Introduction
According to David Baker, insults, such as malnutrition or hormonal disturbances, during critical periods of development may modulate organism physiology and metabolism, favoring noncommunicable diseases later in life, including cardiovascular diseases (CVDs)Reference Barker1. Epigenetic mechanisms underlying cell plasticity and adaptative responses are discussed to guarantee survival upon adverse conditionsReference Reynolds, Gray, Li, Segovia and Vickers2–Reference Langley-Evans4. As mammalian cardiomyocytes continue to differentiate and proliferate at the neonatal period, their hearts remain vulnerable after birthReference Crispi, Crovetto and Gratacos5–Reference Senyo, Lee and Kühn7. Thus lactation, as well as pregnancy, encompasses a critical period regarding cardiovascular developmentReference Langley-Evans4.
Literature reports higher levels of leptin in serum and breast milk of mothers with overweight and obesity. These leptin levels were also positively correlated to each other and maternal body mass indexReference Schuster, Hechler, Gebauer, Kiess and Kratzsch8,Reference Fields, George and Williams9 . Breastfeeding allows this adipokine transference to the offspring, influencing body composition and healthReference Fields, George and Williams9–Reference Teixeira, Passos, Ramos, Dutra and Moura11. Leptin, a hormone secreted by adipocytes, participates in several physiological processes, including energy balanceReference Elias and Purohit12.
Leptin serum concentration in early life seems to be relevant to developmental plasticity thus, the increase in leptin serum lead hyperleptinemia associated with chronic diseases in adulthood in rodentsReference Trevenzoli, Pinheiro and Conceição13,Reference Granado, Fuente-Martín, García-Cáceres, Argente and Chowen14 . Nevertheless, as important as the level itself is the exact moment in which leptin concentration changes in the suckling periodReference Granado, Fuente-Martín, García-Cáceres, Argente and Chowen14. Toste et al.Reference Toste, de Moura and Lisboa15 have demonstrated that leptin administration in newborn male Wistar rats during the first 10 of 21 d of lactation leads to several alterations: hyperleptinemia, hyperphagia, overweight, hyperinsulinemia, and hypertriglyceridemia in adulthood, explained by hypothalamic leptin resistance. Besides, Marques et al.Reference Marques and Rocha16,Reference Marques, Pinto, Nascimento and Scaramello17 have also characterized diastolic dysfunction in this experimental model in youth that evolved to systolic dysfunction in adulthood along with changes in the spontaneous and sympathetic response of isolated heart preparations compatible to heart failure.
CVDs constitute the leading cause of death worldwide18 and sex differences regarding these diseases have already been described by literature, which may affect clinical practiceReference Ventura-Clapier, Dworatzek and Seeland19. Although the cardioprotection addressed to estrogen in females during the reproductive period in animal modelsReference Posa, Szabó and Kupai20, there is a prospective study that reports a decrease of mortality rate due to CVD among men in the past years highlighting the relevance of their outcomes in women.Reference Mehta, Beckie and DeVon21
There are studies that also point to sex dimorphism related to developmental plasticityReference Souza, de Moura and Lisboa22,Reference Pietrobon, Bertasso and Silva23 . Thus, this work aimed to describe the consequences of leptin administration during the first 10 d of lactation on biometric, nutritional, hemodynamic, and cardiac outcomes in female Wistar rats highlighting the hypothesis that leptin programming could also susceptible to sex dimorphism.
Methods
The Ethics Committee for the Use of Animals of Federal Fluminense University (Niterói, Brazil) had previously approved this research before its beginning. The study conduction was also accorded to the Brazilian Society of Animal Science Experimentation (Sociedade Brasileira de Ciência em Animais de Laboratório, SBCAL) guidelines24. The ARRIVE guidelines for reporting animal research have oriented all the steps of the workReference Percie du Sert, Hurst and Ahluwalia25.
Animals and experimental model
All animals had free access to standard chow (Nuvilab®) and tap water at controlled conditions (22°C, 55–65% humidity, 12/12 h light/dark cycle). The breeding laboratory of the university has provided male (n = 5) and primiparous female (n = 10) Wistar rats about 3 months of age used for mating (F0 generation). They had no kinship and after 7 d of mating (two females for each male), the pregnant rats were placed in individual cages. Parameters observed to confirm pregnancy encompass increased abdominal circumference and behavioral changesReference Numan, Knobil and Neill26.
A total of 10–12 puppies were born per dam after 21 d of gestation (postnatal day [PND] 0). Aiming to avoid genetic bias, litters adjustment (six pups per mother) was performed cross-fostering the offspring at PND day 1Reference Li, Guenancia and Rigal27. The offspring were divided into Leptin and Control groups by simple randomization (F1 generation)Reference Bailoo, Reichlin and Würbel28.
Control – six pups/mother (three males and three females)
Leptin – six pups/mother (three males and three females)
The litter adjustment comprised the proportion of one female for each male to ensure the dam’s normal nursing behaviorReference Heijning, Oosting, Kegler and van der Beek29. One dam died during lactation and its offspring were euthanized. Three female offspring have died throughout the experimental period. Male rats were evaluated on a different work. Thus, a total of 24 female rats from the F1 generation were included in this study:
Control – 9 female rats – 4 litters,
Leptin – 15 female rats – 5 litters.
Leptin group received daily subcutaneous injections of mouse leptin (PeproTech Inc., London, UK) diluted in saline within the first 10 of 21 d of lactation (8 µg/100 g of body mass). Control group have received vehicle (0.9% NaCl) throughout the same period insteadReference Marques and Rocha16. Prepubertal and adult female offspring assessment happened at PND 30 and 150Reference Quinn30. Body mass and food intake monitoring comprised all the experimental period (three animals/cage). The conduction of all the assays occurred as described by Marques et al.Reference Marques and Rocha16 and Araújo et al.Reference Araújo, Farias and Pedro31.
Nutritional and biometric analysis
Body mass and food intake monitoring began upon weaning at PND 21. The determination of body weight gain occurred between PND 21–30 and 30–150 (Final body mass−Inicial body mass). The sum of food intake in the same periods allowed the calculation of feed efficiency (Weight gain/Σfood intake).
Nose-to-anus length was collected from anesthetized rats before echocardiography using a tape measure.
Systolic blood pressure recording
The rats were previously acclimated to restraint and tail-cuff inflation throughout 3 d for 10 min in the morning. The determination of systolic blood pressure occurred on the fourth day using awake rats and a noninvasively computerized tail-cuff system (NIBP controller, ML125; ADInstruments) connected to the ADInstruments PowerLab 8/30, ML870 digital–analog converter. Data were analyzed using LabChart 6 Pro software (ADInstruments, Bella Vista, New South Wales, Australia). The final values of systolic blood pressure used were the average of six successful recordings of each animal achieved in the absence of spontaneous tail movement.
Echocardiographic evaluation
The animals were previously anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg) intraperitoneally, and then submitted to the noninvasive transthoracic echocardiography using a portable ultrasound system (Acuson Cypress Plus, Siemens, DEU, Mountain View, CA, USA) and a 10-MHz transducer. Parameters assessed to allow cardiac structure and function evaluation: left ventricle internal diameter (LVID), interventricular septum thickness (IVS), and left ventricle posterior wall thickness (LVPW) in systole and diastole, as well as relative wall thickness (RWT), left ventricle mass (LVM), left atrium-to-aorta ratio (LA/Ao), systolic volume (SV), left ventricle ejection fraction (LVEF), fractional shortening (FS), and mitral deceleration time (DT). All parameters were measured at least three times per animal by a unique researcher. The assay conduction also followed the American Society of EchocardiographyReference Lang, Bierig and Devereux32.
Maximal effort ergometer test
The experiment occurred after 3 d of acclimation (daily exercise sessions of 10 min at 0.7–0.9 km/h). Some animals were nonresponsive and has been categorized as sedentary rats. Because of them, it was not possible to evaluate all animals submitted to previous assays.
The test comprised an adapted treadmill for rats (Imbrasport®, Brasília) and the protocol does not include inclination. The initial speed of 0.9 km/h was followed by progressive increments of 0.3 km/h every 3 min until exhaustion (rats remaining still for at least 10 s despite stimuli). The parameters recorded were distance traveled, time spent, and maximum speed developed in the test.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7.0 software (USA). Shapiro–Wilk test allowed the evaluation of data normality and homoscedasticity and guided the test used to compare Control and Leptin groups within the same age (unpaired Student’s t-test – parametric data – or Mann–Whitney test – nonparametric data). Values are expressed as mean ± standard deviation. Significance accepted comprised a p < 0.05.
Results
Nutritional parameters
Data points differences regarding nutritional parameters only between PND 21 and 30. Table 1 shows that female rats from the Leptin group presented lower food intake (−13.8%, p = 0.0003) and higher feed efficiency (+28%, p = 0.0058).
Table 1. Nutritional parameters
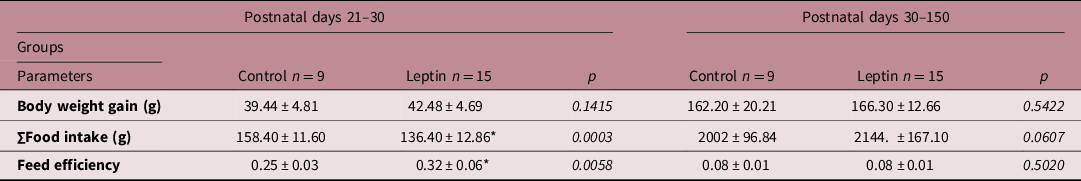
Values are expressed as mean ± standard deviation. Data were analyzed using unpaired t-test or Mann–Whitney test.
* P < 0.05 Leptin versus Control group.
Biometric parameters
Body weight and nose-to-anus length were similar between prepubertal and adulthood females from Leptin and Control groups (Table 2).
Table 2. Biometric parameters
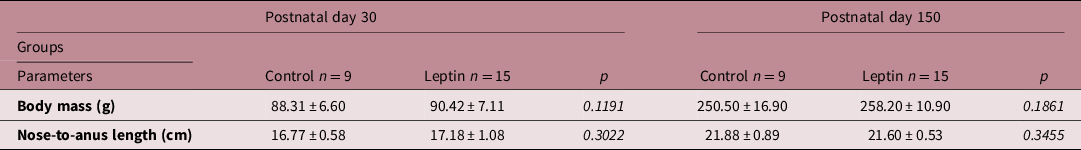
Values are expressed as mean ± standard deviation. Data were analyzed using unpaired t-test or Mann–Whitney test. Significance was accepted if P < 0.05 (Leptin versus respective Control group). No statistical difference were observed between groups at postnatal day 30 nor 150.
Echocardiographic and Hemodynamic analysis
Table 3 highlights some differences between Leptin and Control groups regarding echocardiographic but not hemodynamic data. Prepubertal female rats submitted to leptin administration presented high values of LVIDs (+50%, p = 0.0051) and remained higher in these animals (+33.3%, p = 0.0270) in adulthood. In addition, prepubertal animals from the Leptin group presented lowers values of LVEF (−4.4%, p = 0.0111) and FS (−9.2%, p = 0.0405).
Table 3. Echocardiographic and hemodynamic parameters
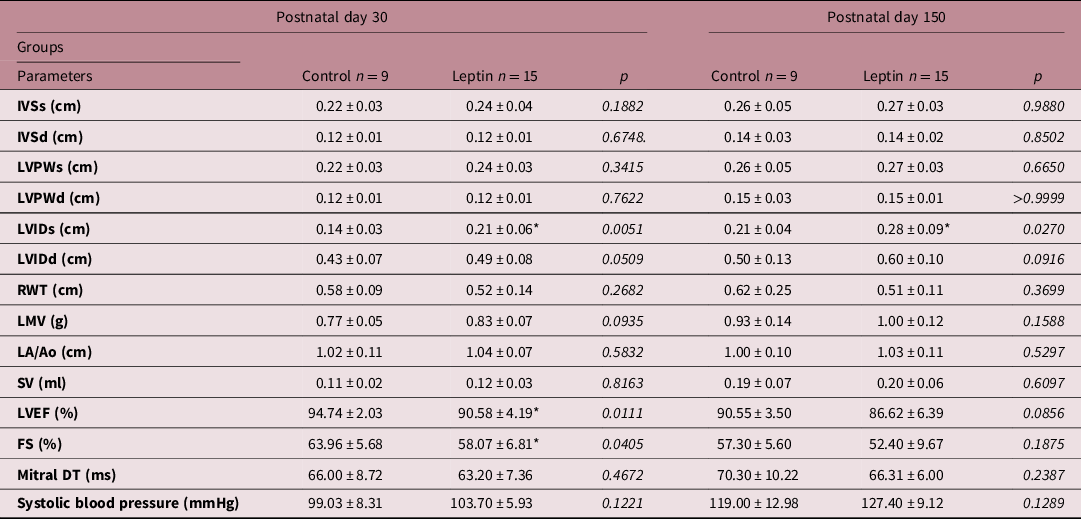
d, diastole; FS; fractional shortening; IVS, interventricular septum thickness; LVPW, left ventricle posterior wall thickness; LVID, left ventricle internal diameter; RWT, relative wall thickness; LVM, left ventricle mass; LA/Ao, left atrium-to-aorta ratio; SV, systolic volume; LVEF, left ventricle ejection fraction; Mitral DT, mitral deceleration time; s, systole.
Values are expressed as mean ± standard deviation. Data were analyzed using unpaired t-test or Mann–Whitney test.
* P < 0.05 Leptin versus Control group.
Maximal effort ergometer test
Within the same age, Leptin and Control groups presented a similar performance on the maximal effort ergometer test in all parameters analysed (Fig. 1a–c).
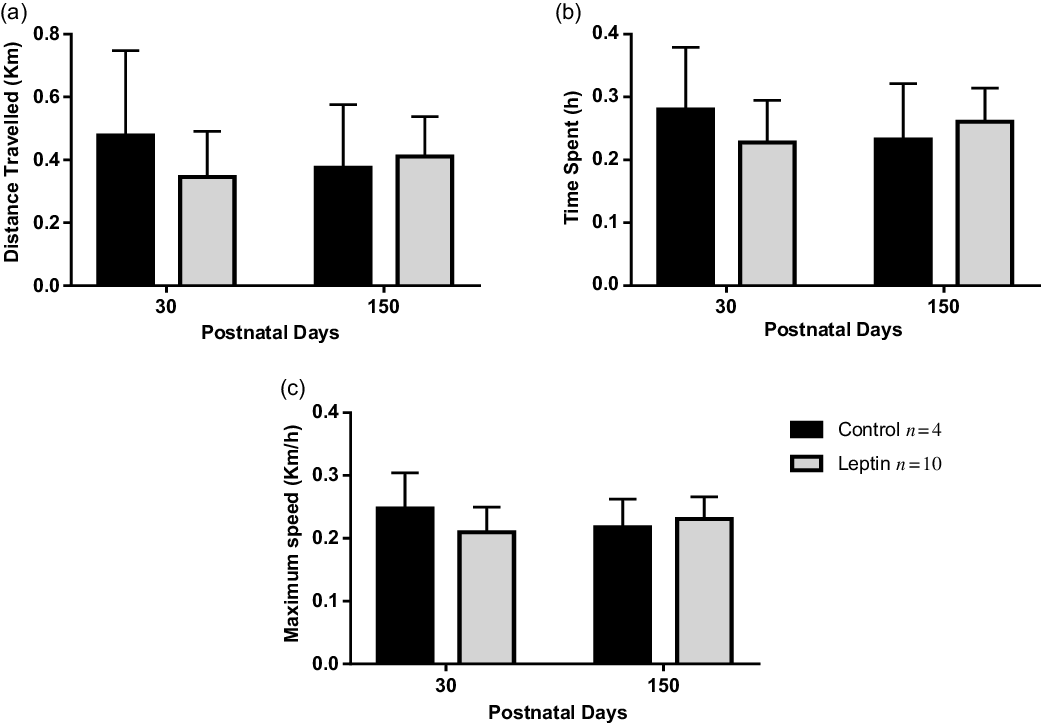
Fig. 1. Data from maximal effort ergometer test (Control n = 4, Leptin n = 10). (a) Distance traveled, (b) time spent, and (c) maximum speed developed. Values are expressed as mean ± standard deviation. Data were analyzed using unpaired t-test or Mann–Whitney test.
*P < 0.05 Leptin versus Control group.
Discussion
In this work, leptin administration led to hypophagia and higher feed efficiency in prepubertal animals. Echocardiographic data showed a slightly reduced LVEF and FS in youth. However, these data do not reflect functional impairment. Independently of the age, the LVIDs was higher in the Leptin group. No differences were observed regarding systolic blood pressure and performance on the maximal effort ergometer test. It is essential to highlight that these outcomes are different from those reported for male rats submitted to the same experimental protocolReference Marques and Rocha16.
The nutritional profile suggests that neonatal leptin administration altered the metabolism of prepubertal female rats but not adult onesReference Wideman and Murphy33. The early decrease in food consumption seems to be accompanied by a reduction in energy expenditure. Toste et al.Reference Toste, de Moura and Lisboa15 reported higher serum levels of insulin due to neonatal leptin administration. The literature describes a role for leptin and insulin on energy balance and food intake, highlighting the interaction between these hormones’ signaling pathways in the hypothalamusReference Elias and Purohit12,Reference Hermsdorff, Vieira and Monteiro34–Reference Maior36 . Nevertheless, unlike male ratsReference Toste, de Moura and Lisboa15, data does not indicate the development of leptin’s central resistance in adult female rats in this experimental model. According to the literature, male rats seem to be more susceptible to obesity than females. This observation may be explained by estrogen’s role in energy homeostasis and neuropeptides secretion, increasing anorexigenic neuropeptides, and decreasing orexigenic neuropeptides levelsReference Litwak, Wilson and Chen37–Reference Sharma and Prossnitz41.
According to the literature, leptin has a stimulating effect over the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. Besides, this adipokine can enhance the secretion of luteinizing (LH) and follicle-stimulating (FSH) hormones by the anterior pituitary, acting directly on estrogens synthesisReference Matysková, Zelezná and Maixnerová42. However, Pietrobon et al.Reference Pietrobon, Bertasso and Silva23 have observed that the relationship between leptin and estrogen levels depends on the experimental model of metabolic programming. While non-pharmacological early weaning promoted hyperphagia and hyperleptinemia without differences regarding estradiol levels in adult females, pharmacological early weaning determined normophagia, normoleptinemia, and reduced plasma estradiol concentrations.
In agreement to literature, body mass and nose-to-anus length can be used as biometric parameters to discuss adiposity in male and female ratsReference Leopoldo, Lima-Leopoldo and Nascimento43–Reference Fouda, Tom, Atsamo, Bonabe and Dimo45. No cutoff points were established for female rats. However, we have observed body mass and nose-to-anus length values similar to Araújo et al.Reference Araújo, Farias and Pedro31 Thus, according to this reference, prepubertal and adult female rats submitted to neonatal leptin administration did not present an increase in cardiometabolic risk related to an adiposity increment. These data are different from those observed by Toste et al.Reference Toste, de Moura and Lisboa15 who studied male rats submitted to neonatal leptin administration.
Besides similar biometric parameters, no difference regarding systolic blood pressure was seen within female Leptin and Control groups independently of the age. These findings may be due to leptin levels/activity. Previously, hyperleptinemia has been related to higher values of systolic blood pressure and heart rate in adult male rats of the same experimental modelReference Trevenzoli, Valle and Machado46. Trevenzoli et al.Reference Trevenzoli, Pinheiro and Conceição13 suggested that hyperleptinemia increases adrenal medullary function through sympathetic nervous system activation. The high leptin levels on lactation program the sympathoadrenal system’s activity in adulthood and it may contribute to the development of adult chronic diseases such as hypertensionReference Trevenzoli, Pinheiro and Conceição13. Leptin signaling seems to be necessary for the increased systolic blood pressure induced by obesityReference Simonds, Pryor and Ravussin47. However, a recent study has dissociated hypertension development in obese individuals and leptin presenceReference von Schnurbein, Manzoor and Brandt48.
Echocardiographic data shows an increased LVIDs in female rats of the Leptin group suggesting decreased ventricular compliance. This abnormality may be a sign of dilated cardiomyopathyReference Lakdawala, Winterfield and Funke49. This structural change was not accompanied by functional injury as the differences seen between prepubertal groups regarding LVEF and FS were insufficient to address systolic dysfunction. The values recorded to Control and Leptin groups are similar to those attributed to normal function by literatureReference Souza, Dos-Santos and Silveira50,Reference Yu, Shun-Guang, Weiss and Felder51 . In contrast, Marques et al.Reference Marques and Rocha16 have shown that leptin administration during lactation programmed cardiac structural and functional changes both in young and adult male rats. These observations may also explain the differences noticed between sex regarding the maximum effort ergometer test. While young and adult male rats submitted to neonatal leptin administration have traveled a shorter distance during a shorter test, developing a lower maximal velocityReference Marques and Rocha16, no differences were noticed between females concerning these parameters. An important symptom of diastolic dysfunction is exercise intolerance, which can be assessed by cardiopulmonary exercise testsReference Kitzman and Groban52. Maximal effort ergometer tests have already been successfully applied previously to assess cardiorespiratory capacity in ratsReference Marques and Rocha16,Reference Araújo, Farias and Pedro31 . Literature provides a linear relationship between maximum speed and oxygen consumptionReference Rodrigues, Figueroa and Mostarda53.
Estrogen has been recognized as a cardioprotective hormone due to its direct and indirect actions on myocardial cells and blood vessels. In animal models of CVD, adult females exhibited lower mortality and vascular injury, cardiac function preservation, and slower progression to decompensated heart failure than males. Estrogen deprivation mitigated this cardioprotectionReference Wang, Keimig and He54–Reference Lagranha, Deschamps, Aponte, Steenbergen and Murphy57. On the other hand, estrogen reposition was able to increase cardiomyocytes’ survival in a murine model of infarction, prevent hypertrophy in cardiomyocytes’ culture, and improve cardiac function in the isolated hearts of gonadectomized ratsReference Patrizio and Marano58,Reference Baka, Hodosy and Krajcirovicova59 . According to literature, the activation of membrane-bound receptor G protein-coupled estrogen receptor (GPER) and estrogen receptor beta (ERβ) modulates Ca2+ homeostasis. The increased expression of sarco/endoplasmic reticulum Ca2+−ATPase and phospholamban improves cardiomyocytes contractilityReference Schuster, Mahmoodzadeh and Dworatzek60,Reference Alencar, da Silva and Lin61 . In addition, GPER activation by specific agonists may reduce infarct size after myocardial ischemia-reperfusion, preserving cardiac function through phosphatidylinositol 3-kinase-dependent signaling pathwaysReference Deschamps and Murphy62.
All data together suggest sex dimorphism concerning different outcomes related to neonatal leptin administration. The measurement of leptin and estrogen levels should contribute to a better understanding of the mechanisms underlying these findings. Although the lack of these data constitutes limitations, it does not compromise the relevance of them. Besides, cardiac outcomes were similar comparing female Leptin and Control groups independent of the age. Literature also reports that sex differences may be related to sex chromosomes, products of genes located on the X and Y chromosomes, not only to gonadal hormonesReference Wang, Bingaman and Huxley63–Reference Ngun, Ghahramani, Sánchez, Bocklandt and Vilain66.
In conclusion, this study suggests that female Wistar rats are less susceptible to cardiac programming due to neonatal leptin administration than male rats, contributing to a neglected research area named Gender Medicine. Further studies are welcome to investigate better sex differences and the underlying cardiac structure and function preservation mechanism in female rats.
Acknowledgments
This study was financial supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (grants numbers E-26/200.964/2017 and E-26/203.400/2015), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (fellowships received by Karyne Pollo de Souza and Emiliana Barbosa Marques), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (fellowship received by Samuel Pedro).
Conflicts of interest
None.
Ethical standards
The authors declare that all proceedings adopted in this study were under the approval of the Ethics Committee of Fluminense Federal University (protocol number CEUA/UFF812-16).






