recorded, and urinary electrolytes measured using a COBAS Integra 400 Plus electrolyte analyzer (n=9–11).
Measurements of BP, heart rate (HR) and activity under basal conditions
Telemetry probes (PA-C10, Data Sciences International, USA) were implanted into a separate group of 6-month-old offspring under isoflurane anesthesia (3–3.5% in O2) to measure BP, HR and activityReference O’Sullivan, Cuffe and Paravicini 24 (n=7–8). Mice were housed individually following surgery, and data acquisition commenced 10 days after surgery. Data (systolic and diastolic pressures, HR and activity) was captured continuously for 10 s every 15 min for a period of 3 days using Dataquest Advanced Research Technology (Data Sciences International).
Cardiovascular responsiveness to stressors
After measuring BP, HR and activity under basal conditions, we investigated whether the cardiovascular responsiveness to stress was altered by maternal Mg2+ deficiency. Responses to stress were assessed by exposing the animals (n=7–8) to aversive (restraint stress, dirty cage switch) and non-aversive stimuli (feeding) that cause changes in cardiovascular parameters, arousal and activity.Reference Chen, Jancovski and Bassi 25 – Reference Davern, Jackson, Nguyen-Huu, La Greca and Head 28 Stressor experiments were performed on 3 consecutive days between 8 am and 12 pm, with continuous data acquisition throughout the experiment and for 60 min before the stressor. For the restraint stress test, each mouse was placed into a well-ventilated clear plastic cylinder (radius ~30 mm, height ~70 mm) for 15 min. For the dirty cage switch test, the experimental mouse was placed into a cage that had been occupied by a different mouse of the same sex and strain for 7 days previously, and data recorded for 90 min. The non-aversive feeding test involved placing a novel food stimulus (~0.5 g of almond) into the home cage, and data were recorded for 10 min.
Tissue collection
Following all experiments, animals were euthanized by cervical dislocation (at PN21) or by CO2 inhalation (6 months) and organs (heart, kidneys, liver and brain) collected, weighed and either fixed in 4% paraformaldehyde or snap frozen in liquid nitrogen for later analysis. Bone samples were processed by microwave/nitric acid digestion, and Mg2+ content measured using inductively coupled plasma atomic emission spectrophotometry. Blood was also collected, centrifuged at 4°C and the plasma stored at −20°C until analysis.
Gene expression
The messenger RNA expression levels of the Mg2+ channel TRPM6 in the kidneys of offspring were measured using quantitative PCR (qPCR). Total RNA was isolated using TRIzol and reverse transcribed using a TaqMan reverse transcription reagents kit (Life Technologies). qPCR was performed using Assay on Demand reagents (TRPM6 Mm00463112) and ribosomal 18S as a housekeeping gene. Data were analyzed using the
![]() $${\rm 2}^{{{\minus}\Delta \Delta C_{T} }} $$
method and expressed relative to the control group (n=6–11/group).
$${\rm 2}^{{{\minus}\Delta \Delta C_{T} }} $$
method and expressed relative to the control group (n=6–11/group).
Statistics
All data are presented as mean±S.E.M. Renal data were analyzed using two-way analysis of variance (ANOVA) with a Tukey’s multiple comparisons test (Graph Pad Prism); P<0.05 was regarded as being significant. Telemetry data were analyzed by repeated measures two-way ANOVA using sex and maternal diet as factors. Responses to stress were calculated by quantifying the area under the curve during the stressor and comparing with an equivalent amount of time during the baseline period.
Results
Offspring growth and organ weights
In the present study, we monitored animals from weaning (PN21) through 6 months of age, and although we found significant differences between sexes, there were no differences in body weights between treatment groups. Animals were not weighed on the day of birth. There was a significant increase in liver weight in the Mg2+-deficient offspring at 6 months of age, however, there were no other differences in organ weights between groups (Table 1).
Table 1 Body and organ weights from 6-month-old offspring

*P<0.05.
Renal function, gene expression and nephron number
Urinary Mg2+ excretion was reduced in Mg2+-deficient male (but not female) offspring when compared with control offspring (Fig. 1a). This was not associated with altered renal expression of the TRPM6 Mg2+ channel (Fig. 1b), nor were there any differences in plasma and bone Mg2+ levels between groups (Fig. 1c and 1d). Although males had significantly more nephrons than females, there was no difference in nephron number between control and Mg2+-deficient groups at PN21 (Fig. 2a). Both male and female Mg2+-deficient offspring showed a significant (P<0.05) increase in urine flow at 6 months of age (Fig. 2b), but there were no statistically significant differences in water consumption between treatment groups (Fig. 2c). All offspring had similar urinary osmolar excretion (Fig. 2d), and plasma/urinary levels of Na, K and Cl (Table 2).
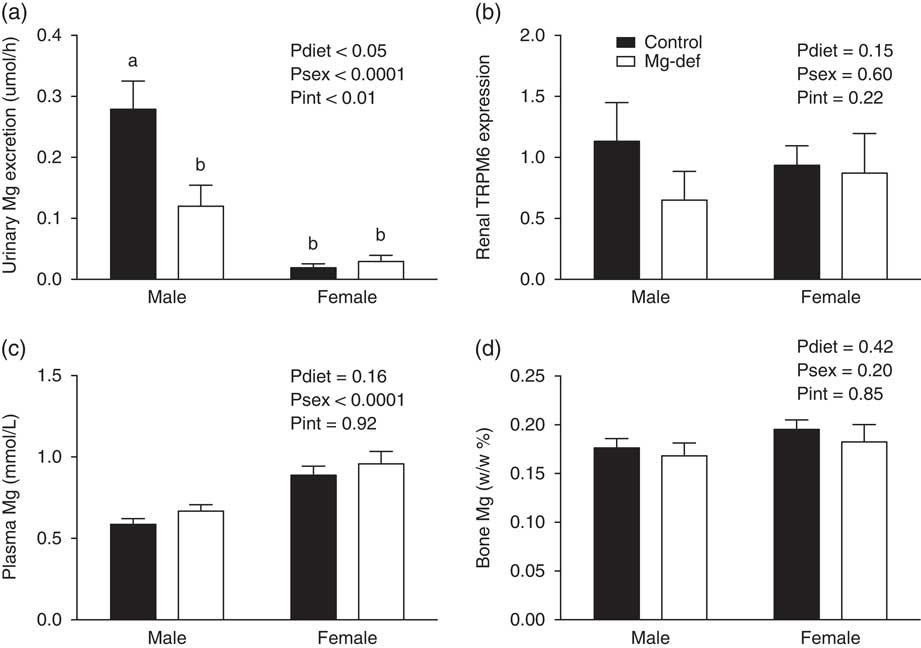
Fig. 1 Mg2+ handling in 6-month offspring. (a) Mg2+ urinary excretion. (b) Renal TRPM6 expression. (c) Plasma Mg2+. (d) Bone Mg2+ (w/w, %). n=6–12 offspring/group per sex. a,bDifferent letters indicate significant differences (P<0.05) between groups.
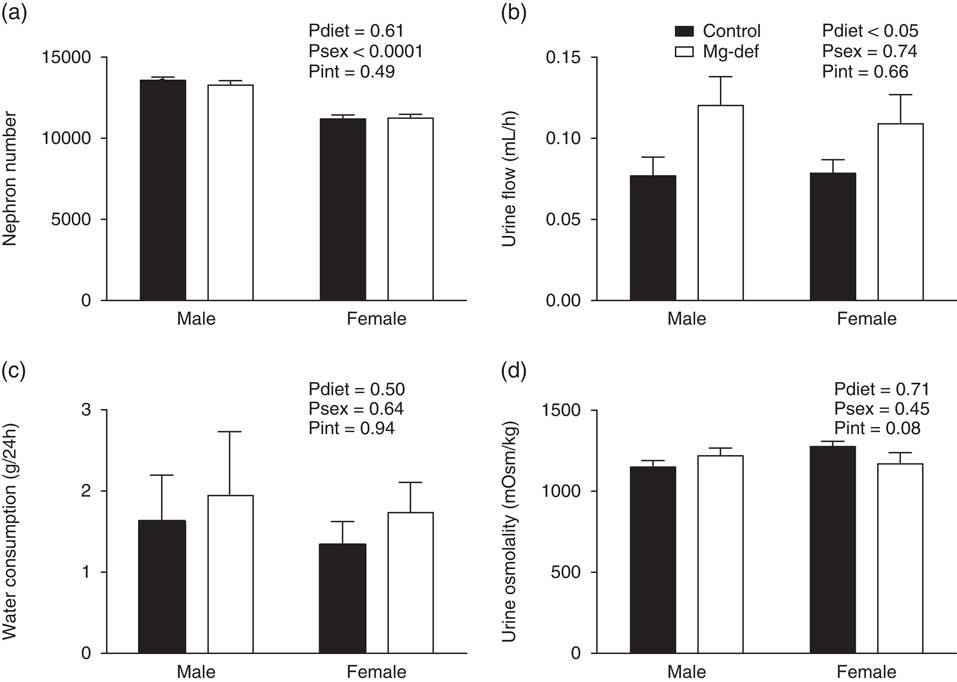
Fig. 2 Renal physiology in offspring. (a) Nephron number at postnatal day 21, (b) urinary flow, (c) water consumption per 24 h and (d) urine osmolality at 6 months of age. n=6–12 offspring/group per sex.
Table 2 Urinary Na, K, Cl excretion, Na/K excretion ratio and plasma Na, K, Cl from 6-month-old offspring

Basal MAP, HR and activity
Analysis of radiotelemetry data revealed no differences in basal MAP or HR between groups during either the day or night periods (Fig. 3). All groups showed normal circadian variations in MAP, HR and activity (Fig. 3). A collective 24-h analysis of radiotelemetry data across all 3 days also showed no treatment-related differences in basal MAP (control males, 116±3 mmHg; control females, 113±2 mmHg; Mg2+-deficient males, 118±1 mmHg; Mg2+-deficient females, 117±2 mmHg), HR (control males, 539±16 bpm; control females, 621±12 bpm; Mg2+-deficient males, 549±10 bpm; Mg2+-deficient females, 583±15 bpm) or activity (control males, 5±1 AU; control females, 8±1 AU; Mg2+-deficient males, 6±1 AU; Mg2+-deficient females, 9±1 AU). Separate analysis of the day and night periods also found no statistically significant differences in these parameters.

Fig. 3 Mean arterial pressure (MAP) in (a) male and (b) female offspring at 6 months of age. Heart rate in (c) male and (d) female offspring in 6-month-old offspring. Activity in (e) male and (f) female offspring in 6-month-old offspring. Data were recorded for 10 s every 15 min and are expressed as a 12 h average of all sampled data across 3 consecutive day (D) and night (N) periods. n=5–8 offspring/group per sex.
Cardiovascular responses to stressors
Restraint stress caused an immediate increase in MAP (Δ23–32 mmHg) and HR (Δ88–163 bpm) above baseline levels. The dirty cage switch also caused an immediate elevation in MAP (Δ16–21 mmHg), HR (Δ140–217 bpm) and activity (Δ25–31 AU) in all groups. The almond-feeding test (non-aversive stimulus) caused an immediate and modest increase in MAP in control (Δ19–20 mmHg) and Mg2+-deficient (Δ12–14 mmHg) offspring (P=0.061). HR (Δ95–138 bpm) and activity (Δ9–12 AU) were also elevated across all groups. There were no significant differences in ΔMAP or ΔHR between groups in response to any of the above stressors (Table 3).
Table 3 Mean arterial pressure (MAP) and heart rate (HR) in 6-month-old offspring subject to cardiovascular reactivity/stress tests

Stressor measurements were calculated by quantifying the area under the curve (AUC) during the stressor (restraint stress, 15 min; dirty cage swap, 60 min; almond feeding, 10 min) and normalized to an equivalent amount of time during baseline (pre-stressor).
*P<0.05.
Discussion
This study has for the first time examined the long-term cardiovascular and renal outcomes of offspring born to Mg2+-deficient mothers. Maternal hypomagnesemia altered renal physiology by increasing urinary flow (in offspring of both sexes) and decreasing Mg2+ excretion (in male offspring only). However, this alteration in urinary Mg2+ excretion was not associated with altered expression of the Mg2+-permeable ion channel TRPM6 in the kidney. Furthermore, we found no differences in nephron number between treatment groups. The basal BPs and HRs of adult offspring were unaffected by maternal Mg2+ deficiency, nor were there any differences in cardiovascular responses to stress. Our current findings demonstrate that maternal Mg2+ deficiency causes minor alterations in renal function but does not cause high BP during later adult life.
Many prenatal dietary deficiencies result in renal dysfunction.Reference Alwasel and Ashton 9 , Reference Lelièvre-Pégorier, Vilar and Ferrier 10 In the present study, Mg2+-deficient offspring had ~30–40% increase in urinary flow rate compared with controls. This was accompanied by an increase in water intake of ~15–25% in Mg2+-deficient offspring. Although this was not statistically different to the control group, the high variability in the water intake data may have prevented the detection of significant changes. In addition, mice consume only small volumes of water over a 24-h period (when compared with larger species such as rats), which may contribute to the lack of accuracy in measuring changes in this parameter, and the subsequent variability in the data. Other models of maternal dietary restriction have demonstrated that the offspring of dams receiving a low-protein diet had an increase in urinary flow rates that was associated with an increase in water intake.Reference Alwasel, Barker and Ashton 29 An increase in water intake is thus a likely explanation for the increase in urinary flow in our model of maternal Mg2+ deficiency. An alternative explanation is that different responses to the metabolic cage-induced stress led to differences in water consumption between groups. However, as we found no differences in cardiovascular responsiveness to stressors, a differential response to metabolic cage stress seems unlikely. Furthermore, if the increased urinary flow rate occurred in the absence of increased water consumption, we would expect to have found a hypovolemic reduction in BP. Instead, radiotelemetry measurements of BP revealed no differences between groups. Taken together, this makes an increase in water intake, which is the most likely explanation for the increased urinary flow in Mg2+-deficient offspring.
In addition to the increased urinary flow rate, male offspring from Mg2+-deficient dams also showed reduced urinary Mg2+ excretion. However, this was not associated with increased levels of Mg2+ in either plasma or bone. Studies have reported that fractional Mg2+ excretion and urinary output of Mg2+ is reduced in offspring from diabetic dams;Reference Bond, Hamilton and Balment 30 , Reference Bond, Sibley, Balment and Ashton 31 a similar phenotype to male offspring from our Mg2+-deficient group. Changes in Mg2+ excretion also have been reported clinically, as Mughal et al.Reference Mughal, Eelloo and Roberts 32 found altered Mg2+ handling in children born to mothers with insulin-dependent diabetes mellitus. However, programmed alterations in Mg2+ homeostasis due to prenatal or perinatal Mg2+ deficiency have not been previously reported. In the current study, we found that the change in Mg2+ excretion in males was not due to altered TRPM6 expression in the kidney, despite this channel being primarily responsible for renal Mg2+ reabsorption and the regulation of Mg2+ excretion.Reference Schlingmann, Waldegger, Konrad, Chubanov and Gudermann 33 Nonetheless, these changes in Mg2+ excretion in males may be a programmed adaptation by which fetuses exposed to reduced Mg2+ levels in utero develop a compensatory and permanent reduction in Mg2+ excretion that is retained during later life in order to maintain normal Mg2+ homeostasis. However, the mechanism(s) responsible for these changes in the renal handling of Mg2+ remain unknown.
Given the association between maternal dietary nutrient restriction and reduced nephron number,Reference Lelièvre-Pégorier, Vilar and Ferrier 10 , Reference Woods, Weeks and Rasch 15 , Reference Hoppe, Evans, Bertram and Moritz 17 we determined nephron number in male and female offspring and were surprised to find that it was unaltered by maternal Mg2+ deficiency. Although numerous models of both mild and severe prenatal nutrient deficiencies have demonstrated reduced glomerular number in offspring,Reference Lelièvre-Pégorier, Vilar and Ferrier 10 , Reference Hoppe, Evans, Bertram and Moritz 17 in our model moderate maternal Mg2+ deficiency did not cause changes in total glomerular number. At 6 months of age there were marked differences in body and organ weights between male and female pups. Interestingly, despite similar kidney weights at P21Reference Schlegel, Cuffe, Moritz and Paravicini 22 males had a greater glomerular number than females. This is consistent with previous studies, both animal and human, that have demonstrated glomerular number is significantly linked to gender.Reference Hoy, Hughson, Bertram, Douglas-Denton and Amann 34 – Reference Jean-Faucher, Berger and Gallon 36 In addition, we saw no changes in 24-h MAP and HR in Mg2+-deficient offspring. These findings were quite unexpected, as maternal deficiencies in other trace elements such as zincReference Tomat, Inserra and Veiras 37 and ironReference Lisle, Lewis and Petry 38 , Reference Bourque, Komolova, Nakatsu and Adams 39 are known to reduce nephron number and increase BP in the adult offspring. The absence of any programmed outcomes from maternal Mg2+ deficiency during gestation and lactation is particularly surprising. We have previously shown that different degrees of maternal dietary Mg2+ deficiency leads to graded fetal growth restriction, and the offspring born to mothers who were moderately Mg2+-deficient were mildly growth restricted during early postnatal development.Reference Schlegel, Cuffe, Moritz and Paravicini 22 Such growth restriction is often associated with adverse impacts on the developing kidneys and cardiovascular system. Although this previous study showed mild growth restriction in moderately Mg2+-deficient offspring, in the present study we did not assess birth weight, placental transport of Mg2+, or Mg2+ plasma levels at PN21 in the same animals that were used for cardiovascular studies at 6 months of age; this may be considered a limitation to our current study. We were unable to detect even small (<5 mmHg) changes in basal 24-h BP, HR or activity through the use of radiotelemetry, the ‘gold-standard’ and highly sensitive method for measuring cardiovascular parameters in mice.Reference O’Sullivan, Cuffe and Paravicini 24 As demonstrated by Woods et al.,Reference Woods, Ingelfinger and Rasch 40 there is evidence that the degree of dietary restriction can be correlated with the adverse programmed outcome. Therefore, it is possible that a more severe dietary Mg2+ deficiency during gestation and development may affect BP in later life. However, the effects of severe Mg2+ deficiency could not be explored in this model due to the high neonatal mortality of offspring born to severely Mg2+ deficient mothers.Reference Schlegel, Cuffe, Moritz and Paravicini 22
In addition to measuring BP under basal conditions, we also investigated whether the cardiovascular responsiveness to aversive and non-aversive stress was affected by maternal Mg2+ deficiency. Clinical research shows that increased cardiovascular reactivity to psycho-emotional stress is a predictor for the development of hypertension and heart disease.Reference Matthews, Woodall and Allen 41 , Reference Rozanski, Blumenthal and Kaplan 42 Altered cardiovascular reactivity can also be developmentally programmed, as rodent models have shown that maternal stress and exposure to prenatal corticosteroids can alter cardiovascular reactivity in postnatal offspring.Reference Igosheva, Klimova, Anishchenko and Glover 43 , Reference O’Sullivan, Cuffe and Koning 44 Despite these studies linking maternal perturbations and altered cardiovascular reactivity, in the present study offspring from Mg2+-deficient dams did not show any changes in cardiovascular reactivity in response to aversive and non-aversive stimuli. Overall, this suggests that moderate maternal Mg2+ deficiency does not put offspring at an increased risk of developing cardiovascular disease during later adult life. Nonetheless, we cannot discount the possibility of other programmable phenotypes (e.g. altered metabolic outcomes) that result from maternal Mg2+ deficiency, or the impact of a ‘second-hit’ (such as poor diet or lifestyle) that may exacerbate or unmask existing conditions or predispositions to disease.
Maternal nutrition plays an important role in the health outcomes of offspring in both the early postnatal period and later in adulthood. Although we have previously demonstrated that maternal Mg2+ deficiency caused fetal and postnatal growth restriction, the present study shows that this did not cause a deficit in glomerular number, or any changes in BP, HR or cardiovascular reactivity. This infers that babies born to women with moderate levels of Mg2+ deficiency during pregnancy are unlikely to suffer from adverse cardiovascular health for this reason alone.
Acknowledgments
R.N.S. is the recipient of an Australian Postgraduate Award scholarship. K.M.M. is a Senior Research Fellow of the NH&MRC. The authors would like to thank the University of Queensland, School of Agriculture and Food Science for performing the inductively coupled plasma atomic emission spectrophotometry for our study.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Australian guides on the care and use of laboratory animals (Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, NH&MRC), and were approved by The University of Queensland Anatomical Biosciences Animal Ethics Committee.









