Introduction
Intrauterine growth restriction (IUGR) impacts up to 15% of pregnancies and increases the risk of obesity, thus contributing to the global obesity epidemic.Reference Botero and Lifshitz 1 , Reference Gluckman, Hanson and Pinal 2 With the frequent consumption of a high-fat diet (HFD) worldwide and the association of obesity with hypertensive disorders of pregnancy, increasingly IUGR infants are born to mothers that consume an HFD or are obese.Reference King 3 – Reference Cinar, Timur and Aksoy 8 In certain populations, even up to half of infants born to obese mothers are IUGR.Reference Radulescu, Munteanu, Popa and Cirstoiu 6 Both fetal exposure to a maternal HFD and IUGR independently predispose the individual to adult-onset obesity.Reference Demicheva and Crispi 9 – Reference Boney, Verma, Tucker and Vohr 12 Importantly, fetal exposure to a maternal HFD and IUGR independently increase the risk of developing obesity even when dietary and lifestyle choices are taken into account, suggesting a role for fetal programming of increased obesity risk.Reference Boney, Verma, Tucker and Vohr 12 – Reference Whitaker 18 Obesity that develops secondary to an adverse in utero environment is often depot-specific, with the visceral adipose tissue (VAT) increasing in excess to the subcutaneous adipose tissue (SAT). Furthermore, in children increased visceral adipose that develops secondary to an adverse in utero environment occurs without changes to the body mass index (BMI), thus making the clinical determination of increased visceral adipose challenging.Reference Rolfe Ede, Loos and Druet 19 Importantly, increased visceral adiposity in childhood increases the risk of developing metabolic syndrome, which increases the risk of cardiovascular disease and early mortality.Reference Kim, van de Wall and Laplante 20 – Reference Owens, Gutin and Ferguson 26 Multiple studies demonstrate the persistence of epigenetic and physiological changes resulting from an adverse perinatal environment both into adulthood and to subsequent generations despite consumption of a healthy diet in the offspring.Reference Wei, Yang and Wei 27 , Reference Vadlamudi, Kalhan and Patel 28 Despite the clinical importance of the perinatal environmental impact on obesity risk, it is unknown whether IUGR that occurs in the setting of a maternal HFD enhances the maternal HFD-induced offspring obesity risk.
Understanding how the in utero environment impacts future adiposity is critical, as current clinical recommendations primarily emphasize maternal weight gain and caloric intake, not diet composition, during pregnancy.Reference Rasmussen, Catalano and Yaktine 29 – Reference Kennedy and Meyers 31 Yet, it is not known if there are overlapping mechanisms or cumulative effects of IUGR and a maternal HFD on developing increased visceral adiposity. One potential mechanism includes disruption to glucocorticoid-mediated fat deposition. Increased adiposity secondary to an increased glucocorticoid effect can be the result of excess secretion of circulating glucocorticoids by the hypothalamic–pituitary–adrenal axis or liver, increased intracellular glucocorticoid receptor (GR) density, or dysregulated intracellular glucocorticoid metabolism via the enzyme 11-β hydroxysteroid dehydrogenase 1 (11-β HSD 1) (as reviewed by Oakley and CidlowskiReference Oakley and Cidlowski 32 ). Conditions that increase circulating glucocorticoid levels increase visceral adiposity,Reference Newell-Price, Bertagna, Grossman and Nieman 33 and increased glucocorticoids modulate adipogenesis and increase the risk of developing metabolic syndrome.Reference Masuzaki, Paterson and Shinyama 34 – Reference Lee, Pramyothin, Karastergiou and Fried 39 Glucocorticoid signaling induces transcription of proadipogenic factors through activation of GR isoforms, including active GR isoform (GR-α).Reference Bamberger, Bamberger, de Castro and Chrousos 40 , Reference Oakley, Jewell, Yudt, Bofetiado and Cidlowski 41 Binding of glucocorticoid to GR-α induces transcription of proadipogenic and lipogenic factors, such as ATP-binding cassette sub-family A member 1 (Abca1).Reference Phuc, Friedman and Schug 42 The adipocyte enzyme 11-β HSD 1 catalyzes the conversion of inactive glucocorticoid (11-dihydrocorticosterone in rodents, cortisone in humans) to active glucocorticoid (corticosterone in rodents, cortisol in humans).Reference Atanasov, Nashev, Schweizer, Frick and Odermatt 43 Via generation of active glucocorticoids, overexpression of 11-β HSD 1 induces visceral adipose accumulation in rodents, while 11-β HSD 1 knock-out mice show reduced visceral adiposity.Reference Masuzaki, Paterson and Shinyama 34 , Reference Kotelevtsev, Holmes and Burchell 44
Increased glucocorticoids also induce the adipocyte differentiation and lipid metabolism regulator, peroxisome proliferator-activated receptor gamma (Pparγ), via increased expression of transcription factors CCAAT/enhancer-binding proteins β and Δ.Reference Rosen, Hsu and Wang 45 Isoforms of Pparγ include Pparγ1 and Pparγ2; both Pparγ1 and Pparγ2 are expressed in adipose tissue.Reference Yanase, Yashiro and Takitani 46 Activation of either isoform of Pparγ induces differentiation of preadipocytes and increases adipocyte storage capacity for circulating lipids, potentially through regulation of adipose triglyceride lipase in adipocytes.Reference Kershaw, Schupp and Guan 47 , Reference Imai, Takakuwa and Marchand 48
The etiology of increased adiposity has not been studied in an animal model that combines IUGR and a maternal HFD. Thus measurement of circulating corticosterone and triglyceride levels and adipose tissue levels adipogenic and lipogenic proteins may provide insight into the mechanism through which IUGR and a maternal HFD increase visceral adiposity both independently and in combination. We hypothesized that the combined in utero insults of IUGR and a maternal HFD would enhance the programmed adipose tissue dysregulation and increase visceral adiposity in adult rat offspring more than either in utero insult alone. Specifically, IUGR combined with a maternal HFD would have greater visceral adiposity, circulating corticosterone and triglyceride levels, and visceral adipose protein levels of GR-α and 11-β HSD 1 than either IUGR rats or rats exposed to a maternal HFD. Because an adverse in utero environment programs obesity independent of lifestyle choices, we also hypothesized that weaning IUGR offspring exposed to a maternal HFD to a standard, regular rat chow would not fully reverse the combined IUGR- and maternal HFD-induced adipose tissue dysregulation.
Methods
Animals husbandry and study design
The University of Utah Animal Care Committee approved all procedures. Rat husbandry and the rat model were as previously described.Reference Zinkhan, Zalla and Carpenter 49 In brief, 45-day-old male and non-pregnant female Sprague Dawley rats were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA). Rats were exposed to 12 h light/dark cycles. Male rats used for breeding were kept on a regular rat chow (TD.8640; Reg, Harlan-Teklad, Madison, WI, USA) throughout the study. Female rats used for mating were fed one of two diets, either a regular rat chow (TD.8640) or a HFD rat chow (TD.110526; Harlan-Teklad). The regular chow had a caloric density of 3 kcal/g and contained 17% kcals from fat, consisting of 60 g/kg soybean oil and 0.03 wt/wt cholesterol, 54% kcals from carbohydrate, and 29% kcals from protein. The HFD had a caloric density of 4.3 kcal/g and contained 44% kcals from fat, consisting of milkfat and 10 g/kg soybean oil, and 1% wt/wt cholesterol, 40% kcals from carbohydrate, and 16% kcals from protein. The fat in the HFD was 65% saturated fat. The HFD also contained 0.5% cholic acid to aid in fat absorption as rats do not have gall bladders. Protein content in the HFD was lower than the regular diet. The protein content was chosen to mimic current protein consumption in the United States,Reference Wright, Wang, Kennedy-Stephenson and Ervin 50 is sufficient for normal mammalian growth, 51 and balances dietary decreases in carbohydrates and protein while increasing fat intake. Further, protein content was significantly higher than levels used in low-protein dietary studies.Reference Boujendar, Reusens and Merezak 52
After 5 weeks on either the regular diet or HFD, at 80–90 days of life, non-pregnant female rats were mated by placing the male rats into the female rat cages. Pregnancy day 0.5 was determined by the presence of sperm on a vaginal swab the following morning. Bilateral uterine artery ligation was performed on day 19 of a 21-day gestation using anesthesia alone as a control, as described previously.Reference Fu, McKnight, Yu, Callaway and Lane 53 We previously reported that maternal caloric intake did not vary due to diet or surgical intervention, with all dams consuming an average of 63 kcal/day.Reference Zinkhan, Zalla and Carpenter 49 Dams were allowed to deliver vaginally and litters were culled to six for rearing consistency, with three males and three females per litter. Only one rat per sex per litter was used in the experiments outlined in this manuscript, with the exception that body weights from birth (P0) were averaged per sex from each litter, and the average body weight per sex from each litter was then averaged across the litters in each group and analyzed between groups. At the age of weaning (P21), offspring from dams fed an HFD were either continued on the HFD or changed to a regular rat chow (TD.8640). For each of the normal non-IUGR (N) and IUGR (I) rats, our study design resulted in three experimental groups: offspring exposed to a maternal regular diet and weaned to a regular diet (RR); offspring exposed to a maternal HFD and weaned to a regular diet (HR); offspring exposed to a maternal HFD and weaned to an HFD (HH) (Fig. 1). All offspring were killed at postnatal day (P) 60 after an overnight fast and anesthetized with isoflurane before decapitation. Tissues were flash frozen in liquid nitrogen and stored at −80°C or fixed in formalin for further analysis. Female offspring were harvested only in estrus to minimize confounders of the estrus cycle.Reference Hubscher, Brooks and Johnson 54
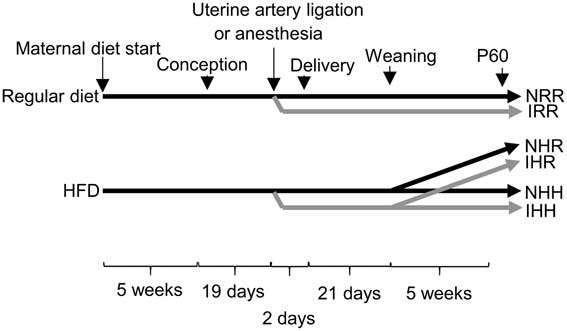
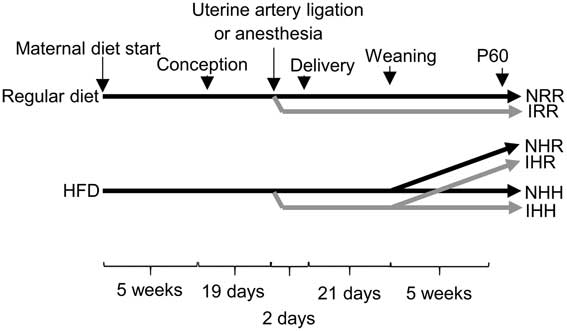
Fig. 1 Schematic of the timeline of dietary and surgical interventions in the rat model. Either a maternal regular diet or a maternal high-fat diet (HFD) were started 5 weeks before conception and continued through gestation and lactation. Nineteen days after conception, either bilateral uterine artery ligation to induce intrauterine growth restriction (IUGR) (I) or anesthesia alone for non-IUGR (N) rats was performed 2 days before delivery. At P21, the normal time of weaning, regular diet rats were weaned to a regular diet (RR), and maternal HFD fed rats were weaned to either a regular diet (HR) or to an HFD (HH). Post-weaning diets were continued for an additional 5 weeks, until the offspring were 60 days old. In the figure, the normal, non-IUGR rat timeline is shown in black lines, and IUGR rats shown in gray lines.
Volumetric assessment of adipose depots
Magnetic resonance imaging (MRI) images of the abdomen and pelvis were obtained and data analyzed as previously described.Reference Joss-Moore, Wang and Campbell 55 In brief, MRI was performed at P60 at the University of Utah small animal imaging core facility. Paired images of fat plus water and water alone were obtained from diaphragm to pelvis. Image analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). MRI images were analyzed using retroperitoneal fat as a representative visceral adipose depot. MRI image analysis was undertaken on four rats from different litters per group. These rats were not used for other analyses in this study.
Analysis of adipocyte size
The left retroperitoneal adipose tissue was used as a representative visceral adipose depot, and the left flank adipose tissue was used as a representative subcutaneous adipose depot. Adipose tissue was fixed in 10% formalin, embedded in paraffin, sliced to 5 µm thickness and stained with hematoxylin and eosin at the University of Utah Dermatopathology laboratory. Adipocyte diameter was quantified using Bioquant True Color Windows Image Analysis System (R&M Biometrics, Nashville, TN, USA). Diameter was measured as the longest distance from one end of the adipocyte to the other. All adipocytes where the entire adipocyte appeared in the field were measured per field from 15 high powered fields taken from six rats for each adipose depot. These rat tissues were not used for other analyses in this study.
Analysis of serum corticosterone, triglycerides, leptin and adiponectin
After an overnight fast, mixed arterial and venous blood was collected in serum separator tubes (BD Vacutainer; BD, Franklin Lakes, NJ, USA) after decapitation and placed on ice. Serum was separated from cellular components within 30 min of collection, flash frozen in liquid nitrogen and stored at −80°C. Serum corticosterone was measured using an ELISA kit (Cayman Chemical Company, Ann Arbor, MI, USA), with a detection limit of 30 ng/ml. Triglyceride levels were measured by ARUP Laboratories (Salt Lake City, UT, USA). Serum leptin and adiponectin were quantified using an ELISA kit (Millipore, St. Charles, MI, USA), with kit detection limits of 0.08 ng/ml for leptin and 0.155 ng/ml for adiponectin. Serum from six rats from different litters per group was analyzed. All six rats used in serum analyses were also used in adipocyte protein and messenger RNA (mRNA) analyses.
Protein analysis
VAT and SAT protein was isolated using whole cell lysates in a buffer containing 150 mM NaCl, 50 mM Tris at pH 7.4, 1 mM EDTA, 0.25% Na-deoxycholate, 1% Igepal CA-630. Total protein was quantified using a BSA standard curve measured by a bicinchoninic assay (Pierce, Rockford, IL, USA); 50 μg protein was run on a 10% SDS-PAGE gel (Bio-Rad, Hercules, CA, USA) and transferred to a polyvinylidene difluoride membrane. Membranes were blocked with 5% milk. Antibodies used to identify and quantify specific protein content included GR-α (Abcam, Cambridge, UK), 11-β HSD 1 (Sigma-Aldrich, St. Louis, MO, USA), leptin (Millipore, Billerica, MA, USA), adiponectin (Cell Signaling Technology, Danvers, MA, USA) and Pparγ (Santa Cruz Biotechnology, Dallas, TX, USA), which detects both isoforms of Pparγ. Hypoxanthine phosphoribosyltransferase 1 (Hprt1) (Proteintech, Rosemont, IL, USA) was used as an internal control as Hprt1 did not vary by intrauterine condition or diet. Protein was detected with Western Lightning-enhanced chemiluminescence (PerkinElmer Life Sciences, Waltham, MA, USA) using goat anti-rabbit horseradish peroxidase secondary antibody from Cell Signaling Technology (Beverly, MA, USA). Blots were quantified using a Kodak Image Station 2000R (Eastman Kodak/SIS, Rochester, NY, USA).
Due to the large number of rat diet and intrauterine condition groups, not all samples were able to be run on a single western blot gel. Therefore, protein samples from NRR and IRR rats were run on every gel along with either samples from both NHR and IHR or both NHH and IHH rats. Each target protein was first compared with the loading control (Hprt1), then protein levels from the NRR rat group were compared between the two western blot gels (one containing NRR, IRR, NHR and IHR protein samples, and other containing NRR, IRR, NHH and IHH protein samples). Target protein levels from the NHR and IHR rats were normalized for differences between the NRR data between the two sets of gels to account for differences in baseline loading and exposure of the gel. Sexes were run on separate gels and analyzed separately. Given the large number of samples, representative western blot images were cropped from the gels in the figures.
Adipocyte mRNA analysis
Total adipocyte mRNA was isolated as described previously using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA, USA).Reference Joss-Moore, Wang and Campbell 55 Total RNA was quantified spectrophotometrically. The complementary DNA (cDNA) was synthesized from 1 µg RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) per manufacturer’s protocol.
Real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Semi-quantitative real-time RT-PCR quantification was performed using Hprt1 as an internal control, as Ct values of Hprt1 did not differ between any of the groups. Relative quantification of PCR products was based on differences between Hprt1 and the target using the comparative Ct method (TaqMan Gold RT-PCR manual; PE Biosystems, Foster City, CA, USA).Reference Livak and Schmittgen 56 Assays were performed with Taqman Gene Expression Assays (Applied Biosystems) for target gene Abca1.
Statistics
Data were expressed as mean ± s.e.m., except for adipocyte diameter, for which data were expressed as median and interquartile range in a box and whisker plot. Two-way analysis of variance with Fisher’s protected least-significant difference was used for data analysis, using variables of diet and intrauterine environment. A P value⩽0.05 was considered statistically significant. Results of statistical analyses are shown for data from IUGR relative to non-IUGR rats of the same maternal and offspring diet, and all groups relative to NRR. Male and female data were analyzed separately (Fig. 1).
Results
Weight gain and food intake
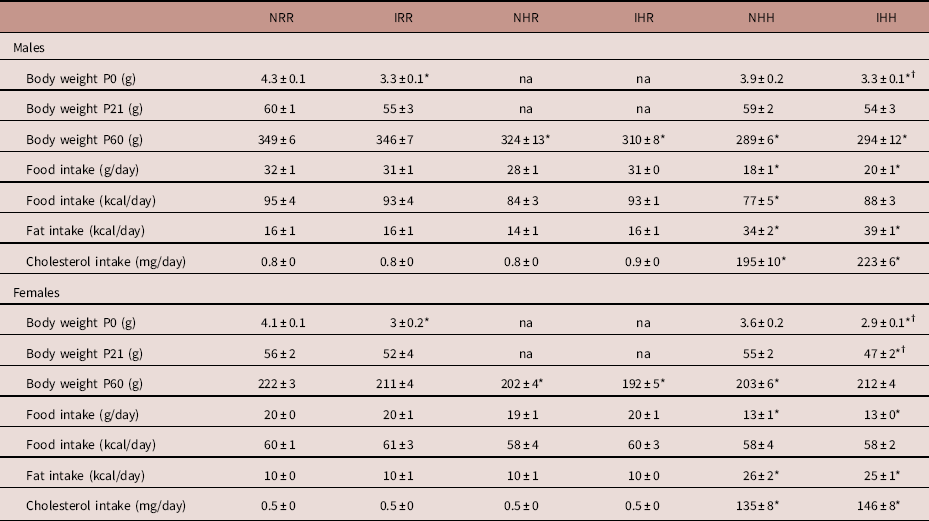
Male NHH rats consumed less kilocalories per day than NRR male rats, but otherwise there were no differences in food or caloric intake in this study. There was no difference between maternal diet groups in the time to achieve pregnancy (regular diet females were pregnant at 1.7±0.2 days, HFD females were pregnant at 2.7±0.5 days), and >95% of female rats with a vaginal swab containing sperm became pregnant in all groups. Maternal diet also did not impact the number of pups per litter (regular diet litter size 10.6±0.8 pups per litter; HFD litter size 12.3±0.8 pups per litter, P>0.05). Maternal HFD decreased the male to female ratio (regular diet male:female ratio 2.3±0.4, HFD male:female ratio 1.2±0.2, P=0.019). While we previously reported that maternal HFD intake induced mild growth restriction of about 5% in both male and female offspring at birth, with a larger number of rats per group in this study, the decreased body weight in the maternal HFD fed rats at P0 no longer reached statistical significance (P=0.1 for both males and females).Reference Dodson, Miller and Powers 57 While only female IUGR rats from maternal HFD fed dams weighed less than regular diet fed dams by P21, by P60, all rats exposed to the maternal HFD weighed less compared with sex-matched maternal regular diet fed rats, regardless of post-weaning diet (Table 1). Decreased rat body weight occurred despite the same total caloric intake, with the exception of decreased caloric intake in NHH male rats. None of the rats in our study ate more than the normal non-IUGR regular diet group (NRR). Both male and female NHH and IHH rats consumed more fat and cholesterol than the other four groups of rats, with no difference between IUGR and non-IUGR within any diet group (Table 1).
Table 1 Growth and food intake at P60
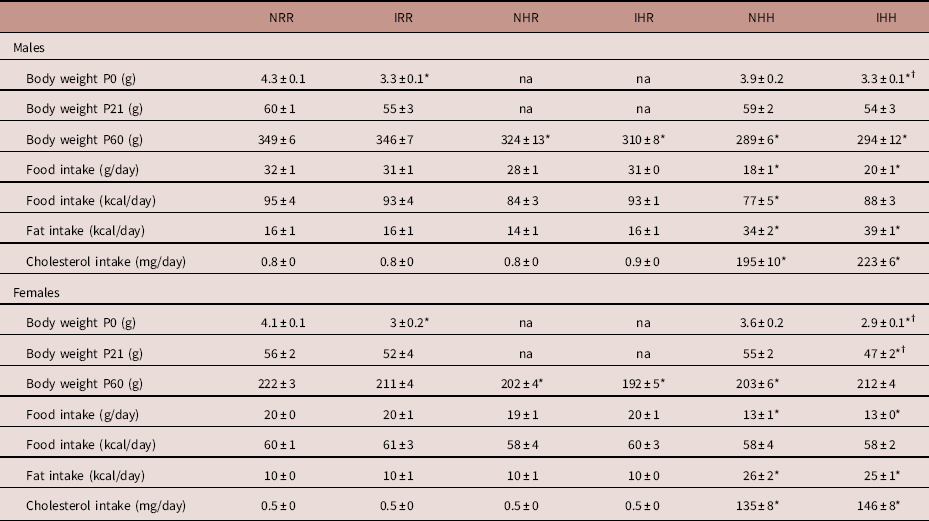
Groups: Body weights from rats at P0 were averaged for all live offspring rats per sex in each litter, and the average body weight per sex from each litter was averaged for 10 litters per group.
Body weights were obtained from the following numbers of offspring rats each from a different litter at P21 and P60.
Normal non-intrauterine growth restriction (IUGR), maternal regular diet, regular diet at weaning (NRR): at P21, n=27 male and n=22 female; at P60, n=24 male and n=22 female.
IUGR, maternal regular diet, regular diet at weaning (IRR): at P21, n=10 male and n=8 female; at P60, n=20 male and n=20 female.
Normal non-IUGR, maternal high-fat diet (HFD), regular diet at weaning (NHR): at P60, n=14 male and n=14 female.
IUGR, maternal HFD, regular diet at weaning (IHR): at P60, n=24 male and n=21 female.
Normal non-IUGR, maternal HFD, HFD at weaning (NHH): at P21, n=10 male and n=13 female; at P60, n=18 male and n=25 female.
IUGR, maternal HFD, HFD at weaning (IHH): at P21, n=7 male and n=9 female; at P60, n=20 male and n=35 female.
P0 and P21 maternal HFD offspring growth data are shown under the NHH and IHH columns, and not-applicable (na) is instead written into the columns under NHR and IHR for this data.
Data presented as mean ± s.e.m. for rats each from different litters.
*Significant difference between the control group and NRR, P⩽0.05; †significant difference between IHH and NHH, P⩽0.05. No difference was found comparing IHR with NHR.
Volumetric assessment of adipose depots and adipocyte size
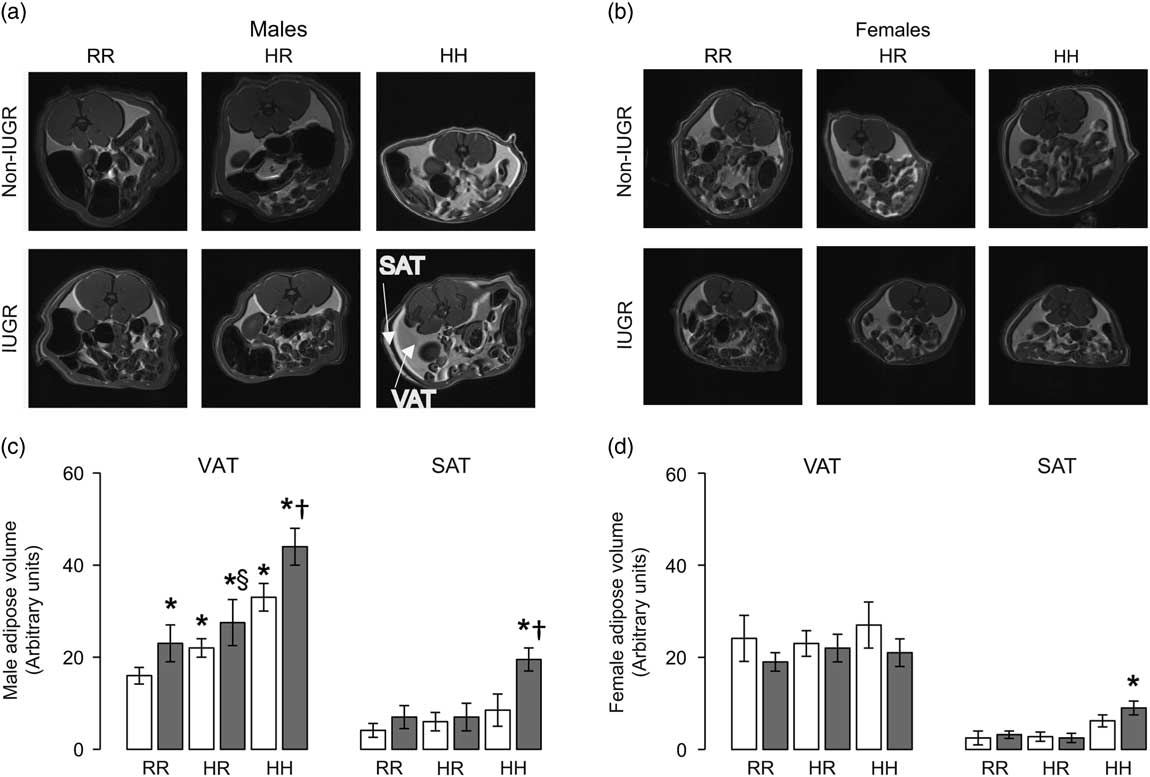
The greatest visceral and subcutaneous adiposity was seen in male offspring in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD (IHH males) (Fig. 2a and 2b). Compared with NRR males, all other male rat groups had greater visceral adiposity (Fig. 2a and 2b). Changing the post-weaning diet to a regular diet decreased visceral adiposity by 35% but did not fully reverse the impact of maternal HFD exposure and/or IUGR on visceral adipose volume in NHR and IHR males (Fig. 2b). IUGR increased visceral adiposity in males compared with respective diet non-IUGR rats (Fig. 2b). The greatest subcutaneous adiposity in female rats was seen in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD (IHH females), findings which were normalized by weaning to a regular diet (Fig. 2c and 2d).
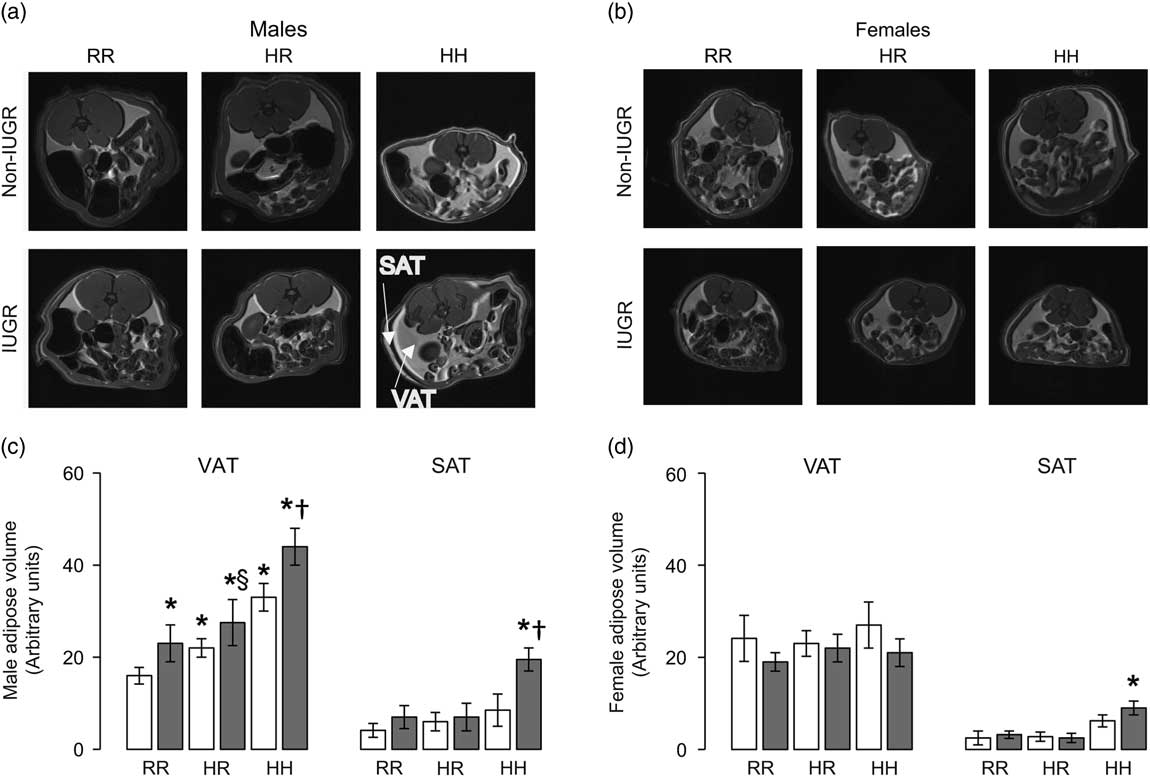
Fig. 2 The combined in utero insults of intrauterine growth restriction (IUGR) and a maternal high-fat diet (HFD) increased visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) in male rats (a,b) and SAT in female rats (c,d). Weaning to a regular rat chow did not normalize IUGR or maternal HFD-induced increased adiposity in male rats. White bars represent normal, non-IUGR (N) rats and gray bars represent IUGR (I) rats. Maternal and offspring diets are denoted below the graphs [regular diet rats were weaned to a regular diet (RR), and maternal HFD fed rats were weaned to either a regular diet (HR) or to an HFD (HH)]. A total of four rats each from different litters per group were used in these analyses. Statistical significance is denoted as follows: *P⩽0.05 for NHR, NHH, IRR, IHR and IHH rats compared with sex-matched NRR rats; § P⩽0.05 for IHR rats compared with NHR sex-matched rats; † P⩽0.05 for IHH rats compared with sex-matched NHH rats.
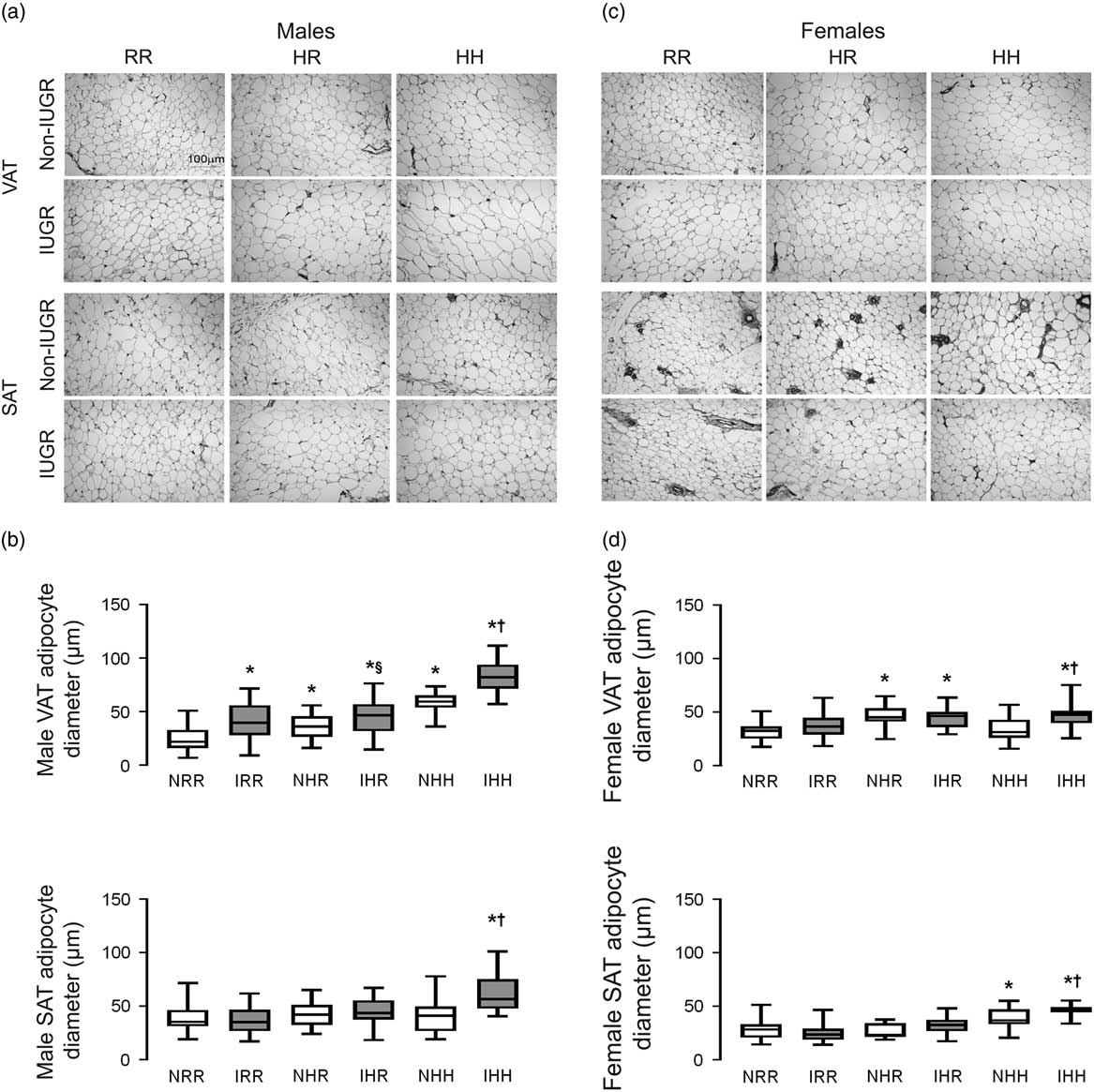
The greatest visceral and subcutaneous adipocyte size was seen in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD in both sexes (IHH males and females) (Fig. 3a–3d). Weaning to a regular diet decreased visceral adipocyte size by 38% but did not fully reverse the impact of maternal HFD exposure in NHR and IHR males (Fig. 3a and 3b). IUGR increased visceral adipocyte size in males compared with respective diet non-IUGR rats (Fig. 3b). Weaning maternal HFD fed rats to a regular diet normalized adipocyte size in the subcutaneous adipose but not the visceral adipose in both sexes.
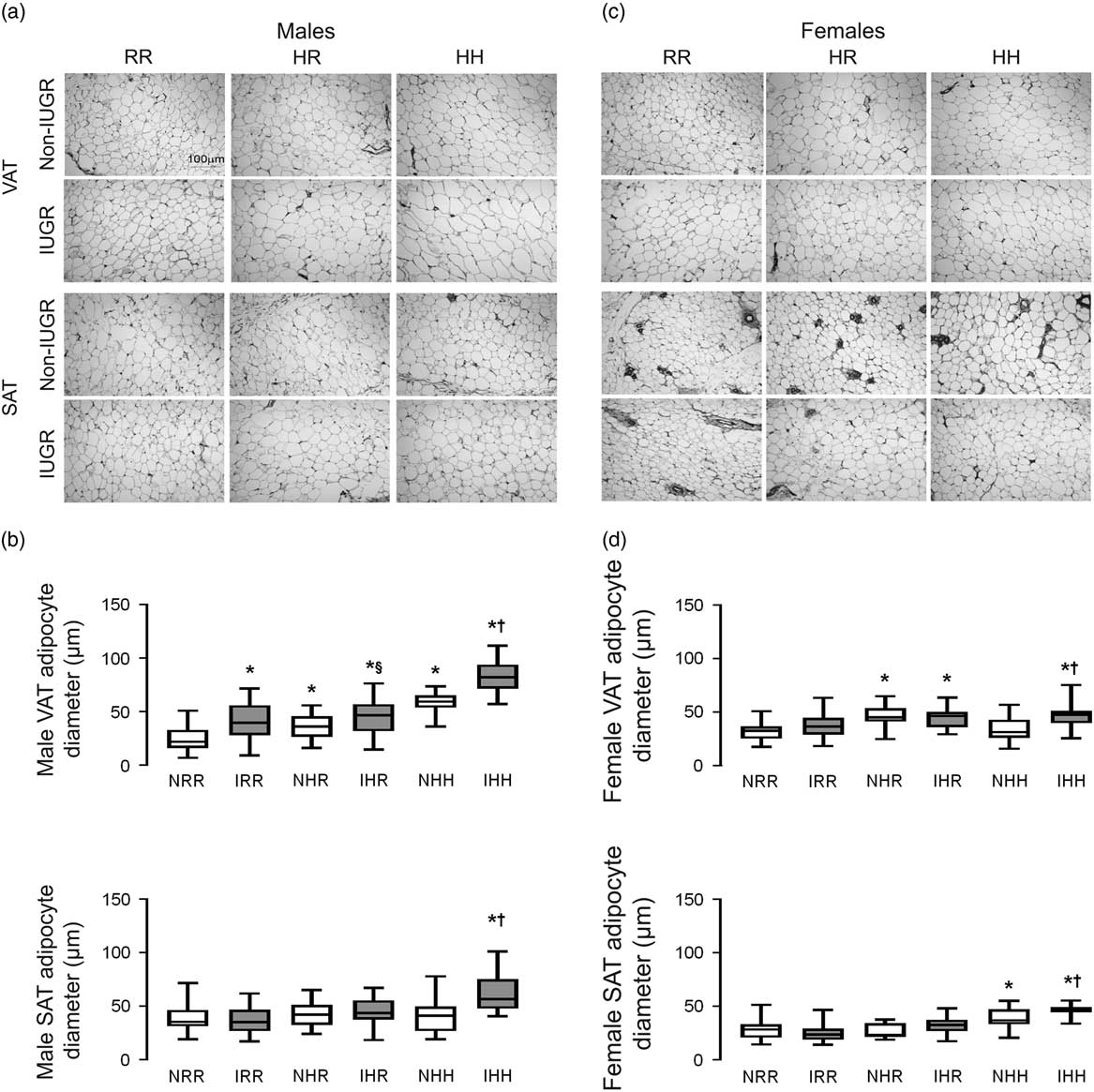
Fig. 3 The greatest adipocyte size was found in the combined in utero insult of intrauterine growth restriction (IUGR) and a maternal high-fat diet (HFD), which does not normalize by weaning to a regular diet in the visceral adipose tissue (VAT). Representative hematoxylin and eosin stained adipocyte images of VAT and subcutaneous adipose tissue (SAT) for normal non-IUGR (N) and IUGR (I) male (a) and female (c) rats; and quantification of adipocyte size is shown below the representative images for male (b) and female (d) VAT and SAT. Scale bar in the upper left panel represents 100 µm. White bars represent normal non-IUGR rats and gray bars represent IUGR rats. Quantification of adipocyte diameter data shown as box and whisker plots, with median and 25–75% interquartile range. Maternal and offspring diets are denoted below the graphs [regular diet rats were weaned to a regular diet (RR), and maternal HFD fed rats were weaned to either a regular diet (HR) or to an HFD (HH)]. A total of six rats from different litters per group were used in these analyses. Statistical significance is denoted as follows: *P⩽0.05 for NHR, NHH, IRR, IHR and IHH rats compared with sex-matched NRR rats; § P⩽0.05 for IHR rats compared with NHR sex-matched rats; † P⩽0.05 for IHH rats compared with sex-matched NHH rats.
Analysis of serum corticosterone, triglycerides, leptin and adiponectin
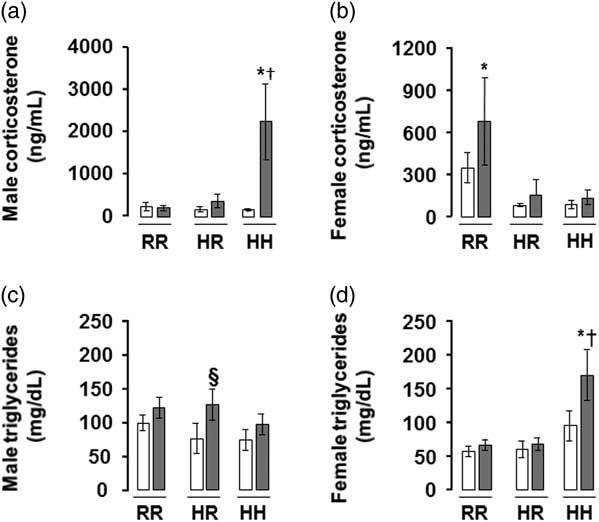
The greatest serum corticosterone was seen in males rats in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD (IHH males), findings which were normalized by weaning to a regular diet (Fig. 4a). The greatest serum triglyceride level was seen in female rats in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD (IHH females), findings which were normalized by weaning to a regular diet (Fig. 4d). The greatest serum adiponectin was seen in female rats in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD (IHH females), findings which were normalized by weaning to a regular diet (Supplemental Fig. 1).
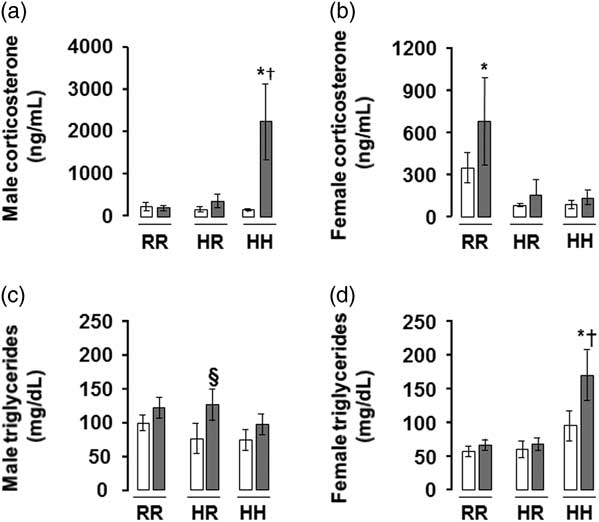
Fig. 4 The greatest corticosterone level was in the combined in utero insult of intrauterine growth restriction (IUGR) and a maternal high-fat diet (HFD) in male rats, and the greatest triglyceride level was in the combined in utero insult of IUGR and a maternal HFD in female rats, findings which were normalized by weaning to a regular diet. Fasting serum corticosterone levels shown in (a,b) and triglycerides in (c,d). White bars represent normal non-IUGR (N) rats and gray bars represent IUGR (I) rats. Maternal and offspring diets are denoted below the graphs [regular diet rats were weaned to a regular diet (RR), and maternal HFD fed rats were weaned to either a regular diet (HR) or to an HFD (HH)]. A total of six rats from different litters per group were used in these analyses. Statistical significance is denoted as follows: *P⩽0.05 for NHR, NHH, IRR, IHR and IHH rats compared with sex-matched NRR rats; § P⩽0.05 for IHR rats compared with NHR sex-matched rats; † P⩽0.05 for IHH rats compared with sex-matched NHH rats.
Adipose depot protein quantification
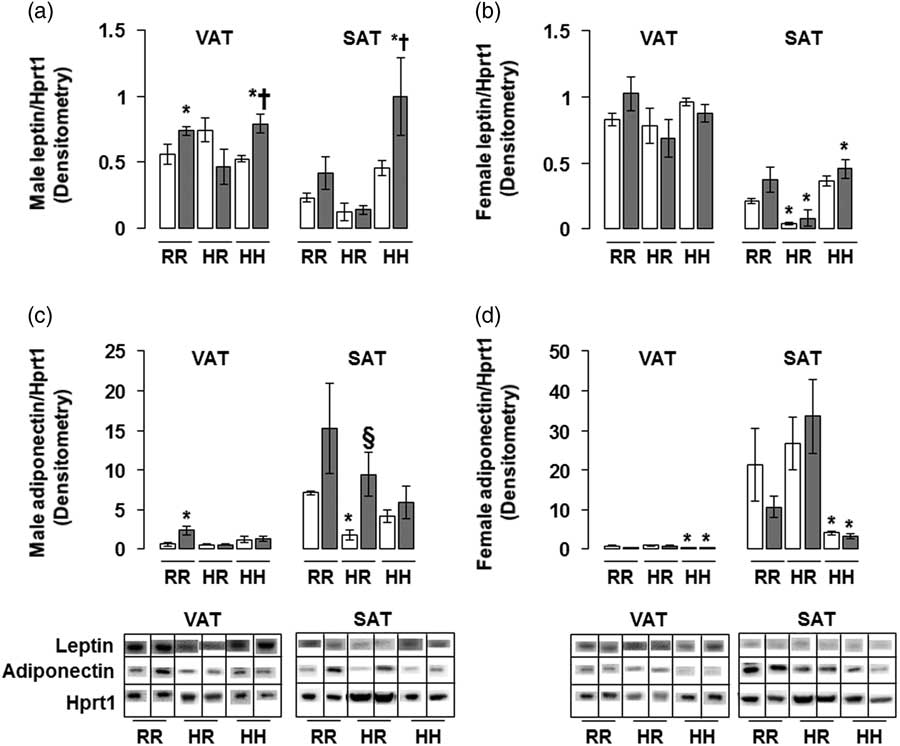
The greatest amount of leptin protein was found in male rats in the combined in utero insult of IUGR and a maternal HFD when weaned to an HFD (IHH males) in both VAT and SAT, findings which were normalized by weaning to a regular diet (Fig. 5a). The lowest amount of adiponectin protein was found in non-IUGR and IUGR female rats exposed to a maternal HFD when weaned to an HFD (NHH and IHH females) in both VAT and SAT, with no difference between NHH and IHH female adiponectin protein levels (Fig. 5d). This increased adiponectin protein in was normalized by weaning to a regular diet (Fig. 5d).
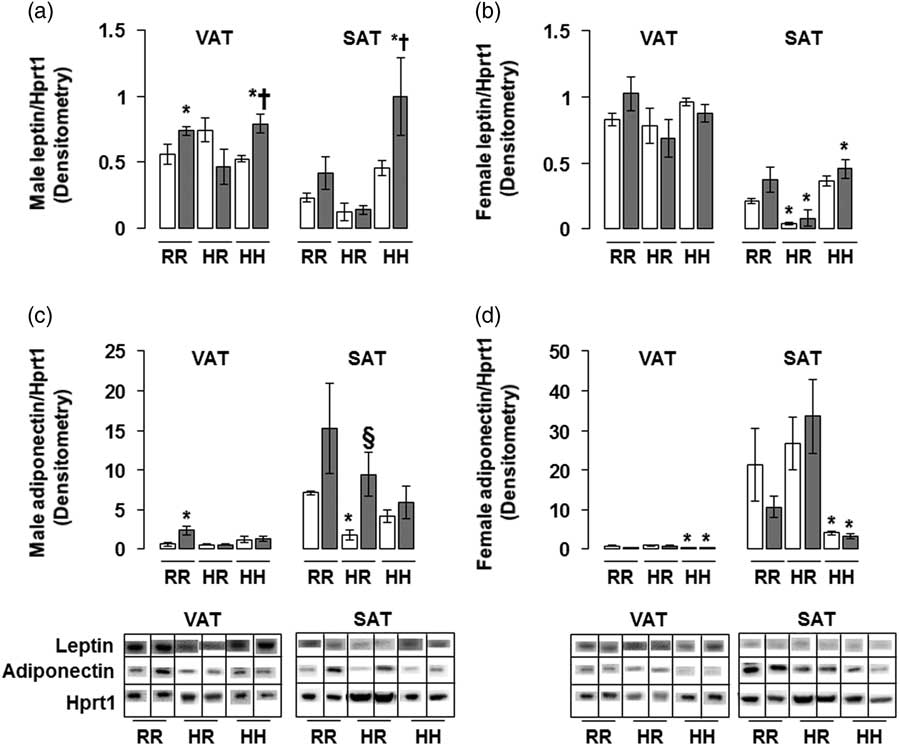
Fig. 5 The greatest leptin protein was found in the combined in utero insult of intrauterine growth restriction (IUGR) and a maternal high-fat diet (HFD) in male visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT); and the least adiponectin protein was found in the combined in utero insult of IUGR and a maternal HFD in female VAT and SAT. These findings were normalized by weaning to a regular diet. Western blot protein quantification of VAT and SAT leptin (a,b) and adiponectin (c,d). Due to the large number of samples, western blots were run as follows per sex: western blots with NHH and IHH samples were run on one gel, and NHR and IHR were run on a second gel. NRR and IRR samples were run on both western blots. All samples were first normalized to hypoxanthine phosphoribosyltransferase 1 (Hprt1) control protein levels, then controlled for variation in NRR protein levels between blots. Representative western blot images are shown below the graphs, with one representative lane shown per rat group. White bars represent normal non-IUGR (N) rats and gray bars represent IUGR (I) rats. Maternal and offspring diets are denoted below the graphs [regular diet rats were weaned to a regular diet (RR), and maternal HFD fed rats were weaned to either a regular diet (HR) or to an HFD (HH)]. A total of six rats from different litters per group were used in these analyses. Statistical significance is denoted as follows: *P⩽0.05 for NHR, NHH, IRR, IHR and IHH rats compared with sex-matched NRR rats; § P⩽0.05 for IHR rats compared with NHR sex-matched rats; † P⩽0.05 for IHH rats compared with sex-matched NHH rats.
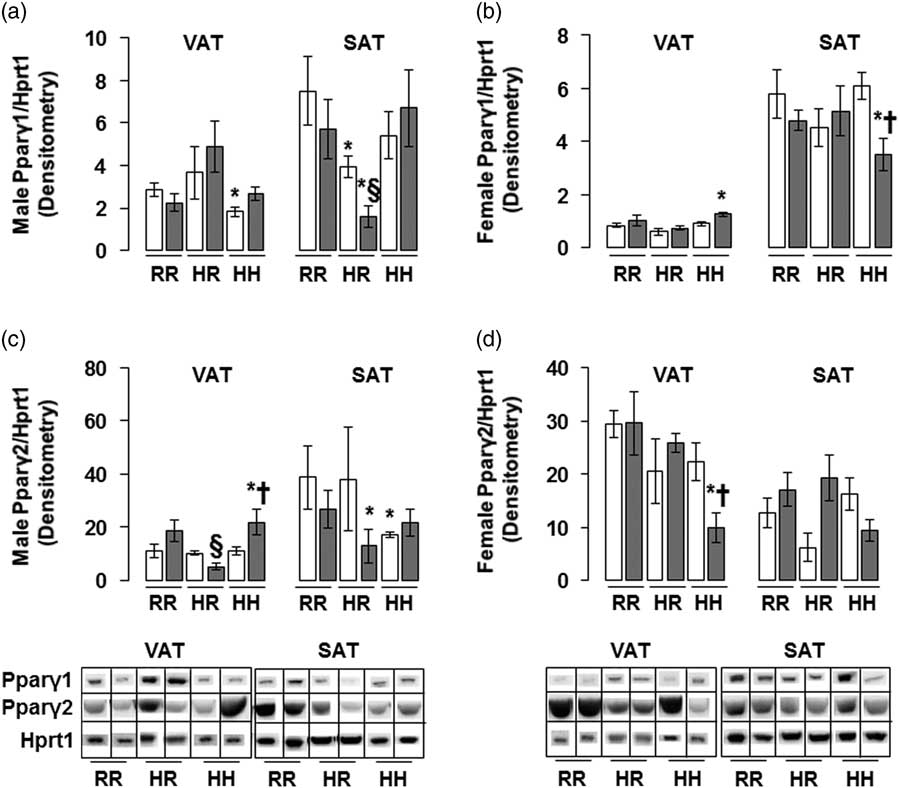
The greatest amount of Pparγ2 protein in male rats was found in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD (IHH males), findings which were normalized by weaning to a regular diet (Fig. 6c). The lowest Pparγ1 and Pparγ2 protein levels in female rats were seen in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD (IHH females), findings which were normalized by weaning to a regular diet (Fig. 6b and 6d). Normalizing maternal HFD fed rats to a regular diet decreased Pparγ1 protein levels in male subcutaneous adipose, regardless of in utero IUGR insult (Fig. 6a).
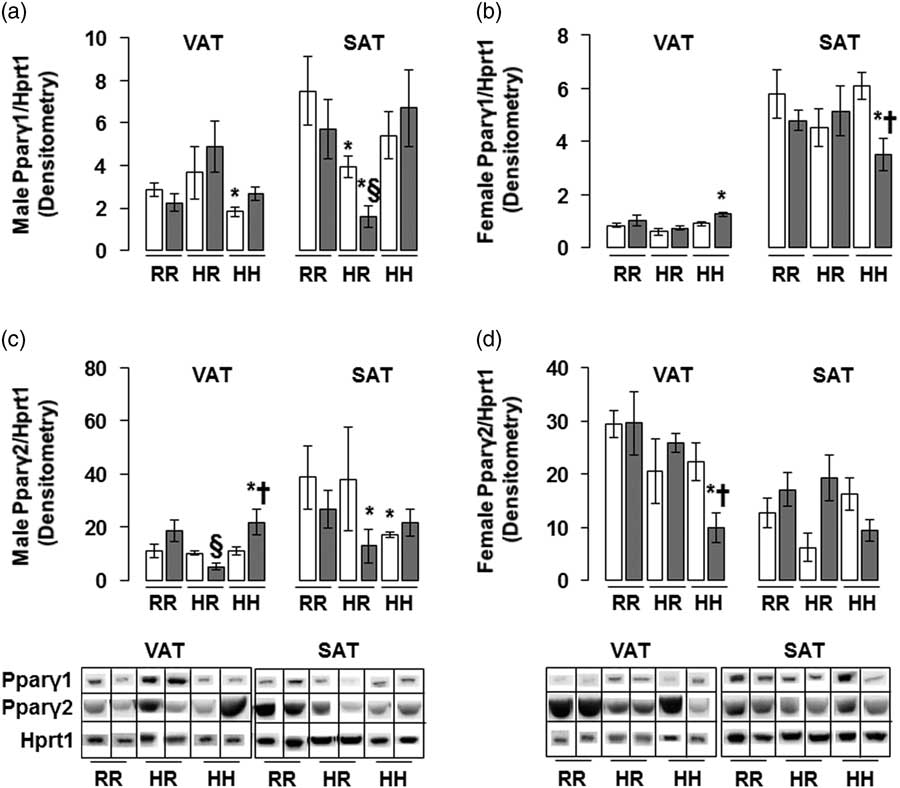
Fig. 6 The greatest peroxisome proliferator-activated receptor gamma 2 (Pparγ2) protein was found in the combined in utero insult of intrauterine growth restriction (IUGR) and a maternal high-fat diet (HFD) in male visceral adipose tissue (VAT), while the least Pparγ1 protein was found in the combined in utero insult of IUGR and a maternal HFD in female subcutaneous adipose tissue (SAT) and Pparγ2 protein was found in the combined in utero insult of IUGR and a maternal HFD in female VAT. These findings were normalized by weaning to a regular diet. Western blot protein quantification of VAT and SAT Pparγ1 (a,b) and Pparγ2 (c,d). Due to the large number of samples, western blots were run as follows per sex: western blots with NHH and IHH samples were run on one gel, and NHR and IHR were run on a second gel. NRR and IRR samples were run on both western blots. All samples were first normalized to hypoxanthine phosphoribosyltransferase 1 (Hprt1) control protein levels, then controlled for variation in NRR protein levels between blots. Representative western blot images are shown below the graphs, with one representative lane shown per rat group. White bars represent normal non-IUGR (N) rats and gray bars represent IUGR rats (I). Maternal and offspring diets are denoted below the graphs [regular diet rats were weaned to a regular diet (RR), and maternal HFD fed rats were weaned to either a regular diet (HR) or to an HFD (HH)]. A total of six rats from different litters per group were used in these analyses. Statistical significance is denoted as follows: *P⩽0.05 for NHR, NHH, IRR, IHR and IHH rats compared with sex-matched NRR rats; § P⩽0.05 for IHR rats compared with NHR sex-matched rats; † P⩽0.05 for IHH rats compared with sex-matched NHH rats.
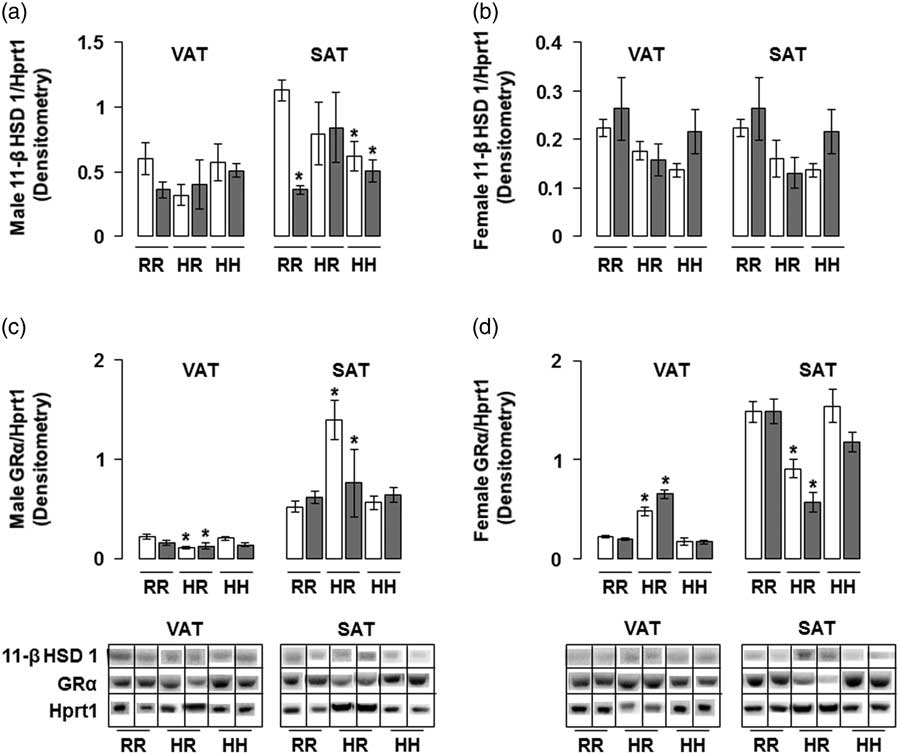
The combined in utero insults of IUGR and a maternal HFD did not change 11-β HSD 1 protein levels in any male rat groups in the visceral adipose and in any female rat groups in VAT and SAT (Fig. 7a and 7b). Combined maternal and offspring HFD decreased 11-β HSD 1 protein levels in male rat subcutaneous adipose, regardless of in utero IUGR insult (Fig. 7a). The combined in utero insults of IUGR and a maternal HFD did not change GR-α protein levels in male or female rat VAT and SAT (Fig. 7c and 7d). Normalizing maternal HFD fed rats to a regular diet decreased GR-α protein levels in male visceral and female subcutaneous adipose, and increased GR-α protein levels in male subcutaneous and female visceral adipose, regardless of in utero IUGR insult (Fig. 7c and 7d).
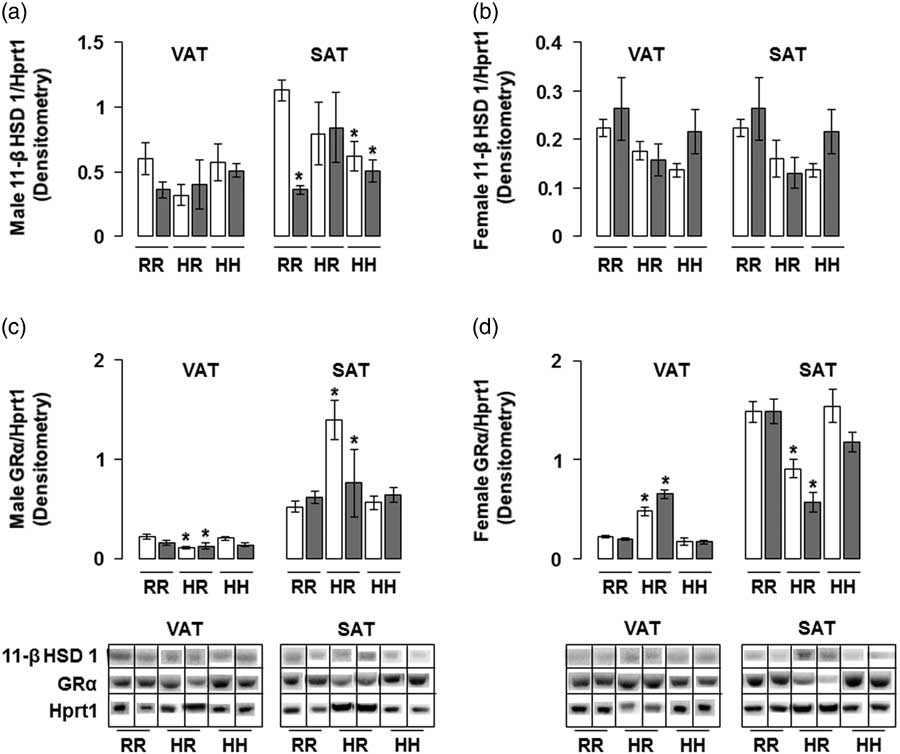
Fig. 7 The combined in utero insults of intrauterine growth restriction (IUGR) and a maternal high-fat diet (HFD) did not change 11-β hydroxysteroid dehydrogenase 1 (11-β HSD 1) protein levels in any male rat groups in the visceral adipose tissue (VAT) and in any female rat groups in VAT or subcutaneous adipose tissue (SAT) (a,b). The combined in utero insults of IUGR and a maternal HFD did not change active glucocorticoid receptor (GR-α) protein levels in male or female rat VAT or SAT (c,d). Western blot protein quantification of VAT and SAT 11-β HSD 1 in (a,b) and GR-α in (c,d). Due to the large number of samples, western blots were run as follows per sex: western blots with NHH and IHH samples were run on one gel, and NHR and IHR were run on a second gel. NRR and IRR samples were run on both western blots. All samples were normalized to hypoxanthine phosphoribosyltransferase 1 (Hprt1) control protein levels, then controlled for variation in NHR protein levels between blots. Representative western blot images are shown below the graphs, with one representative lane shown per rat group. White bars represent normal non-IUGR (N) rats and gray bars represent IUGR (I) rats. Maternal and offspring diets are denoted below the graphs [regular diet rats were weaned to a regular diet (RR), and maternal HFD fed rats were weaned to either a regular diet (HR) or to an HFD (HH)]. A total of six rats from different litters per group were used in these analyses. Statistical significance is denoted as follows: *P⩽0.05 for NHR, NHH, IRR, IHR and IHH rats compared with sex-matched NRR rats. No difference was found between IHR and NHR sex-matched rat data, or between IHH and NHH sex-matched rat data.
Adipose depot mRNA quantification
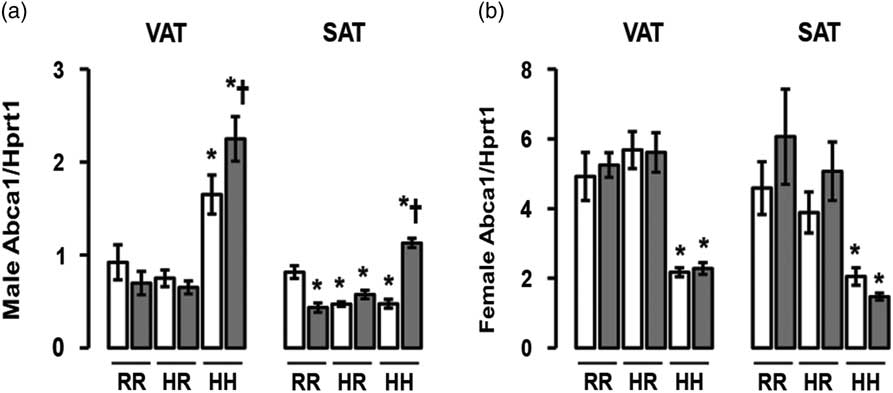
The greatest Abca1 mRNA levels were seen in male rats in the combined in utero insults of IUGR and a maternal HFD when weaned to an HFD (IHH males), findings which were normalized by weaning to a regular diet (Fig. 8a). Abca1 mRNA levels were decreased in all other male rat subcutaneous adipose (Fig. 8a). The least Abca1 mRNA levels were seen in female rats exposed to a maternal HFD and weaned to an HFD (NHH and IHH females), findings which were normalized by weaning to a regular diet (Fig. 8b).
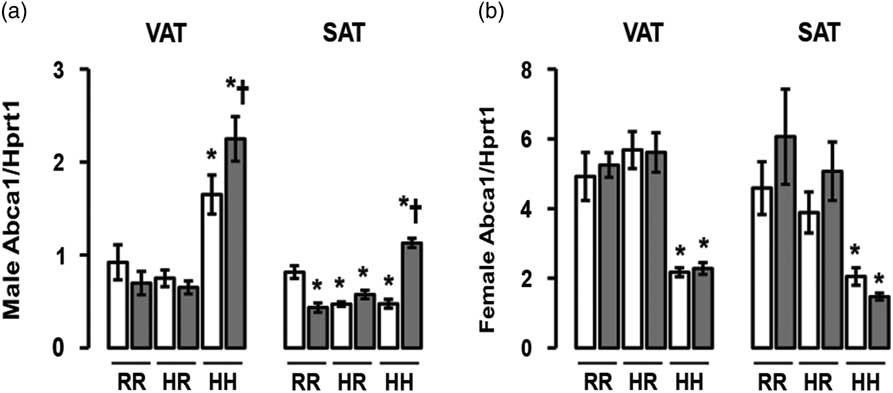
Fig. 8 The greatest ATP-binding cassette sub-family A member 1 (Abca1) messenger RNA (mRNA) was found in the combined in utero insult of intrauterine growth restriction (IUGR) and a maternal high-fat diet (HFD) in male visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), and the least Abca1 mRNA was found in IUGR (I) and non-IUGR (N) rats from a maternal HFD in female VAT and SAT. These findings were normalized by weaning to a regular diet. mRNA quantification of VAT and SAT Abca1 mRNA (a,b). White bars represent normal non-IUGR rats and gray bars represent IUGR rats. Maternal and offspring diets are denoted below the graphs [regular diet rats were weaned to a regular diet (RR), and maternal HFD fed rats were weaned to either a regular diet (HR) or to an HFD (HH)]. A total of six rats from different litters per group were used in these analyses. Statistical significance is denoted as follows: *P⩽0.05 for NHR, NHH, IRR, IHR and IHH rats compared with sex-matched NRR rats; † P⩽0.05 for IHH rats compared with sex-matched NHH rats. No difference was found between IHR and NHR sex-matched rat data.
Discussion
In our study, the combined in utero insults of a maternal HFD and IUGR increased visceral and subcutaneous adiposity, serum corticosterone and serum triglyceride levels in a sex-dependent manner. Weaning offspring to a regular diet did not fully normalize the increased adiposity secondary to the combined in utero insults. Increased circulating corticosterone levels and increased levels of downstream GR transcription targets suggests that increased adiposity may be in part secondary to increased circulating corticosterone in IHH male rats. Increased adiposity secondary to the combined in utero insults likely occurs through multiple mechanisms and in a sex-specific manner.
Ample evidence now exists demonstrating that maternal obesity or HFD intake programs obesity in her children. When adjusted for sex, age and socio-economic factors, a study of 504 children in India born to mothers with normal glucose tolerance demonstrated that increased maternal adiposity predicted increased adiposity in her children.Reference Veena, Krishnaveni, Karat, Osmond and Fall 58 When controlling for lifestyle factors, maternal obesity more than doubled the risk of obesity in 2–4-year-old children as found in a study of 8494 children.Reference Whitaker 18 Further, a longitudinal study of 179 children ages 6–11 years old showed increased metabolic syndrome in children exposed to maternal obesity compared with those born to women with a normal pre-pregnancy body weight.Reference Boney, Verma, Tucker and Vohr 12 Similar to epidemiological studies, rodent studies of maternal HFD intake show increased offspring adipose tissue depot volume. A maternal HFD in non-IUGR mice induced increased adiposity, which was not reversed by a standard chow postnatally and further exacerbated by HFD feeding.Reference Fante, Simino and Reginato 59 Maternal HFD feeding in rats also induced increased adiposity in offspring, which was not improved by fish oil supplementation.Reference Oliveira, Souza and Souza 60 Offspring from non-obese, maternal HFD fed dams had increased adiposity, indicating that in the absence of maternal obesity, an HFD alone can increase offspring adiposity.Reference Frihauf, Fekete, Nagy, Levin and Zorrilla 61 Our study also showed that a maternal HFD alone increased visceral adipose depot volume and adipocyte size in male rats. We then showed that these results are further exacerbated by weaning to an HFD and are not normalized by weaning to a regular chow. Increased caloric consumption, particularly of fat, increases body weight and fat deposition.Reference Golay and Bobbioni 62 Unlike in humans, it is common for rodents to decrease or maintain their food intake when fed an HFD, although this finding is not universal.Reference Samuelsson, Matthews and Argenton 63 , Reference Siemelink, Verhoef, Dormans, Span and Piersma 64 The reason behind the discrepancy in HFD food intake between studies is unknown, but may be secondary to the species studied or to the specific components of the diet and palatability. Diet composition of HFDs with or without additional sucrose enhanced weight gain and adiposity differentially depending on the age of the rat.Reference Cheng, Ton, Phang, Tan and Abdul Kadir 65 The male rats in our study consumed the same total calories as the NRR males, except for decreased caloric intake of the NHH males, a finding that was associated with decreased body weight but not decreased visceral adiposity. Following the rats in our study to an older age may show increased body weight in addition to increased adiposity.
IUGR alone impacts the development of adult-onset obesity. At the age of 50 years, women exposed during fetal life to the Dutch famine of 1944–1945 had increased BMI and waist circumference.Reference Ravelli, van Der Meulen, Osmond, Barker and Bleker 13 Further, without exposure to famine, low birth weight was associated with increased waist to hip ratios independent of lifestyle in a study of 1084 adult men born between 1920 and 1943.Reference Law, Barker, Osmond, Fall and Simmonds 17 Studies in rodents also indicate that adverse in utero environments resulting in IUGR increase adipose deposition in the offspring.Reference Joss-Moore, Wang and Campbell 55 , Reference Zinkhan, Lang and Yu 66 Spontaneous IUGR occurs in piglets and is associated with increased body fat at 12 months of age.Reference Poore and Fowden 67 Decreased placental nutrient transfer in sheep leads to increased visceral adiposity in adolescence.Reference De Blasio, Gatford, Robinson and Owens 68 Our data presented here supports the growing body of literature that IUGR even without a maternal HFD increases visceral adiposity, with all male IUGR rat groups having increased adiposity compared with diet-matched non-IUGR male rats.
The role that catch-up growth, or the rapidity with which a growth-restricted individual’s weight matches that of non-growth-restricted peers, impacts the development of obesity. Rapid catch-up growth increases the risk of developing obesity in both humans and animal models.Reference Martin, Connelly, Bland and Reilly 69 – Reference Bol, Delattre, Reusens, Raes and Remacle 72 We previously reported that at postnatal day 21, male IUGR rats from either dams on an HFD or dams on a regular diet had caught up in body weight to the non-IUGR male rats, while IUGR female rats weighed less than non-IUGR females.Reference Dodson, Miller and Powers 57 In our current study, regardless of post-weaning diet and despite having increased visceral adiposity, maternal HFD exposed rats weighed less than regular diet non-IUGR rats at P60. Similar results are found in the literature, where maternal HFD fed offspring rats showed normal body weight early in life but increased adiposity with aging.Reference Frihauf, Fekete, Nagy, Levin and Zorrilla 61 In another study, adolescent rat offspring of HFD fed dams weighed the same as offspring from normal diet fed dams, but by adulthood the maternal HFD fed offspring weighed more.Reference Howie, Sloboda, Kamal and Vickers 73 Taken together, these data suggest that a latent predisposition to increased adiposity may exist even if offspring do not weigh more in early life. Following our IUGR and HFD fed rats to an older age may reveal eventual weight gain in addition to increased adiposity. Further, IUGR has been shown to increase the preference for palatable foods, particularly sweet-tasting food, which is thought to play a role in catch-up growth and eventual formation of obesity.Reference Laureano, Dalle Molle and Alves 74 In our study, IUGR rats fed an HFD did not consume more calories than the non-IUGR regular diet fed rats. Discrepancy in food intake and weight gain in IUGR individuals from the literature and from our study may be due to the lower carbohydrate content in the HFD compared with the regular diet, suggesting that the HFD was not more palatable than the regular diet.
The mechanisms for increased adiposity depend on sex, type of in utero environment, and post-weaning diet. One mechanism for increased adiposity despite similar caloric intake is exposure to excess endogenous or exogenous glucocorticoids. In humans with cortisol excess, visceral adiposity is increased more than subcutaneous adiposity.Reference Geer, Shen and Gallagher 75 Similarly in animal models, excess corticosterone administration increases visceral adiposity in rats.Reference Campbell, Peckett, D’Souza, Hawke and Riddell 76 , Reference Rebuffe-Scrive, Walsh, McEwen and Rodin 77 The impact of the in utero environment on circulating glucocorticoids can be seen in IUGR rodent studies. Maternal food restriction in rats followed by HFD feeding of the offspring impacts GR and 11-β HSD 1 levels, while increasing white adipose tissue volume and leptin levels.Reference Lukaszewski, Mayeur and Fajardy 78 Similar to our current results, IUGR male rats exposed in utero to maternal tobacco smoke also have increased visceral adiposity in association with increased circulating corticosterone without changing GR-α protein levels.Reference Zinkhan, Lang and Yu 66 Offspring HFD feeding alone did not change circulating corticosterone levels or 11-β HSD 1 or GR-α mRNA levels in adult rats.Reference He, Lv and Ding 79 In GR knock-out mice, obesity resulting from HFD feeding does not require the GR, whereas obesity resulting from exogenous glucocorticoid administration requires an intact GR, emphasizing the importance of multiple pathways to increased adiposity.Reference Shen, Roh, Kumari and Rosen 80 In our current study, a maternal HFD with or without a post-weaning HFD in non-IUGR male rats (NHR and NHH) increased visceral adiposity and adipocyte size, without changing circulating corticosterone or visceral adipose 11-β HSD 1 protein. Despite increased circulating corticosterone in female IUGR rats on a regular diet (IRR females), visceral and subcutaneous adiposity, GR-α protein and Abca1 mRNA levels are unchanged, suggesting that mildly increased circulating corticosterone alone does not necessarily increase adiposity or glucocorticoid signaling. Conversely, female non-IUGR and IUGR rats exposed to a maternal HFD and weaned to a regular diet (NHR and IHR) did not have increased circulating corticosterone, but had increased GR-α protein in VAT and increased visceral adipocyte diameter. NHR and IHR female rats did not have increased downstream transcription target Abca1 mRNA levels in VAT, suggesting that the increased adipocyte diameter may be due alterations in non-glucocorticoid pathways. GR-α protein was decreased in NHR males, which may indicate an appropriately decreased response to normal levels of circulating glucocorticoids as a result of increased visceral adiposity. Male IHH GR-α protein is unchanged from NHH in both visceral and subcutaneous adipose depots, which combined with the significantly increased circulating glucocorticoids may in part explain the etiology of increased visceral and subcutaneous adipose depot volume and adipocyte size. Increased circulating corticosterone and stable GR-α protein may allow for increased GR signaling and thus increased visceral adipose in the IHH male rats, as suggested by increased GR transcription target Pparγ2 protein and Abca1 mRNA levels, and similar to results found in our previous study of maternal tobacco smoke exposure on offspring adiposity.Reference Zinkhan, Lang and Yu 66
The perinatal environment also impacts adipocyte expression of Pparγ. Increased adiposity and adipocyte size is associated with increased Pparγ levels in HFD fed rats, particularly when fed a HFD from the time of weaning.Reference Cheng, Ton, Phang, Tan and Abdul Kadir 65 Further, adipose tissue Pparγ protein levels are increased by both maternal HFD feeding and IUGR throughout life.Reference Desai, Jellyman, Han, Lane and Ross 81 In our study, maternal HFD feeding alone and IUGR alone did not increase adipose tissue Pparγ protein levels. However, we found that the combination of a maternal HFD and IUGR increased Pparγ2 protein and visceral adiposity in male rats without increasing serum triglycerides. In males, maternal HFD or IUGR rats may compensate for increased serum fatty acids by appropriately depositing the fat into adipocytes, a physiologic storage depot for circulating fatty acids, thus increasing adipocyte hypertrophy. Increased visceral adipose Pparγ2 protein in IHH males may have contributed to increased circulating lipid deposition into the visceral adipocytes. Conversely, IHH female rats had increased SAT volume, adipocyte size and serum triglycerides, and decreased visceral and subcutaneous adipose Pparγ isoforms. Translational repression of Pparγ via administration of microRNA miR-130b in HFD fed obese mice reduced fat deposition, a process suggested to be mediated by increased lipolysis.Reference Pan, Yang and Jia 82 Taken together, our findings suggest that IHH females have either decreased uptake of serum lipids into this physiologic storage depot or enhanced lipolysis.
Many of the findings of our study were sex-specific. Sex-specific differences in outcomes were also seen with a sex-bias toward female offspring secondary to a maternal HFD intake during pregnancy in our model. Sex-bias during pregnancy can be due to multiple factors including maternal diet, fat source, stress and animal genetics, though the mechanisms through which this occurs remain unclear.Reference Fountain, Mao and Whyte 83 – Reference Obel, Henriksen, Secher, Eskenazi and Hedegaard 88 Often these stressful events result in a greater proportion of female offspring at birth. While we do not have data on maternal stress in our rat model to determine whether stress may have played a role in sex-bias at birth, placing the female rats on an HFD before conception could have contributed to the decreased male:female sex ratio. Long-term metabolic outcomes are also impacted after exposure to a HFD or IUGR. Male mice exposed to a maternal HFD had higher corticosterone levels than male mice exposed to a maternal control diet, while female mice remained unaffected.Reference Chin, Schmidt and Martel 89 Further, despite higher corticosterone levels, male mice exposed to a maternal HFD did not have changes in 11-β HSD 1 protein levels.Reference Chin, Schmidt and Martel 89 Increased adipose depot volume and adipocyte size often occur with decreased circulating or local production of adiponectin or increased leptin due to leptin resistance.Reference Pelleymounter, Cullen and Baker 90 , Reference Aoki, Kawada and Sugimoto 91 Consistent with increased adipose depot volume and adipocyte size, increased leptin protein was found in the visceral and subcutaneous adipose of IHH male rats while decreased circulating adiponectin was found in the IHH female rats. The reason for the differential metabolic effects between male and female rats remain unclear, but may be secondary to differential hormonal regulation of the hypothalamic–pituitary–adrenal axis.Reference Chen, Wang, Yu, Liu and Pearce 92 , Reference Nelson, Hendy and Shukin 93 While we have not examined the potential role of sex-specific neuroendocrine alteration in our rat model, other authors have shown that increased sex steroids can induce systemic cortisol release.Reference Porter, Lincoln and Naylor 94
Differential effects between VAT and SAT were seen in our study, including increased visceral adiposity outpacing that of the subcutaneous adiposity in male rats. VAT remains more strongly correlated with metabolic risk. Increased VAT was more highly correlated with metabolic syndrome than increased SAT in 3001 healthy men and women from the Framingham Heart Study.Reference Fox, Massaro and Hoffmann 95 The mechanism through which visceral adipose excess may confer a greater metabolic risk is not fully defined and likely multifactorial, possibly including increased metabolic activity with secretion of vasoactive substances, inflammatory markers, adipocytokines, growth factors and markers of fibrinolysis.Reference Yatagai, Nagasaka and Taniguchi 96 – Reference Miyazawa-Hoshimoto, Takahashi, Bujo, Hashimoto and Saito 102 Further, the protein and mRNA expression profiles between VAT and SAT vary.Reference Giusti, Suter, Verdumo, Gaillard, Burckhardt and Pralong 103 – Reference Perrini, Laviola and Cignarelli 105 The inherent differences between VAT and SAT and sex-specific responses of these tissues to perinatal insults likely contributed to the increased depot-specific adiposity seen in this rat model.
Several potential limitations of this study must be addressed. First, the HFD rat food contains less protein than the regular diet food. The HFD protein content is sufficient for normal mammalian growth, and was selected to be similar to the typical American diet and to prevent severe decreases in carbohydrate intake in this model.Reference Wright, Wang, Kennedy-Stephenson and Ervin 50 , 51 Dietary changes in carbohydrate, fat and protein intake have been shown to be associated with persistent metabolic changes in the offspring, therefore we anticipate that the alteration of carbohydrate, fat and protein each contributed to the metabolic changes in our HFD fed rats.Reference Vadlamudi, Kalhan and Patel 28 , Reference Han, Won and Kwon 106 , Reference Xie, Zhang and Rasmussen 107 However, despite decreased protein and carbohydrate intake in the dam, offspring from dams fed a HFD weighed the same at birth as offspring from dams fed a regular diet, suggesting that the maternal HFD was sufficient for normal growth. Second, we used an anesthesia control group to generate our non-IUGR rats because sham surgery induces mild growth restriction,Reference Zinkhan, Zalla and Carpenter 49 therefore we surmise that data obtained from a sham surgery group would be partway between that of the bilateral uterine artery ligation and current anesthesia control. Thus, inclusion of a separate sham surgery group may not further enrich our knowledge of how IUGR and a maternal HFD impact development of obesity. Third, we have not manipulated glucocorticoid signaling in this model to demonstrate a more definitive mechanism through which IUGR and a maternal HFD increase adiposity. Lastly, our data only focuses on one time-point (P60) to measure early adulthood onset of obesity. Studies are underway to determine when adiposity increases in this rat model and if increased adiposity secondary to an adverse in utero environment persists.
In conclusion, the combined in utero insults of IUGR and a maternal HFD increased visceral and subcutaneous adiposity and serum corticosterone and triglycerides in adult rat offspring more than either in utero insult alone and in a sex-specific manner. Weaning offspring from the combined in utero insults to a regular rat chow did not fully normalize the increased visceral adiposity seen in male rats. Our findings suggest that the increased systemic but not locally produced corticosterone in IHH male rats may have increased glucocorticoid signaling, leading to increased visceral and subcutaneous adiposity despite stable caloric intake. Further, increased visceral and subcutaneous adiposity occurred without increasing body weight, making body weight gain a poor indicator of adiposity in our rat model. Implications of this work are two-fold; first, body weight remains a poor indicator of visceral adipose accumulation resulting from perinatal insults, and second, even when consuming a low-fat diet, the impact of an adverse perinatal environment on increased visceral adiposity persists into early adulthood.
Acknowledgments
The authors would like to thank Osama Abdullah and the University of Utah Small Animal Imaging Core for performing MRI scans, and Merica Hale, Summer Bowser, Daniel Curtis and Heather Hansen at the University of Utah, Department of Dermatology, Dermatopathology Laboratory for assistance with fixed tissue preparation and staining.
Financial Support
Funding for this study was provided by the Primary Children’s Hospital Foundation Early Career Award to E.K.Z.
Conflicts of Interest
None.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174418000016