Introduction
Current evidence suggests that the Developmental Origin of Health and Disease or DOHaD in humans and animals is not only due to nutritional deficiencies but also due to fetal hypoxia, as a result of environmental or placental factors.Reference George and Granger1–Reference Parraguez, Urquieta and Pérez3 Increasing evidence also links DOHaD to epigenetic changes in progeny with older parents. Numerous studiesReference Momand, Xu and Walter4 have identified such a link between older fathers and their offspring, and similar evidence is beginning to emerge in older mothers and their offspring.Reference Sandovici, Smith and Nitert5, Reference Adkins, Thomas and Tylavsky6 These epigenetic changes in the progeny may lead to metabolic disorders in adulthood.Reference Wiener-Megnazi, Auslender and Dirnfeld7, Reference Stene and Gale8 If this association between DOHaD and parental age is true, it is likely to worsen, as modern living is associated with postponement of childbearing.Reference Huang, Sauve and Birkett9 This postponement is associated not only with epigenetic changes in offspring related to DOHaD, but also with decreased fertility in both males and females, as well as adverse pregnancy outcomes in women.Reference Mills, Rindfuss and McDonald10, Reference Wong-Taylor, Lawrence and Cowen11
The trend toward greater postponement of childbearing highlights the importance of researching the effects of parental age on DOHaD. Researchers are increasingly turning to large animal models, because of several advantages.Reference McMillen, Adam and Mühlhäusler12–Reference Gonzalez-Bulnes, Pallares and Ovilo16 Studies of prenatal programming in large animal species may provide valuable insights not only translationally as a model for human beings but also directly for improving animal production, health and welfare.Reference Reynolds, Borowicz and Caton14, Reference Wu, Bazer and Wallace17
The cow offers an excellent model system for studying prenatal programming and also has several characteristics particularly useful: singleton pregnancies; a developmental trajectory similar to that of humans, including 9-month gestation; and, like other ruminants, cotyledonary placentation with the same villous type as the human placenta and with similar vascular development and morphology.Reference Barry and Anthony18 Among bovine breeds, the Holstein is particularly useful for research in developmental programming because of the potential dual pay-off for improving dairy animal management. The modern Holstein, the world’s most productive dairy animal, is the result of intensive genetic programs to enhance productivity.Reference VanRaden19, Reference Pritchard, Coffey and Mrode20 In fact, genetic selection has produced Holstein individuals capable of producing more than 10,000 kg of milk/year on high-producing farms, which translates to more than 33 kg/day.Reference Ferguson and Skidmore21, Reference Astiz and Fargas22 As a result of the intensely scientific approach to Holstein management, breeding programs around the world routinely collect large amounts of longitudinal data on pedigree, phenotype and environmental information. Thus, the Holstein cow offers an outstanding opportunity for discerning how genetic and environmental factors affect adult life through developmental programming.
Most studies of how genetic and environmental factors influence life performance have assessed the effects of nutrition and metabolic status;Reference Sova, Leblanc and McBride23 health status (mastitis and lameness Reference de Graaf and Dwinger24–Reference Eicher, Lay and Arthington30) and environmental and handling factors, including climate, social stress, bedding and stall type.Reference Fulwider, Grandin and Garrick31–Reference Tao and Dahl35 As a result, many studies neglect the well-known fact that adequate health, management and nutrition from birth to puberty and during first pregnancy and calving also influence adult performance.Reference Davis Rincker, Vandehaar and Wolf36–Reference Eaglen, Coffey and Woolliams38 Indeed, increasing evidence indicates that prenatal conditions affect adult phenotype and productivity.Reference Funston, Larson and VonNahme39, Reference Hinde, Carpenter and Clay40
The possible influence of prenatal programming on milk productivity in dairy cows is of particular economic importance. Dairy cows are unique among large mammals in that early embryonic development and subsequent fetal development overlap with maternal lactation. Conception typically occurs around the lactation peak, within 3 months after parturition and lactation onset; as a result, ∼75% of the gestation period occurs concurrently with lactation. Not only does the metabolic environment, during which the lactation begins, but also the preimplantational stages of the next pregnancy strongly affect embryo survival and development.Reference Wu, Bazer and Wallace17, Reference Bach37, Reference Funston, Larson and VonNahme39, Reference Robinson, Sinclair and Mcevoy41 The more milk the dam produces, the lower is the survival capacity of her offspring.Reference Berry, Lonergan and Butler42 González-Recio et al.Reference González-Recio, Ugarte and Bach43 have also provided evidence that the greater is the mother’s milk yield at conception, the lower will be the daughter’s yield and longevity.
The objective of the current study was to determine the effects of Holstein maternal age at conception on the metabolic efficiency and both productive and reproductive life performance of the offspring, independently of other genetic, physiological and environmental pre- and postnatal factors. The results should provide translational insights into DOHaD in humans and direct insights into DOHaD in bovines, helping guide efforts to improve the profitability and responsible management of the Holstein breed.
Material and methods
Animals and management
Experimental data on developmental, reproductive and productive features of 404 Holstein cows on a commercial dairy farm in eastern Spain (SAT More, Betere, Spain) were collected over 7 years and included the two first complete lactations. During the study period (2007–2013), the herd included 1216 cows of which 37% were primiparous cows. The replacement rate was 30%. Holstein cows reach puberty at around 11 months of age and give birth for the first time at a mean age of 24 months.Reference Schillo, Hall and Hileman44, Reference Hoffman45 On intensive farms, where metabolic challenge remains high throughout the Holstein’s life, each cow averages 2.6–3.2 calvings, corresponding to 5–6 years of age. Hence, a Holstein cow that has given birth three or more times (>5 years old), is considered a mature, or even aged, animal.Reference Brickell and Wathes46
All animals were managed in the same way. Cows were milked three times daily; mean daily milk production was 35.6 kg/cow. The herd was fed twice daily with a total mixed ration (TMR) that was balanced following to recommendations for lactating dairy cows.47 The TMR consisted of brewer’s grain, alfalfa or corn silage, orange, corn, cotton, soybean hulls, straw and soybean, as well as bicarbonate and corrector salts. All animals had ad libitum access to water.
For reproductive management, first pregnancy heifers were first artificially inseminated (AI) at a mean age of 13.3 months after observed estrus. In adult cows, after a 90-day waiting period (days after parturition), ovulation synchronization was carried out based on the G6G protocolReference Bello, Steibel and Erskine48 in all cows. This protocol consists of administering 500 µg of a PGF2α analog (cloprostenol, Cyclix; Virbac SA, Barcelona, Spain), then 100 µg of gonadotropin-releasing hormone 2 days later (Cystoreline; Ceva SA, Barcelona, Spain) and finally Ovsynch 6 days later. A pedometer system was used to detect natural estrus after first AI as effectively as possible. Non-pregnant cows were resynchronized by re-initiating the G6G protocol on the day of negative pregnancy diagnosis if not seen in estrus before.
Inclusion criteria, data collection and confounding variables
Over the inclusion period (2007–2009), 782 heifers were born on the farm. Of these, 20 (2.6%) died during the first 2 months of life, 16 (2.1%) died between 2 and 13 months of age, 47 (6%) died or were culled between 13 and 24 months of age, 117 (15%) died or were culled during their first lactation and 125 (16%) died or were culled during their second lactation. Cows culled during the first and second lactations were culled for reasons other than productivity (involuntary culling), primarily because of infertility (60%), mastitis (10%) and lameness (6%). A total of 53 cows had not finished their second lactation by the last day of data collection. Thus, the final analysis included the complete first and second lactations of 404 dairy cows.
All weekly yield records from 404 first lactations and 404 second ones were included in the analysis to generate performance clusters. There was an average of 52 weekly yield records per first lactation for a total of 21,271 records, while the average per second lactation was 48 weekly yield records for a total of 19,542 records. Performance clusters were analyzed using the following variables: number of parturitions of the dam, maternal age in years, father, total yield in liters during lactation of the gestation of the dam (Y-Gest); average yield/lactation in liters in life of the mother (Y-life); date of birth, serum protein at birth (SP, mg/ml), weight at birth (WB, kg), weight at 1 month of age (W1, kg); weight at 2 months of age (W2, kg), growth rate during the 1st month of age (GR1, g/day), growth rate during the 2nd month of age (GR2, g/day), growth rate during the first 2 months of life (GRs, g/day), age in days at weaning (DW, day), height at withers at first AI (H-AI, cm), age at first AI (AFAI, month), height at first calving (H-FC, cm), number of AIs per pregnancy for the first pregnancy (AI1), weight at first calving (WFC, kg), age at first calving (AFC, month), total fat yield in the first lactation (F1, kg), total protein yield in the first lactation (P1, kg), total milk yield in the first lactation (MY1, l), length of the first lactation (L1, week), length of the dry period (DP; day), interval between calving and fecundating insemination (ICFAI, day), number of AIs per pregnancy for the second pregnancy (AI2), age at second calving (ASC, month), total fat yield in the second lactation (F2, kg), total protein yield in the second lactation (P2, kg), total milk yield in the second lactation (MY2, l) and length of the second lactation (L2, week).
Possible effects of maternal genetics on performance were assessed by calculating the probability that daughters of the same dam fell into the same performance cluster. Possible effects of paternal genetics were assessed by determining the distribution of daughters from different fathers among the performance clusters. Individual influence was observed through the probability that a daughter showed similar milk productivity in the first and second lactations. Influence of calving season on milk productivity during subsequent lactations was assessed by comparing calving during the cold season (October to June) and hot season (July, August and September).
Statistical analyses
The present study relied on cluster analysis,Reference Hartigan49 a mathematical approach rarely used in biomedical research but widely used in numerous other fields, including machine learning, pattern recognition, image analysis, information retrieval and bioinformatics.Reference Lebart, Morineau and Piron50, Reference Elvira, Hernandez and Cuesta51 Cluster analysis involves grouping individuals into ‘clusters’ based on characteristics, such that members of one cluster are more similar to one another than to members of the total population. The resulting clusters can then be analyzed to uncover similarities (intra-cluster) and differences (inter-cluster). Cluster analysis has recently been used to define clusters of lactation curves Reference Elvira, Hernandez and Cuesta51, Reference Cardenas52 and thereby identify factors in the animals or their environment associated with particular types of lactation curve. The cluster method was performed as described in Elvira et al. Reference Elvira, Hernandez and Cuesta51 In brief, SPAD.N (‘Système Portable pour l’Analyse des Données’, version 5.6; DECISIA, France) was used through a ‘mixed strategy,’ which combines divisive and agglomerative techniques and is the recommended method for such a large amount of data.Reference Lebart, Morineau and Piron50 The distance observed, in order to discriminate clusters or types was Euclidean squared. The aggregation criterion for data was the minimal variance of Ward. The amount of clusters is determined by the aggregation process that describes the hierarchical tree (Fig. 1) and the histogram of aggregation. Finally, a ‘consolidation’ process follows, which consists of re-allocating each case to the most nearest cluster, by the k-average method. Afterwards, the characteristics in study were analyzed within each cluster and compared. The characterization of the clusters consists of the comparison of the values of the variables studied in the n k cases of the cluster C k with the values of the same variable in the total set (studied population). The comparison is performed between the mean value of the variable in the cluster and that of the population through the following statistical process:

Fig. 1 Hierarchical tree obtained after cluster analysis of weekly milk yield records for 404 first and 404 second complete lactations of 404 dairy cows. Four performance clusters or types were identified.
With ![]() $$\bar{V}_{j} $$
being the average of the variable V j calculated based on the total set of n cases and with
$$\bar{V}_{j} $$
being the average of the variable V j calculated based on the total set of n cases and with ![]() $$s_{j}^{2} $$
being the related variance.
$$s_{j}^{2} $$
being the related variance.
With ![]() $$\bar{V}_{k} $$
being the average of n k cases from the characterized cluster.
$$\bar{V}_{k} $$
being the average of n k cases from the characterized cluster.
Under the null hypothesis that the n k cases were placed by random into the cluster C k, extracting without re-emplacement among the n possible cases, the expected value and variance of ![]() $$\bar{V}_{k} $$
are calculated:
$$\bar{V}_{k} $$
are calculated:
Then, if n and n k are not too small, the following expression
follows approximately a normal distribution N(0,1).
When the null hypothesis is not rejected for one variable, this variable has no interest for the characterization of the cluster C k, because their values are not different from the n k values randomly chosen from the total set.
The method calculates a statistic for comparisons called the ‘T-value;’ this value is compared with a normal distribution N(0,1) to determine the significance level of the difference between the cluster average and the global average. In the case of categorical variables, the method works in a similar way. It characterizes the clusters based on frequency distribution of values of the categorical variables that are higher or lower than the global frequency. The significance level for differences between the cluster percentage and global percentage is assessed by comparing the T-value with a normal distribution N(0,1). In our study, significance was defined as P<0.001.
In addition, a cluster analysis was performed in the same form excluding lactations from daughters of primiparous cows (n=249), whose mothers were not lactating during pregnancy, in order to discard the metabolic advantage of non-lactating gestating dams. Relationships between continuous variables were assessed using Pearson correlation analysis. For these statistical analyses, SPSS® 19.0 (IBM, NY, USA) was used with a threshold of P<0.05 for significance and of P⩽0.10 for tendencies. All results are reported as mean±s.d.
Results
Characterization of cows
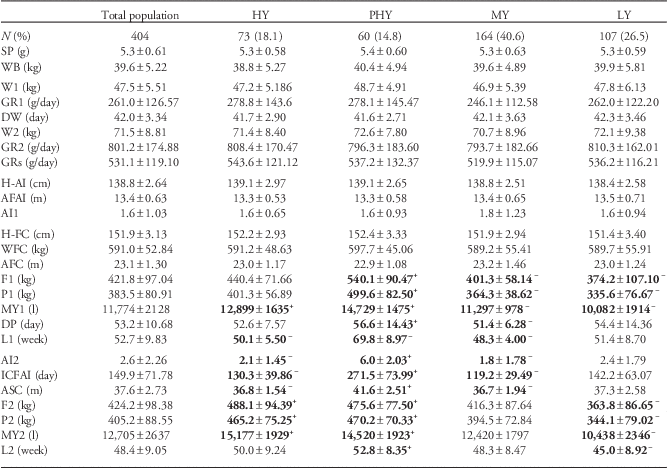
Table 1 summarizes data on growth patterns, metabolic performance (fat and protein yield per lactation) and reproductive performance of 404 dairy cows during the first two lactations. This corresponded to a mean period of 4.4 years of lifespan. Data are presented for the entire study population, as well as separately for the four performance types identified by cluster analysis (Fig. 2). High-yielding (HY) cows (n=73, 18.1% of the total) showed high milk yields in both lactations, that is, a mean of 12,899 l in the first and 15,177 l in the second. Persistently high-yielding (PHY) cows (n=60, 14.8%) showed not only high milk yields in both lactations, with a mean of 14,729 l in the first and 14,520 l in the second, but also high persistence in the first lactation. Lactation persistence can be defined as the ability of the cow to maintain milk yield (milk, fat and proteins) after achieving the maximum milk production. Medium-yielding (MY) cows (n=164, 40.6%) showed mean milk yields of 11,297 l in the first lactation and 12,420 l in the second. Low-yielding (LY) cows (n=107, 26.5%) showed mean milk yields of 10,082 l in the first lactation and 10,438 l in the second.

Fig. 2 Mean lactation curves for each cow performance cluster identified by analysis of 404 first lactation curves and 404 second lactation curves of Holstein cows, daughters of primi- and multiparous cows (a) and of 249 first lactation curves and 249 second lactation curves of Holstein cows daughters from multiparous cows (b). HY, high-yielding cows; PHY, persistently high-yielding cows; MY, medium-yielding cows; LY, low-yielding cows.
Table 1 Characterization of four Holstein dairy cow performance types: HY, PHY, MY and LY.

HY, high-yielding cows; PHY, persistently high-yielding cows; MY, medium-yielding cows; LY, low-yielding cows; SP, serum protein at birth; WB, weight at birth; W1, weight at 1 month of age; GR1, growth rate during the 1st month of age; DW, days at weaning; W2, weight at 2 months of age; GR2, growth rate during the 2nd month of age; GRs, growth rate during the first 2 months of life; H-AI, height at first artificial insemination; AFAI, age at first artificial insemination; AI1, number of artificial inseminations per pregnancy for first pregnancy; H-FC, height at first calving; WFC, weight at first calving; AFC, age at first calving; F1, total fat yield during the first lactation; P1, total protein yield during the first lactation; MY1, total milk yield during the first lactation; DP, length of the dry period; L1, length of the first lactation; AI2, number of artificial inseminations per pregnancy for second pregnancy; ICFAI, interval between calving and fecundating insemination; ASC, age at second calving; F2, total fat yield during the second lactation; P2, total protein yield during the second lactation; MY2, total milk yield during the second lactation; L2, length of the second lactation.
Data are shown as mean±s.d.
Superscripts ‘+’ and ‘−’ indicate values significantly larger or smaller than the corresponding mean value for the total study population (P<0.01). Significant values are highlighted in bold.
Differences in growth patterns, metabolic efficiency and life performance among different performance types
No relationship was found between growth pattern and cow performance, either across the entire study population or within each performance cluster separately (Table 1). However, we found a significant effect of maternal age at pregnancy on the metabolic efficiency and life performance of the offspring (Table 2).
Table 2 Maternal age and parity and productivity for the four Holstein dairy cow performance types: HY, PHY, MY and LY.
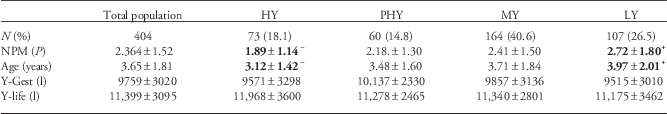
HY, high-yielding cows; PHY, persistently high-yielding cows; MY, medium-yielding cows; LY, low-yielding cows; NPM, number of lactations by the mother; Age, maternal age at parturition (years); Y-Gest, total yield during lactation of gestation of the daughter under study; Y-life, average yield/lactation in life.
Data are shown as mean±s.d.
Superscripts ‘+’ and ‘−’ indicate values significantly larger or smaller than the corresponding mean value for the total study population (P<0.01). Significant values are highlighted in bold.
HY and PHY cows showed significantly higher total milk yield in both lactations than the average (P<0.0001), as well as higher total fat and protein yields during the second lactation than the average (P<0.0001). PHY cows showed significantly greater persistence than the average in the first lactation (P<0.0001) and slightly greater persistence in the second lactation (P<0.01). MY cows gave significantly lower total yield, total fat and total protein than the average, and their first lactation was significantly shorter (all P<0.0001). The corresponding values for the second lactation, however, were similar between MY cows and the population average. LY cows produced significantly less total milk, fat and protein during both lactations than the average, and their second lactation was significantly shorter (all P<0.0001).
The four performance clusters showed similar ages at first fertile estrus and therefore at first calving (Table 1), indicating similarities in onset of reproductive life. However, the clusters showed significantly different ICFAI and therefore significantly different ages at second calving. HY cows showed shorter ICFAI than the population average (130.3±39.86 v. 149.9±71.78 days; P<0.001) and shorter ASC than the average (36.8±1.54 v. 37.6±2.73 months; P<0.005). Conversely, PHY cows showed a larger ASC than the average (41.6±2.51 months; P<0.0001). This longer ASC was attributable to a longer ICFAI (271.5±73.99 days; P<0.0001) and to the higher-than-average number of inseminations needed to achieve the second pregnancy (AI2; 6.0±2.03 v. 2.6±2.26; P<0.0001) and was independent of lactation persistence. MY cows showed the shortest ASC (36.7±1.94 months; P<0.0001), attributable to shorter-than-average ICFAI (119.2±29.49 days; P<0.0001) and AI2 (1.8±1.78; P<0.0001). LY cows achieved reproductive values similar to the average (Table 1).
Influence of parturition season
As research has yet to determine whether parturition season can affect dairy cow performance, we explored the distribution of parturition season among the performance clusters. The distribution was similar across all four clusters for the first calving (P>0.05), with approximately two-thirds of births occurring during the cold season: HY, 69.9%; PHY, 71.7%; MY, 59.8%; and LY, 72.0%. During the second calving, 81.5% of births occurred during the cold season, and this proportion was significantly higher in HY cows (90.1%) and significantly lower in PHY cows (58.3%; P<0.0001) than the average.
To assess for covariation in parturition seasonality across both the first and second calving, the percentage of the total study population falling into the following four groups was determined: CC, which had both calvings in the cold season, 57.9%; CH, which had the first calving during the cold season and the second during the hot season, 8.5%; HC, 23.7%; and HH, 10.0%. Compared with these population averages, PHY cows calved significantly less often in CC seasons (35.0%; P<0.0001) and significantly more often in the CH season (36.7%; P<0.0001). MY cows calved significantly less often during hot-cold season (1.8%) and more often in the HH season (14.0%; P<0.0001). LY cows showed a distribution among the four seasonal groups similar to that of the total population. Maternal parity did not significantly affect the seasonality of calving by daughters (data not shown).
Effects of parental and progeny genetics on progeny performance
To explore whether paternal genetics influences the life performance of daughters, we examined how the offspring of the 17 sires that fathered the 404 animals in the study population were distributed among the four performance clusters. All but two sires fathered similar numbers of cows of the four performance types. One exception was sire 12, who fathered significantly more LY cows than HY and MY cows (P=0.001). This sire fathered a total of 48 animals. The distribution of these animals was 3 in the HY cluster (4.1%); 10 in the PHY (16.7%); 12 in the MY group (7.3%) and 23 in the LY cows (21.5%). However, specifically this sire was improving milk yield in average, with a mean milk yield/lactation of their daughters of 12,011.6 l.
Another exception was sire 5, who fathered significantly more PHY cows than cows of the three other performance types (P=0.004). This sire fathered a reduced number of daughters (20 heifers) with the following distribution: two daughters in the cluster HY (2.7%); seven in the PHY (11.7%); five in the MY (3.0%) and six in the LY cluster (5.6%).
To explore whether maternal genetics influences the life performance of daughters, we analyzed the total of 27 cows that gave birth to two daughters included in the study population. Among these dams, 11 (40.7%) gave birth to two daughters who fell into the same performance cluster (HY, 2; MY, 7; LY, 2; P>0.05). The remaining dams gave birth to two daughters in different performance clusters.
In order to explore the individual offspring’s influence on performance, we examined whether performance indexes from the first and second lactations showed any relationships. This correlation analysis showed a significant relationship between the two lactations in terms of milk yield (r=0.357), total fat yield (r=0.211) and total protein yield (r=0.277; all P<0.0001).
Finally, no difference was found in milk yield during the gestation of the dam (Y-Gest), nor in the life milk yield of the mother (Y-life), among the different performance clusters (Table 2).
Influence of maternal age on the metabolic efficiency and life performance of daughters
The mothers had a mean age of 3.65±1.81 years when they conceived the cows in this study, corresponding to 2.36±1.52 lactations (Table 2). LY cows were born from the oldest cows (3.97±2.01 years, 2.72±1.80 lactations; P<0.005), whereas HY cows were born from the youngest cows (3.12±1.42 years, 1.89±1.14 lactations; P<0.005).
These effects of maternal age were further supported by cluster analysis, which showed significant differences in the distribution of maternal ages across the four performance types (P<0.005). The proportion of daughters of the youngest mothers (primiparous) was significantly higher in the HY cluster (49.3%) than in the total study population (38.4%). The HY cluster also contained a significantly smaller proportion of daughters of the oldest dams (11.0%) than did the study population (22.5%). At the other end of the performance spectrum, daughters of the oldest mothers were overrepresented in the LY cluster (30.8%) than in the population as a whole (22.5%).
To clear the difference between metabolic challenge suffered by the lactating pregnant dam from the age effect clusters were performed with the daughters of multiparous cows (only lactating dams during pregnancy). Clustering gave similar results with four clusters, with similar lactation curves (Fig. 2). The maternal age was again significantly lower in the cluster HY (2.93 years old; P=0.032) and significantly higher in the cluster of LY cows (3.55 years old; P=0.030).
In order to clear the effect of the age of the mother from the overall improvement observed in the herd during this time (this improvement includes genetic merit progress, as well as general management improvement in general), the average milk yield/lactation of the herd during the years of the study (2007–2013) and the average improvement of the herd, respective to 2007 (begin of the study), was compared (Fig. 3).

Fig. 3 Left: milk yield/lactation (line) of the herd during the years of the study (2007–2013) and average improvement of the herd respective to 2007 (bars). Right: milk yield/lactation of the first (black square) and second lactation (white dot) of the different clusters of cows and average improvement of each cluster respective to the mean average of the herd in 2007 (black and white bars for first and second lactation, respectively). HY: high-yielding cows; PHY: persistently high-yielding cows; MY: medium-yielding cows; LY: low-yielding cows.
Discussion
The present work provides a most rigorous and extensive exploration of DOHaD to date using a bovine model system. We analyzed individuals on a high-producing dairy farm where paternal and maternal genetic features were well known and optimized. The animals’ environment, including nutrition, health, season and stress, was strictly maintained within optimal conditions during pre- and postnatal life. This helps ensure that the findings are of direct value to other high-producing Holstein farms and of translational value to humans as a model of conditions in modern, high-income societies. However, an important difference between human societies and dairy herds has to be taken into account. This is the high genetic variability among humans, when compared with the homogeneity of Holstein population. Third, the powerful approach of cluster analysis, which defines ‘types’ within a general population and uncovers revealing differences, was successfully applied to genetic and other pre- and postnatal conditions to determine their influence on adult phenotype.
In the present study, we used cluster analysis to identify four Holstein cow performance types. Approximately 33% of our study population was classified as superior yielding, 41% as average yielding and 26% as low yielding. We then set out to determine whether these performance clusters differed in parental genetics, daughter genetics or environmental and developmental characteristics. Although our sample size was too small to detect paternal or maternal genetic influences, we did find evidence that the genetics of daughter cows affect their life performance. We observed a significant correlation of yields between the first and second lactations, supporting the possibility of modeling lifetime performance, as suggested earlier.Reference Hargrove, Salazar and Legates53–Reference Togashi and Lin55 In contrast, we failed to detect any influence of environmental and developmental features on adult performance and metabolic efficiency under these optimized living conditions.
In this way, our results support the findings of Swali and WathesReference Swali and Wathes56 that an animal’s birth weight does not affect its future performance. We found a similar lack of association with the level of passive immunity in the neonate. These findings may reflect the effective management practices on the farm under study, which may have masked the positive effects of sufficient passive immunity transfer.Reference Weaver, Tyler and VanMetre57 Indeed, the population average for passive immunity in our study was 5.3 g/dl, while the lower limit for sufficient passive transfer was 5.2 g/dl.Reference Windeyer, Leslie and Godden58 Further evidence for effective animal and environmental management on the farm in our study is that during the study period, overall mortality was 2.6% up to weaning and an additional 2.1% up to the first insemination; these values are lower than in studies of dairy calves in other parts of Europe and in North AmericaReference Donovan, Dohoo and Montgomery59–Reference Trotz-Williams, Leslie and Peregrine61, although they are similar to studies in Canada.Reference Windeyer, Leslie and Godden58, Reference Trotz-Williams, Leslie and Peregrine61
We failed to detect a relationship between the juvenile growth rate and adult performance, in contrast to several other studies.Reference Gardner, Smith and Park62–Reference Bach65 BachReference Bach65 observed that a growth rate of more than 0.8 kg/day between 12 and 65 days of age was associated with greater probability of completing the second lactation. Our failure to detect this relationship may be related to the specific characteristics of our study population or to our study design. In contrast to the study by Bach and co-workers, the animals in our study lived under optimal management and environmental conditions and they were included in the analysis only if they completed the second lactation. In fact, the growth rate in our animals between 30 and 60 days of age was 0.8 kg/day, consistent with the findings of Bach.Reference Bach65 Nevertheless, the total growth rate of our animals during the first 2 months of life was slower than that reported by those authors.
We did not detect any relationship between productive and reproductive characteristics during adulthood. The four performance clusters showed similar fertility (measured as inseminations needed to reach first pregnancy) and age at first parturition. This may again reflect effective management practices on the farm under study, as an average of only 1.6 inseminations were needed to achieve the first pregnancy and heifers were an average of 23.1 months old at the first parturition, which is within the interval of 22–24 months considered optimal for first parturition to maximize performance and longevity.Reference Lin, McAllister and Batra66–Reference Haworth, Tranter, Chuck, Cheng and Wathes69
The two most productive cow clusters, HY and PHY, differed significantly in their reproductive performance (fertility) for a second pregnancy when the cows were still in their first lactation. HY cows were more fertile, required fewer inseminations per pregnancy and became pregnant more quickly than did the average PHY cows, conversely, showed the longest interval to pregnancy and the highest number of inseminations per pregnancy of all four performance clusters. These findings suggest that higher milk production is not always associated with lower fertility; instead, the relationship between these variables is likely to be complex, as proposed by others.Reference Bello, Steibel and Erskine48
One might predict that the only way in which environment affects dairy cow performance under tightly controlled conditions like those on the farm under study is through seasonality. Previous studies have reported negative effects of heat stress during the last months of gestation and/or parturition in the hot season.Reference Tao and Dahl70 In the present study, season did not exert any significant effects on first calving. For the second calving, however, HY cows calved more often during the cold season than did the study population as a whole, whereas PHY cows calved more frequently during the hot season. LY cows did not show any significant seasonal effect for either calvings. Our results indicate that seasonal effects, although they exist, are not primary determinants of life performance.
Taken together, our findings suggest that for Holstein dairy cows living under well-controlled, optimal conditions, the primary determinants of adult performance are not developmental or environmental factors. Instead, as we show first using cluster analysis with lactations of all 404 cows and then confirm using clustering with lactation only from daughters of multiparous cows, maternal age appears to be the single most important determinant of the life performance and metabolic efficiency of the daughter. In the study population, which included all ages from the youngest (primiparous) dams to mothers aged 12.34 years, the metabolically efficient and high-producing HY cows were born from the youngest dams. Persistently high-producing PHY cows were born from the next youngest mothers, and the least efficient LY cows were born from the oldest. The genetic merit could hardly be the only cause of the fact that youngest dams give birth to most productive daughters, as productivity improvement found in this study has been 27–50% comparing the productivity of the dams (2007) with that of the daughters (2013; Fig. 3). ‘It is not easy to genetically improve milk yield, being a triumph of quantitative genetics that rates of genetic improvement in dairy cattle are now about 1% of the mean per year’.Reference Brotherstone and Goddard71 Moreover, the maternal life production as well as the maternal average production during gestation did not differ among clusters of daughters (Table 2). Finally, the improvement of the best clusters overcame notably the mean improvement of the herd achieved during this period (2007–2013), attributed to genetic and management improvement (19%; Fig. 3). Anyway, in order to discard genetic merit confounding effects, this issue should be deeply explored in future studies with a broader population.
Whether maternal age exerts these effects in the daughter through epigenetic changes or other mechanisms remains unclear. An epigenetic mechanism was suggested by studies linking maternal age with precocity of puberty onset.Reference Deng, Tao and Liu72 Several other studies, however, indicate that this association is not easily heritable, and that precocity of puberty onset is most likely associated with other heritable characteristics such as body development capacity.Reference Shiotsuki, Silva, Tonhati and Albuquerque73, Reference Deardorff, Berry-Millett and Rehkopf74 Thus, maternal age may be considered an ‘environmental’ factor that helps to determine fetal development by conditioning its metabolic environment. To draw this conclusion with more confidence, we would need to separate out the effects of maternal age from the effects of parity. This question is particularly important for relating animal models of DOHaD to humans, who in affluent societies are postponing childbearing later and later. The cows in our study are not models of late childbearing cows, as all first became pregnant at an early age. However, the evidence of the influence of the age of the mother on their offspring, even by second or third gestations need to be taken into account.
Our finding of a link between maternal age and the adult performance and viability of offspring is consistent with preliminary data reported in the fruit fly Drosophila spp.Reference Kern, Ackermann and Stearns75 and in bison.Reference Hamel, Craine and Towne76 Together, these studies support the DOHaD hypothesis that adult phenotype and performance are determined by the interaction between genetic predisposition and nutritional and metabolic conditions of the offspring during pre- and early postnatal development.Reference Hanson, Godfrey and Lillycrop77 Maternal aging in humans has been found to be associated with both metabolic alterationsReference Stene and Gale78 and deficiencies in placental developmentReference Kenny, Lavender and McNamee79, leading to adverse reproductive outcomes.Reference Kenny, Lavender and McNamee79, Reference Nelson, Telfer and Anderson80 The results of the current study provide a basis for clarifying systemic or intrauterine conditions that impair adult performance in the progeny of older females. At the same time, our findings may help improve management practices for high-yielding dairy farms.
Acknowledgments
The authors would like to thank Ramón Morla and the farm workers who managed the herd for their collaboration. A.G.B. is a member of the EU COST-Action BM1308 ‘Sharing Advances on Large Animal Models (SALAAM)’.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
None.