Introduction
Oligohydramnios or decreased amniotic fluid volume (AFV) occurs in approximately 0.5%–5% of pregnancies.Reference Rossi and Prefumo1 Several etiologies may cause oligohydramnios including fetal causes (e.g., genitourinary anomalies), placental causes (e.g., placental insufficiency),Reference Apel-Sarid, Levy, Holcberg and Sheiner2 maternal causes (e.g., hypertensive disease and renal disease), pharmacological agents (e.g., angiotensin-converting enzyme inhibitors and nonsteroidal anti-inflammatory drugs),Reference Guron and Friberg3–Reference Abou-Ghannam, Usta and Nassar6 and premature rupture of membranes (PROM).Reference Moore7–Reference Harman9 Isolated oligohydramnios (IO) also known as idiopathic oligohydramnios accounts for less than half of the oligohydramnios casesReference Feldman, Friger, Wiznitzer, Mazor, Holcberg and Sheiner8,Reference Gizzo, Noventa and Vitagliano10 and is believed to arise from decreased placental perfusion and hypoxemia, leading to a diminished generation of fetal urine.Reference Underwood, Gilbert and Sherman11
While some studies reported that IO is not associated with adverse perinatal outcomes,Reference Magann, Chauhan, Kinsella, McNamara, Whitworth and Morrison12–Reference Ott14 other studies suggest differently. Previously reported perinatal complications associated with oligohydramnios include meconium-stained amniotic fluid, low Apgar scores, admissions to neonatal intensive care unit, congenital malformations, and even neonatal death.Reference Hsieh, Hung, Chen, Hsieh, Lo and Chiu15,Reference Leibovitch, Kuint, Rosenfeld, Schushan-Eisen, Weissmann-Brenner and Maayan-Metzger16 Increased rates of labor induction and cesarean deliveries were also shown to be associated with IO.Reference Shrem, Nagawkar, Hallak and Walfisch17,Reference Rabie, Magann, Steelman and Ounpraseuth18
However, little has been reported regarding long-term outcomes in children born after pregnancies complicated by IO, especially when assessed as an independent variable. Long-term complications of IO were previously reported by Chien et al.Reference Chien, Chiou, Wang, Yeh and Chen19, in which an increased risk of respiratory-related hospitalizations was suggested in IO exposed offspring. In addition, an increased risk for cerebral palsy (CP) or even death at 18 months was described by Sasahara et al.,Reference Sasahara, Ishii and Umehara20 though, unlike our study, this study was designed for preterm small for gestational age infants born with oligohydramnios. Unfortunately, no validated evidence regarding a possible association between IO and long-term neurological morbidity of the offspring was found.
This study was constructed due to the high index of suspicion based on these reports as well as the overall limited evidence implying short- and long-term complications, and based on the notion in which an inappropriate environment for the fetus may project on its well-being and development,Reference Godfrey and Barker21–Reference Wallack and Thornburg23 thus drafted in search of evidence for long-term offspring neurological morbidity in pregnancies complicated with IO.
Methods and materials
A population-based retrospective cohort study approved by the Institutional Review Board, including all births that occurred between the years 1991 and 2014 at a tertiary university medical center (“Soroka” – SUMC) was conducted. SUMC is the single tertiary and birth center in the region, with a computerized database which includes data on all deliveries that occurred at the facility since 1991, including pregnancy follow-up and newborn information. Although these data were collected prospectively, it is analyzed as a retrospective design. All deliveries occurring in SUMC are documented by the in-charge obstetrician and later validated by designated medical secretaries. For the current study, data were retrieved from the obstetric computerized database combined with the pediatric hospitalization dataset. The pediatric dataset contains all emergency room (ER) visits and hospitalizations up to 18 years of age. Neurological-related hospitalizations of offspring were compared between the IO exposed group and all other pregnancies (“unexposed”). Pregnancy complications that are associated with oligohydramnios were excluded: multiple pregnancies, any type of diabetes mellitus, intrauterine growth restriction, pregnancy-related hypertensive disorders, congenital malformations, placental abruption, RH-isoimmunizations, and PROM, as well as polyhydramnios. Thus, the IO group contains idiopathic cases only without multiple pregnancy complications cases. All neurological diagnoses received during any hospitalization of all offspring up to 18 years of age were included in the analysis. These were classified using the international classification ICD-9 system (as shown in Supplementary Table S1).
All pediatric ER visits and hospitalizations are documented in the patient’s medical record. Diagnosis of all offspring morbidities mentioned in any pediatric medical records was collected and stratified by system into several main categories, including all neurological morbidities. These morbidities are defined according to the ICD-9 (as shown in Supplementary Table S1). All neurological diagnoses received during any hospitalization of all offspring up to 18 years of age were included in the analysis.
Follow-up time was defined as the time between the date of birth and the first neurological-related hospitalization or until censored. Censoring occurred in case of death (during hospitalization, other than neurological-related) or at age 18 (which was calculated for each child based on date of birth). Only the first hospitalization for each child was included in the analyses.
Background maternal characteristics included age at delivery, parity, and gravidity. The index pregnancy and obstetrical covariates included gestational age upon delivery, preterm delivery (defined as occurring before 37 weeks of gestation and before 34 weeks of gestation), and labor induction. The perinatal covariates analyzed included fetal gender, birthweight, low birthweight (<2500 g), macrosomia (>4000 g), and low Apgar score at 1 and 5 min (<7).
Since SUMC is the only tertiary medical center in the Negev region (south of Israel) which serves all the Negev residents, the study population was nonselective.
Statistical analysis
Since this was a retrospective cohort study, the “exposed” offspring were compared to the “unexposed” offspring. All offspring with IO were defined as exposed, and all offspring without this diagnosis served as the comparison group. The dependent variable was considered a first hospitalization with neurological diagnosis (event), while the independent variable was the presence or absence of oligohydramnios (exposed vs. unexposed).
Univariable analysis was performed to compare dependent and background characteristics between the two study groups. Cumulative incidence rates were compared using a Kaplan–Meier survival curve, and the log-rank test was used to determine significant differences. The Weibull survival parametric model (which adjusted for maternal multiple occurrences within the database, i.e., sibling) was conducted to compare neurological-associated hospitalizations risk by status of oligohydramnios (yes/no). The model adjusted for potential confounders based on the univariable analysis and on clinical relevance. The final model was chosen based on best fit and minimal −2log likelihood.
Results
During the study period, 190,259 singleton deliveries met the inclusion criteria, of which 2.13% (n = 4063) were complicated with IO and composed the exposed group. The number of neurological-related hospitalizations of offspring up to the age of 18 years, for both the exposed and unexposed pregnancies, was 5655 (2.97%).
Table 1 presents the background characteristics of the study groups. Primiparity was more common in the IO group, while maternal age did not significantly differ between the study groups. The IO group also demonstrated a higher rate of labor induction (62.6% vs. 21.2% in the unexposed group, p < 0.001) and infants in the IO group exhibited lower mean birthweight as compared to normal AFV (3107 and 3240 g, p < 0.001).
Table 1. Characteristics of the study groups

AFV, amniotic fluid volume; SD, standard deviation.
All numbers in table represent n (%) except where otherwise mentioned.
Table 2 details selected long-term neurological diagnoses of the offspring according to the presence or absence of IO. The study groups demonstrated a significant difference in total neurological-related hospitalizations (3.7% in the IO group and 3.0% in the comparison group, p = 0.005). Specific neurological diagnoses found to be more common in the exposed group included pervasive developmental disorder (PDD), movement disorders, developmental disorders, and degenerative and demyelization disorders.
Table 2. The association between amniotic fluid volume (isolated oligohydramnios vs. normal) and offspring long-term neurological morbidity

AFV, amniotic fluid volume; RR, relative risk; CI, confidence interval; PDD, pervasive developmental disorder; CP, cerebral palsy; ADHD, attention-deficit hyperactivity disorder.
Fig. 1 depicts the cumulative hazard for any neurological hospitalization of the offspring, accounting for hospitalizations occurring within the child’s first 18 years of age. A higher cumulative hospitalization incidence rate was demonstrated in the IO group as compared with the unexposed group (log-rank, p = 0.001).
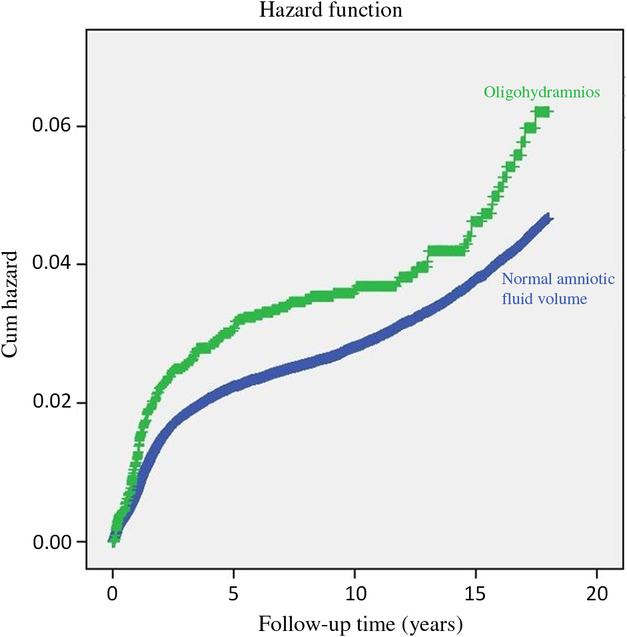
Fig. 1. Survival curve demonstrating the cumulative incidence of neurological hospitalization in the offspring to pregnancies complicated by isolated oligohydramnios and normal amniotic fluid volume. *Log-rank (Mantel–Cox) p = 0.001.
Table 3 presents the results of the Weibull multivariable analysis evaluating the association between AFV and neurological-associated hospitalization risk, while adjusting for gestational age (in weeks), maternal age, and induction of labor. Exposed offspring were 1.203 (95% CI 1.022–1.417) more likely to be hospitalized due to a neurological cause as compared with their unexposed counterparts.
Table 3. Multivariate analysis for prediction of offspring long-term neurologic morbidity

Adj. HR, adjusted hazards ratio.
Discussion
The impact of oligohydramnios with no known underlying pathology on long-term outcomes of offspring remains uncertain. Most of the previously reported evidence refers to perinatal short-term consequences, and little evidence is proposed regarding any potential long-term impact this exposure may bare on offspring. The main finding of the study is a confirmation of an independent association between pregnancies complicated with IO and long-term neurological-related hospitalizations of the offspring, as compared with unexposed offspring.
The specific neurological diagnoses found significantly more often in the IO group were PDD, movement disorders (including epilepsy), developmental disorders, and degenerative and demyelization disorders. No difference was found, however, in CP rates between the groups, as opposed to a previous report by Sasahara et al.Reference Sasahara, Ishii and Umehara20 The discrepancy may be attributed to the inclusion of preterm births only in Sasahara’s report. In that report, the study group comprised of deliveries between 22 and 33 weeks of gestation, while an independent risk factor for long-term unfavorable neurological outcome.Reference Moster, Lie and Markestad24,Reference Raju, Pemberton and Saigal25 The current study did not exclude preterm births, though as shown in Table 1 the incidence of preterm births <34 weeks was not significantly different to that with normal AFV, 0.8% and 0.6% in the exposed and unexposed groups, respectively. Interaction effect between preterm births and IO showed an association between IO and CP only in the presence of preterm births, and for this reason, this association was not found in our study.
The association between long-term neurological morbidity and IO may have several possible underlying causes. The first is related to the role of amniotic fluid in fetal development. It is well established that the amniotic fluid is important for fetal growth and protection, both for cushioning and for immunity.Reference Underwood, Gilbert and Sherman11 In fact, the effects of scant space and mechanical pressure in utero caused by decreased amniotic fluid on limb or lung development are discussed widely in both animal models and humans.Reference Chien, Chiou, Wang, Yeh and Chen19,Reference Wu, Chen and Chou26–Reference Christianson, Huff and McPherson29 We suggest that an abnormal uterine environment in which the fetus grows exposed to decreased volume of amniotic fluid and limited space may also cause subsequent neurodevelopmental abnormalities.Reference Roberts, Nwosu and Walkinshaw30 This insufficient space may affect neurodevelopment in utero, directly or indirectly, and manifest only later in childhood.
A second pathophysiologic explanation for the association between IO and long-term neurologic morbidity relates to the etiology of IO, believed to arise from decreased placental perfusion and hypoxemia, leading to diminished generation of fetal urine.Reference Underwood, Gilbert and Sherman11 This mechanism may provide another evidence for a stressful fetal environment, granted diminished perfusion. The lack of oxygen and blood flow redistribution is thought to have a negative effect on fetal development, and particularly the nervous system.Reference Altshuler31
SUMC is a sole tertiary medical center serving the entire population of the Negev region in Israel, providing both maternity and pediatric services and hospitalizations. This enables long-term follow-up of major health issues of the offspring and little loss to follow-up. Together with the large cohort size, these are the current study’s significant advantages. Several limitations to the study should also be acknowledged with the main limitation being its retrospective nature. As such, this study cannot suggest causation, but only association between IO and long-term neurological complications in the offspring. Moreover, several variables that may have potential significance such as parental neurological illness and other environmental or social factors in the life of the affected offspring cannot be assessed. In addition, the dataset included only neurological-related hospitalizations and lacks cases of ambulatory care or diagnoses that did not require hospitalizations during the disease course and treatment. This limitation may further explain the difference between CP prevalence in a previous studyReference Sasahara, Ishii and Umehara20 and the current study. There may be a degree of underdiagnosis in our study, since many CP cases do not require hospitalization and the question of a selection bias may evoke. The inclusion of hospitalized patients to the current study may evoke the question of a selection bias. While there is a possible selection bias, it would be non-differential and not dependent on the exposure. Therefore, our results maybe an underestimation of the true association. Finally, the study was based on defining oligohydramnios as Amniotic Fluid Index (AFI) of less than 5 cm, a system widely used, though there is some debate concerning the best way to assess AFV as part of fetal examination.Reference Nabhan and Abdelmoula32–Reference Chauhan, Sanderson, Hendrix, Magann and Devoe34 And within medical records limitations, we were unable to quantify the AFV from which an impairment is expected.
In conclusion, we report an association between pregnancies complicated by IO and neurological-related hospitalizations of the offspring. Further studies are essential to establish this finding and better understand the association. Only prospective studies will answer the question of causation, thereby suggesting methods of valuable interventions.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S2040174419000795
Acknowledgments
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Avital Dorot wrote the first draft of the manuscript. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Conflict of interest
None.
Ethical standards
This study was conducted as part of the requirements for MD degree from the Goldman Medical School at the Faculty of Health Sciences, Ben-Gurion University of the Negev.







