Introduction
The prevalence of obesity worldwide has more than doubled between 1980 and 2014, 1 and is now a global health issue. Not only is obesity more prevalent in adults, but the prevalence of infant, childhood and adolescent obesity is increasing at an alarming rate around the world, affecting many low- and middle-income countries. In 2016, an estimated 41 million children under 5 years of age were overweight [body mass index (BMI)⩾85th percentile and <95th percentile)] or obese (BMI⩾95th percentile); a dramatic increase from 32 million globally in 1990. 2 , 3 In Australia, 26% of children aged 5–14 are considered overweight or obese 4 and Aboriginal and Torres Strait Islander children (aged 2–14 years) are more likely than non-Indigenous children to be overweight or obese (30 v. 25%). 5 It is undeniable that being overweight or obese in childhood and adolescence has adverse health consequences, increasing the likelihood of many health problems in adult life, including cardiovascular disease, cancer, diabetes, osteoarthritis and chronic kidney disease.Reference Ng, Fleming and Robinson 6 – Reference Umer, Kelley and Cottrell 8
The intrauterine environment can have long-lasting consequences for the infant related to obesity and future development of chronic disease.Reference Barker 9 , Reference Warner and Ozanne 10 Being born premature (⩽37 weeks gestation) or small at birth (below the 10th percentile for gestational age) is associated with greater adiposity and an increased risk of being overweight or obese throughout all stages of life and contributes to the increased risk of metabolic disease, cardiovascular disease and diabetes in adult life.Reference Whincup, Kaye and Owen 11 – Reference Mathai, Derraik and Cutfield 18 Furthermore, low birth weight (LBW) premature infants exhibit ‘catch up growth’ in the early years of their life and experience a steep gain in weight.Reference Casey 19 Excess weight gain during infancy increases the likelihood of developing chronic diseases such as obesity, cardiovascular disease and diabetes as an adult.Reference Gluckman, Hanson, Cooper and Thornburg 20 , Reference Martin, Connelly, Bland and Reilly 21
Maternal overweight or obesity and maternal hyperglycemia (including gestational diabetes) also increases the likelihood of offspring obesity during infancy, childhood and later in life,Reference Yu, Han and Zhu 22 – Reference Eriksson, Sandboge, Salonen, Kajantie and Osmond 28 and may ‘program’ later cardio-metabolic health in offspring.Reference Boney, Verma, Tucker and Vohr 29 In the Helsinki Birth Cohort Study of individuals born during 1934–1944, higher maternal BMI was associated with greater adiposity,Reference Eriksson, Sandboge, Salonen, Kajantie and Osmond 28 and an increased risk of cardiovascular disease [hazard ratio (HR) 1.026; P=0.002], type 2 diabetes (HR 1.04; P=0.004) among offspring.Reference Eriksson, Sandboge, Salonen, Kajantie and Osmond 30 This emphasizes the importance of ensuring the optimal nutritional health of women of reproductive age and early preventive interventions for overweight and obesity.
The Aboriginal and Torres Strait Islander peoples of Australia (or the Indigenous community) are one of the most socially disadvantaged populations in the country. They continue to experience poorer health outcomes and a life expectancy 10 years lower than non-Indigenous Australians due to generations of social and economic disadvantage. 31 The high rates and burden of preventable chronic disease is a significant contributor to premature mortality and the large disparity in life expectancy. Indigenous mothers are 1.6 times more likely to be overweight or obese, as well as 1.6 times and 3.5 times more likely to have gestational diabetes and pre-existing diabetes, respectively, than non-Indigenous mothers. 32 A recent systematic review reported that up to 22% of Indigenous infants aged 2–4 years are overweight or obese.Reference Dyer, Gomersall and Smithers 33 In total, 14% of Indigenous Australian babies are born preterm, compared with 8% of babies of non-Indigenous mothers, and 14.1% are born small for gestational age (SGA), compared with 9.1% of babies of non-Indigenous mothers. 32
Examination of the associations between being born preterm or exposed to an obesogenic intrauterine environment and risk of childhood obesity has direct relevance for the Aboriginal and Torres Strait Islander population, given the elevated rates of preterm birth, LBW, maternal obesity and chronic diseases. 5 , Reference Vos, Barker, Begg, Stanley and Lopez 34 This link has been explored within other populations, but current evidence for an association in Aboriginal and Torres Strait Islander Australians is lacking.Reference McNamara, Gubhaju, Chamberlain, Stanley and Eades 35
Therefore, the objectives of the current study were: (i) to determine any associations between maternal adiposity or non-fasting plasma glucose and offspring BMI and adiposity in early childhood (1–3 years); and (ii) to determine any associations between preterm birth or Gestation Related-Optimal Weight (GROW) centiles and offspring BMI and adiposity in early childhood (1–3 years) in Indigenous Australian children.
Methods
Ethics
The Gomeroi gaaynggal program has received ethics approval from the Hunter New England Human Research Ethics Committee (Ref. No. 08/05/21/4.01), New South Wales Human Research Ethics Committee (Ref. No. HREC/08/HNE/129) and Aboriginal Health and Medical Research Ethics Committee (Ref. No. 654/08).
Study design and setting
The Gomeroi gaaynggal ArtsHealth program is a prospective pregnancy through to childhood longitudinal cohort where mothers and their infants are followed until the child reaches 5 years of age. The study is based at two locations in New South Wales (NSW), Australia. These are the rural community of Tamworth, NSW, and the smaller remote community of Walgett, NSW. Further details of the Gomeroi gaaynggal study have been published elsewhere.Reference Ashman, Collins and Weatherall 36
Participants
Pregnant women who identified as Indigenous Australians or who were carrying an Indigenous infant were eligible to participate. The study has been recruiting since 2010 and recruitment is ongoing at the time of publication. Pregnant women are eligible to participate at any time in gestation. Participants are eligible to consent provided they were at least 16 years of age. If under 16 years, consent is also required from their guardian. Recruitment is undertaken by Indigenous research assistants in antenatal clinics in each community who spend time explaining the study to the potential participant and any family members. The additional time spent in recruitment is a cultural necessity and many participants will not consent to the study unless this time is taken. This additional time assists in building trustful relationships between the research team and the participant and is an essential component to long-term engagement in the study. Women also provide written consent to participate in the follow-up study.
Timing of study visits
Although every effort was made to see study participants at every study visit, this was not always feasible. Study visits were timed to see participants once per trimester. For the purpose of this study maternal measures (non-fasting plasma glucose, body fat percentage and visceral fat area) were collected at their first visit, regardless of when in gestation this occurred. Follow-up visits with infants occurred at 3 (range 1.6–4.3 months), 6 (4.5–7.8 months), 9 (8–10.8 months) and 12 (11.4–18.4 months) months in the first year of life and annually until the child reached 5 years. For the purpose of this analysis, we have only included data from follow-up visits with infants up until the age of 3 years (ranges of 2 and 3 years of 19–29.9 months and 33.1–43.3 months, respectively). Data collected at each time point for pregnant women and their offspring participating in the Gomeroi gaaynggal study is outlined in Supplementary Table S1. More detailed information has been published elsewhere.Reference Ashman, Collins and Weatherall 36
Measures
Pregnancy and delivery measures
All participants were asked to provide information on their obstetric history and self-report other pre-existing conditions including diabetes, asthma and hypertension. Maternal BMI was calculated from measured height (ht) and self-reported pre-pregnancy weight (wt) at their first visit during pregnancy [wt (kg)/ht (m2)] and each participant was subsequently categorized as being underweight (BMI<18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2) or obese (BMI⩾30.0 kg/m2) according to World Health Organization categories. 37 Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg. For participants who could not recall their pre-pregnancy weight, their pre-pregnancy BMI was calculated using weight measured at less than 12 weeks’ gestation.
All participants had their body composition (percentage body fat and visceral fat area) obtained at each study visit using the InBody 720TM body composition bio-impedance scales (Biospace Co., Seoul, South Korea). InBody 720 (Biospace, Korea) is a multi-frequency body composition analyzer with four pairs of electrodes (octapolar technology) embedded into the handles (thumb and palm electrodes) and floor scale (ball of foot and heel electrodes) of the analyzer. By using low- and high-frequency electric currents which flow at different rates through the body, depending on body composition,Reference Dehghan and Merchant 38 the impedance measures are used to estimate total body water, fat free mass, % body fat and other body composition components.Reference Ellis, Bell and Chertow 39 Participants step onto the foot electrodes barefoot and stand still until body weight is measured. Then the participants are asked to grasp the hand electrode cables and hold the thumb and palm electrode gently. Hands are held ~15° away from the body and the participant is asked to stay still until measurements are complete. Numerous previous studies have evaluated the reliability and accuracy of using different reference methods to determine body composition, for example bioelectrical impedance analysis (BIA) and dual-energy X-ray absorptiometry DEXA, or in different populations.Reference Pietrobelli, Rubiano, St-Onge and Heymsfield 40 – Reference Bedogni, Malavolti and Severi 44 Maternal blood samples were collected at each antenatal visit, centrifuged and non-fasting plasma glucose was tested via the Abbott Architect Automated analyser. Fasting blood sugars and oral glucose tolerance test are currently not accessible in this study, thus the non-fasting plasma glucose measured during routine antenatal visits were reported and used.
Gestational age and estimated date of delivery were calculated by a qualified ultrasonographer using a Portable Phillips CX50 Ultrasound unit with a convex 5 MHz transducer at the first study visit during pregnancy. Birth weight, length and head circumference measures were taken from the data recorded by hospital staff at the time of delivery. A birth weight centile was calculated for the infant using the GROW Customized Birth Weight Centile calculator.Reference Gardosi and Francis 45 This calculator individually adjusts for gestational age, maternal height, weight, parity, ethnicity and infant sex. Where variables were missing, for example maternal height, partial customization was undertaken automatically in the calculator by using an estimate or population average.
Postpartum infant measures
An Accredited Practicing Dietitian (APD) with a Level 1 Anthropometry certification from the International Society for the Advancement of Kinanthropometry (ISAK) undertook all anthropometry measures of mothers and their infants. Using Harpenden skinfold calipers (Baty International, RH15 9LR, England, CE 0120), infant skinfold thicknesses were measured at the following sites: subscapular, biceps, iliac crest, front thigh and medial calf. Circumferences were measured at infant’s head, mid-upper arm, abdomen, mid-thigh and calf. Skinfold thicknesses were taken sequentially and then repeated in that same order. If two skinfold measurements differed by more than 0.5 mm, a third measurement was repeated. The two measurements that were within 0.5 mm of each other were averaged and used for the analysis. Infant weight was measured in light clothing to the nearest 0.01 kg using digital baby scales (model BD-590; Tanita Corporation, Tokyo, Japan) and infant length was measured crown-to-heel without shoes to the nearest 0.1 cm using a recumbent length board (model MZ 10027; Wedderburn, Germany) if they were unable to stand. Once able to stand, the infant was weighed in light clothing to the nearest 0.01 kg using the InBody Scales and infant standing height was measured without shoes to the nearest 0.1 cm using a wall-mounted stadiometer with a headboard (model 0123; Seca, Germany) with the child’s head positioned in the Frankfort plane. The definition of overweight and obesity at age 2–3 years was defined using the International Obesity Task Force cut-offs for BMI, which defines BMI according to age- and gender-specific z-score cut-points for children.Reference Cole 46
Nutritional assessment of infant
Measures of dietary intake of infants are collected via the Infant Feeding Recall form. It collects information on initiation and duration of breastfeeding and if infants have regularly consumed and age of initiation of the following items: infant formula, cows’ milk, milk substitutes and solid foods. This information is collected at each postpartum follow-up visit.
Statistical analysis
Statistical analyses were performed using the statistical software package Intercooled Stata, version 14 (Stata Corp LP, College Station, TX, USA). Variables used in the analyses were tested for normality. P values<0.05 were considered statistically significant.
The χ2 statistics for categorical data were used to compare the birth outcomes between male and female infants. Two sample t-tests or the Kruskal–Wallis test for continuous data were used to compare offspring anthropometry measurements at 1–3 years in preterm and term infants. Analysis of variance testing, with Bonferroni correction for multiple comparisons, was undertaken to assess the differences in offspring anthropometry measurements at 1–3 years between SGA, large for gestational age (LGA) and appropriately grown for gestational age (AGA) infants.
Multiple linear regression models were used to assess how much the maternal characteristics (percentage body fat, visceral fat and non-fasting plasma glucose) during pregnancy explain the variation in offspring anthropometry measurements at birth and at 1–3 years, while adjusting for maternal height, smoking and fetal sex. Similarly, multiple linear regression models were performed to assess how offspring GROW birth weight centiles explain the variation in offspring anthropometry measurements at 1–3 years, while adjusting for maternal height, smoking and fetal sex. Adjusted R 2 values and coefficients (95% CI) are reported, with R 2⩾0.26 considered large, ⩾0.13 to <0.26 medium and ⩽0.02 small.Reference Cohen 47
Results
Maternal characteristics
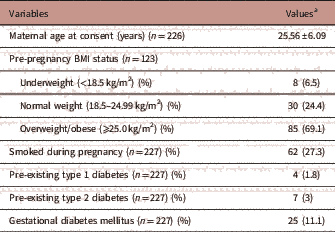
The maternal characteristics of the participants are outlined in Table 1. The mean maternal age of the participants was 25.56±6.09 years (mean±s.d.) at the time of consent (n=226). In total, 6.5% (n=8/123) of mothers were underweight (BMI<18.5 kg/m2), 24.4% (n=30/123) were within the normal weight range (BMI 18.5–24.99 kg/m2) and 69.1% (n=85/123) were overweight/obese (BMI⩾25.0 kg/m2). The proportion who self-reported smoking during pregnancy was 27.3% (n=62/227). Within the cohort, 1.8% (n=4/227) had pre-existing type 1 diabetes, 3% (n=7/227) had type 2 diabetes and 11.1% (n=25/227) developed gestational diabetes mellitus during their pregnancy.
TABLE 1 Maternal characteristics
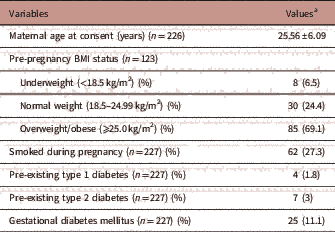
a Values are mean±s.d. or n (%).
The median maternal non-fasting plasma glucose levels were 4.1 (3.7, 4.95) mmol/l [interquartile range (IQR)] (n=184), measured at a mean gestational age of 23.1 weeks (range 8–40.4). For trimester 1 (mean gestational age of 11.1 weeks), the median maternal non-fasting plasma glucose levels were 4.55 mmol/l (IQR: 4, 5.3, n=14). For trimester 2 (mean gestational age of 20.3 weeks), the median maternal non-fasting plasma glucose levels were 4 mmol/l (IQR: 3.6, 4.8, n=116). For trimester 3 (mean gestational age of 32.1 weeks), the median maternal non-fasting plasma glucose levels was 4.4 mmol/l (IQR: 3.9, 5, n=54).
To assess maternal adiposity, we examined measures of percentage body fat and visceral fat area at the first antenatal study visit. The mean percentage body fat of pregnant women in the cohort was 41.3% (s.d. 12.75, n=133) and the mean visceral fat area was 163.31 cm2 (s.d.: 96.8, n=132), both measured at mean 23.7 weeks gestation (range 5.8–38.7 weeks).
Birth outcomes
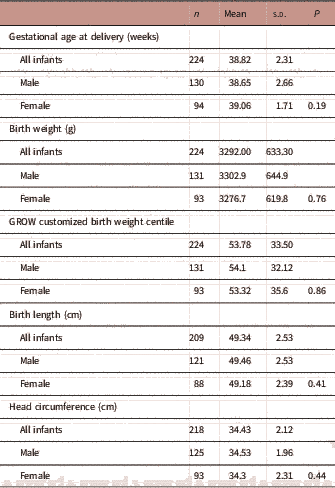
As of August 2017, there were 245 infants born from the Gomeroi gaaynggal cohort with known birth outcomes, 18 of these were twins and were excluded from further analyses. Of the singleton infants (n=227), 58.6% of were male. The mean birth weight, length, head circumference and gestational age at delivery of infants in the cohort are detailed in Table 2. There was no significant difference in the mean gestational age at delivery, birth weight, length, GROW customized birth weight centile, or head circumference between male and female infants (Table 2). A total of 11.9% of singleton infants were born preterm (n=27/227) with the rate tending to be higher among male infants; 15% of male (20/133) and 7.5% of female (7/94) infants were born preterm (P=0.082).
TABLE 2 Birth outcomes of Indigenous infants (n=227) in the Gomeroi gaaynggal cohort study
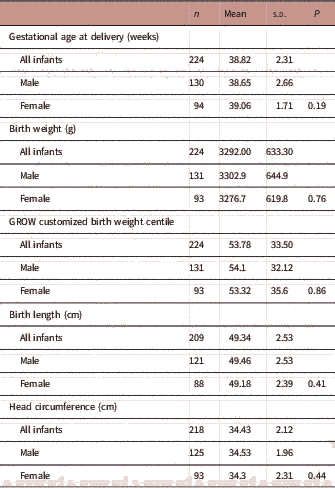
Of all infants, 14.3% were born SGA (32/223) and rates were similar between male and female offspring (13.8 and 15%, respectively, P=0.80). Conversely, 19.7% of babies were born LGA (44/223) with rates in female and male infants being similar (23.6 and 16.9%, respectively, P=0.21). The majority of the babies (64%) were delivered by normal vaginal birth.
Association between maternal body composition and non-fasting plasma glucose in pregnancy and offspring birth outcomes
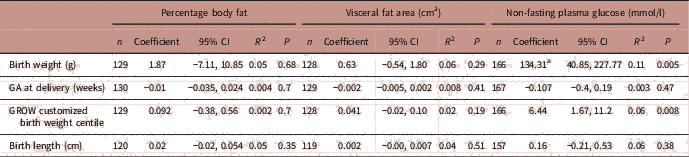
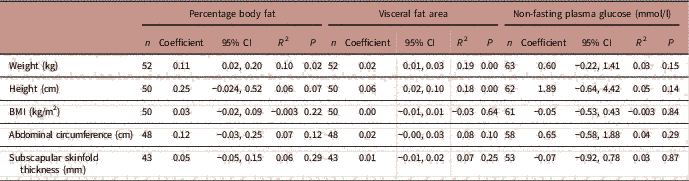
There were no associations between maternal percentage body fat or visceral fat area and offspring birth weight or length (Table 3). In contrast, maternal non-fasting plasma glucose concentrations were positively associated with infant birth weights (P=0.005) and GROW adjusted birth weight centiles (P=0.008), but not with gestational age at delivery nor infant length at birth.
TABLE 3 Associations between maternal measures of adiposity and glucose levels and birth outcomes

GA, gestational age; CI, confidence interval; R 2, partial correlation coefficient.
Adjusted for smoking, fetal sex, maternal height.
a Interpreted as one unit increase in maternal non-fasting plasma glucose is associated with 134.3 g increase in birth weight.
Infant anthropometry measurements from birth to 3 years
The mean infant anthropometry measurements at 3, 6 and 9 months and early childhood anthropometry measurements at 1, 2 and 3 years are outlined in Supplementary Table S2. Overall there was little difference between male and female anthropometry measurements up to 3 years of age. Using the BMI cut-off points sourced from Cole et al.,Reference Cole 46 at 2-years follow-up, the majority of the children (n=27/36, 75%) were within the normal weight range, 8% (n=3/36) were underweight, and 16% (n=6/36) were overweight or obese. At 3-year follow-up, 68.8% (n=11/16) were within the normal weight range whereas 31.3% (n=5/16) were overweight or obese. Mean anthropometric measures (weight-for-age percentiles, length-for-age percentiles, head circumference-for-age percentiles) of infants indicate appropriate growth trajectories with no apparent stunting or wasting (Supplementary Figures S1 and S2).
Infant dietary intake
Of the whole cohort, infant feeding data were available for 96/227 infants and indicates that 20 infants were never breastfed (20.8%), 76 (79.2%) were breastfed, with 27 (35.5%) of these infants breastfed and formula-fed concurrently. Of those who were breastfed, 47 (61.8%) were breastfed for⩽3 months, 3 (3.9%) were breastfed for between 4 and 6 months, and 26 (34.2%) were breastfed for⩾6 months.
Association between gestation at delivery and birth weight on offspring anthropometry measurements at 1–3 years of age
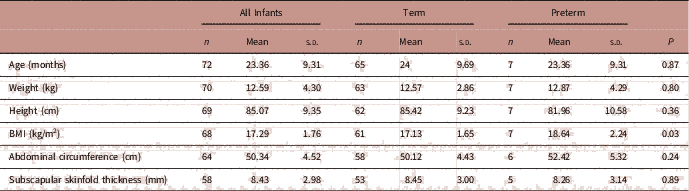
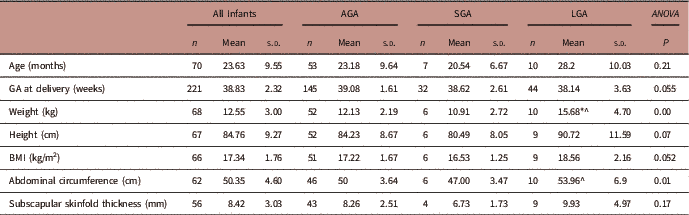
To assess the impact of preterm delivery and birth weight on early childhood growth, data from follow-up visits between 1 and 3 years were pooled and repeated measures on the same offspring were removed, using only data from the latest follow-up visit. The mean age of early childhood follow-up was 23.4 months. Overall 73 infants had at least one follow-up visit between 1 and 3 years of age; 7 of these were born preterm. There was no difference in height, weight, abdominal circumference or subscapular skinfold thickness at 1–3 years of age in infants born preterm or term (Table 4). However, BMI in early childhood (1–3 years) was significantly higher in offspring born preterm compared with those born at term (P=0.03, Table 4).
TABLE 4 Associations between preterm delivery and early childhood anthropometry measurements at 1–3 years
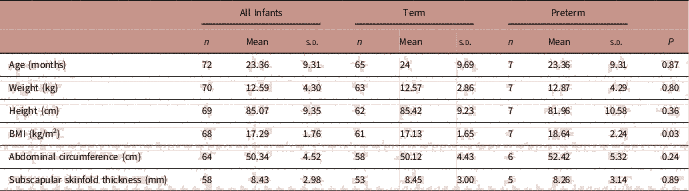
Adjusted for sex, maternal height, maternal smoking in pregnancy.
Being born SGA or LGA significantly affected early childhood weight, and abdominal circumference (ANOVA, Table 5). After testing for multiple comparisons, children who were born SGA had significantly lower body weights (P=0.004) and abdominal circumferences (P=0.008) than children born LGA at mean age of 23.4 months. Children who were born LGA had significantly higher body weight in early childhood than those who were born AGA (P=0.001). Table 6 summarizes the adjusted linear regression analyses between GROW customized birth weight centiles and early childhood anthropometry measurements (1–3 years). In the adjusted regression model (adjusted for smoking, fetal sex and maternal height), GROW customized birth weight centiles significantly explained the variation in body weight, BMI and abdominal circumference in early childhood (1–3 years).
TABLE 5 Associations between small for gestational age (SGA) and large for gestational age (LGA) and early childhood anthropometry measurements at 1–3 years
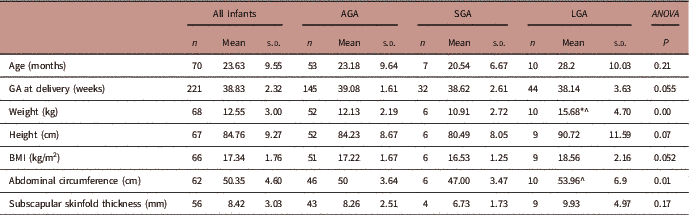
AGA, appropriate for gestational age.
*Significant difference to AGA; ^ significant difference to SGA.
TABLE 6 Associations between Gestation Related-Optimal Weight (GROW) customized birth weight centile and early childhood anthropometry measurements at 1–3 years
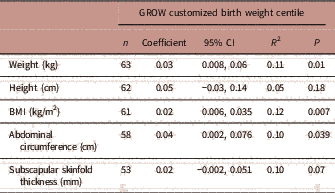
CI, confidence interval; R 2, partial correlation coefficient.
Adjusted for smoking, fetal sex, maternal height.
Association between maternal adiposity and non-fasting plasma glucose levels in pregnancy on offspring anthropometry measurements at 1–3 years of age
Table 7 summarizes the adjusted linear regression analyses between maternal percentage body fat, visceral fat area and non-fasting plasma glucose and early childhood anthropometry measurements (1–3 years). In the adjusted regression model (adjusted for smoking, fetal sex and maternal height), maternal percentage body fat significantly explained the variation in infant body weight while maternal visceral fat area significantly explained the variation in infant body weight and height.
TABLE 7 Associations between maternal adiposity and non-fasting plasma glucose levels and early childhood anthropometry measurements at 1–3 years
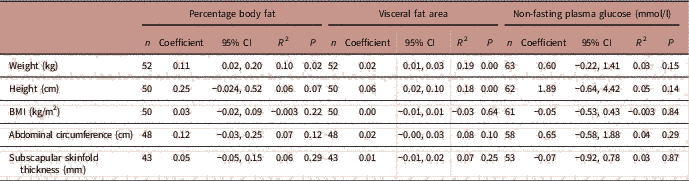
CI, confidence interval; R 2, partial correlation coefficient.
Adjusted for smoking, fetal sex, maternal height.
Discussion
A consistent body of evidence demonstrates that being overweight or obese in childhood and adolescence has adverse consequences on overall health and leads to premature mortality and increased physical morbidity in adulthood.Reference Reilly and Kelly 48 Identification of early-life risk factors for developing obesity in Indigenous Australian children is therefore essential for the development of public health interventions and policy to reduce childhood obesity in this high-risk population group. Although there have been previous descriptive reports from the Gomeroi gaaynggal cohort outlining the various maternal determinants of pregnancy outcomes, including inflammation, cigarette smoke exposure and pre-pregnancy BMI,Reference Pringle, Weatherall and Roberts 49 , Reference Pringle, Rae and Weatherall 50 none have reported on the associations between maternal adiposity, preterm birth or birth weight and early childhood weight status, as has been examined in the current study.
This study demonstrates in an Australian Indigenous population cohort, that being born preterm is associated with an increase in BMI in early childhood (mean age 23.4 months) placing these preterm infants on a trajectory for an increased risk of metabolic disease in later life. Although an association of this type has been demonstrated in a variety of populations,Reference Thomas, Parkinson and Hyde 16 – Reference Mathai, Derraik and Cutfield 18 this has never before been investigated in Indigenous Australian infants. ~12% of Indigenous Australian infants in this cohort and 14.3% of Indigenous Australian babies nationally were born preterm, compared with 8.3% of babies of non-Indigenous mothers. 32 This significant disparity is of great concern. The risk factors for preterm birth, which include smoking during pregnancy, poor nutrition and psychosocial stress related to economic disadvantage, are more prevalent in Indigenous women. Thus, the importance of prioritizing health for Indigenous women during the prenatal and antenatal periods cannot be over-emphasized.
We have also demonstrated, for the first time, that being born LGA is associated with higher BMI in early childhood (mean age 23.4 months) among Aboriginal and Torres Strait Islander children. The association between birth weight and early childhood BMI is similar to that reported in a large systematic review of 282 studies which demonstrated that high infant birth weight is consistently associated with later childhood obesity.Reference Woo Baidal, Locks and Cheng 51 Kapral et al. Reference Kapral, Miller, Scharf, Gurka and DeBoer 52 demonstrated that there is a 70–130% increased risk of overweight or obesity among children of high birth weight compared with children of normal birth weight. Furthermore, it has been demonstrated that children who are LGA at birth and exposed to an intrauterine environment of either maternal diabetes or obesity are at increased risk of developing metabolic syndrome.Reference Kapral, Miller, Scharf, Gurka and DeBoer 52 Boney et al.Reference Boney, Verma, Tucker and Vohr 29 highlighted that children born LGA have an increased risk of metabolic syndrome diagnosis by age 11 years (HR 2.19, 95% CI: 1.25–3.82; P=0.01). In the current cohort, there is a higher percentage of infants who are of LGA (19.7%) compared with those born SGA (14.3%). The rate of Indigenous infants who were SGA in the current cohort is similar to that reported nationally (14.1%) and is 1.5 times higher than that for non-Indigenous infants (9.1%). 32 To the best of our knowledge, there is currently no available information regarding the rate of LGA in Indigenous and non-Indigenous Australian babies nationally to compare with. Being born LGA is also associated with adverse long-term health outcomes such as obesity, cancer, asthma and diabetes.Reference Hadfield, Lain and Simpson 53 Increased attention to and follow-up of Indigenous children who are born at higher birth weights is therefore warranted. Since there is a relatively low sample size in the current study, results should be interpreted with caution. Ideally the prevalence of both SGA and LGA should be examined in a larger cohort with infant measurements also adjusted for gestational age.
In this prospective follow-up study of mothers and offspring from the Gomeroi gaaynggal cohort, we found that maternal adiposity, measured as percentage body fat and visceral fat area, are positively associated with early childhood weight, up to a mean age of 2 years. In the literature, maternal pre-pregnancy BMI or obesity is positively associated with children’s overweight or obesity risk,Reference Yu, Han and Zhu 22 , Reference Castillo-Laura, Santos, Quadros and Matijasevich 54 and there is also compelling evidence from animal studies that maternal obesity alters offspring phenotype in a similar way.Reference Long, George and Uthlaut 55 – Reference Samuelsson, Matthews and Argenton 57 However, our study was unable to find an association between maternal percentage body fat, visceral fat area and offspring abdominal circumference or subscapular skinfold thickness at a mean age of 2 years. The relatively small cohort and the early childhood follow-up age of 1–3 years may have limited our ability to detect an association, thus longer-term follow-up and increase in cohort size of the Gomeroi gaaynggal cohort will endeavor to answer this question. The relatively small sample size together with characteristics related to socioeconomic indices, family income, educational level, maternal smoking during pregnancy, and duration of breastfeeding are potential confounding variables which may limit the ability to detect relationships as statistically significant in the current study. From a recent systematic review,Reference Castillo-Laura, Santos, Quadros and Matijasevich 54 it is clear that the majority of studies assessed the relationship between pre-pregnancy BMI and the offspring’s body composition in terms of childhood adiposity obtained by indirect methods, for example DEXA. Further research that assesses the association between maternal body composition (other than maternal weight and BMI) and the offspring’s body composition obtained by skinfold thickness are required, especially in lower socioeconomic communities where certain factors, such as cost and accessibility, limit the use of reference laboratory methods.
According to the developmental over-nutrition hypothesis, higher concentrations of glucose, free fatty acids and amino acids are delivered to the developing fetus, resulting in permanent alterations in appetite control, neuroendocrine functioning and/or energy metabolism in the developing fetus.Reference Drake and Reynolds 58 Additionally, not only are the infants larger at birth, they have a higher risk of adiposity in later life.Reference Dabelea, Hanson and Lindsay 25 , Reference Clausen, Mathiesen and Hansen 59 , Reference Boerschmann, Pflüger, Henneberger, Ziegler and Hummel 60 The relationship between maternal glycemia and obesity in the offspring is of interest. In line with this association, our study has shown a positive correlation between maternal non-fasting plasma glucose concentrations at a mean gestational age of 23 weeks and offspring birth weight and GROW customized birth weight centile. However, we did not detect any association between maternal non-fasting plasma glucose levels in pregnancy and early childhood BMI or adiposity. Similarly, the Belfast Hyperglycemia and Adverse Pregnancy Outcome (HAPO) follow-up study found little association between maternal plasma glucose (fasting glucose P=0.08; 1-h glucose P=0.22; 2-h glucose P=0.36) during pregnancy at 28 weeks’ and obesity in 2-year-old offspring.Reference Pettitt, McKenna and McLaughlin 61 It is possible that the relationship between maternal diabetes and later childhood adiposity only becomes evident at a later age,Reference Krishnaveni, Hill and Leary 62 thus further prospective studies with longer-term follow-up are required. Some previous studies have reported that intrauterine exposure to maternal hyperglycemia (i.e. gestational diabetes) is associated with greater levels of abdominal fat in older children and adolescent youthReference Crume, Ogden and West 26 , Reference Krishnaveni, Hill and Leary 62 , Reference Wright, Rifas-Shiman and Rich-Edwards 63 and is also associated with greater risk of adverse health consequences such as type 2 diabetes and cardiovascular disease.Reference Bergman, Kim and Catalano 64 For example, the Pima Indian population has been studied extensively due to their high rates of chronic disease. The strongest risk factor predicting obesity development in this population is intrauterine exposure to maternal diabetes, independent of maternal obesity and infant birth weight.Reference Dabelea and Pettitt 65 In a study of almost 10,000 children, Hillier et al.Reference Hillier, Pedula and Schmidt 66 found that the highest quartile of maternal hyperglycemia on the 50-g 1-h glucose challenge test during pregnancy, was associated with a significantly higher level of childhood obesity (85th and 95th percentiles) when compared with the lowest quartile of glucose concentration (P trend<0.0001). In this cohort, 15.9% of the women developed gestational diabetes or had pre-existing diabetes during pregnancy, placing the infants at increased risk of being born LGA, developing obesity in early childhood and increasing the risk of metabolic disease in later life.
This is of concern given the high rates of, and increasing trends in, both obesity and diabetes among Aboriginal and Torres Strait Islander women of reproductive age. 32 Potentially, this incurs intergenerational cycle of increased risk of obesity and metabolic syndrome in their offspring. Our findings suggest that to reduce the disease and mortality burden among the next generation of Aboriginal and Torres Strait Islander children is to improve the health of their mothers. This would require accessible, affordable, effective and culturally acceptable antenatal care which include social and psychological support for the Indigenous women during pregnancy.
BMI is a commonly used measure of adiposity in clinical and epidemiological studies. It can be used to estimate the prevalence of obesity within a population and to assist in developing public health and nutrition policy and to prioritize interventions. Although universal BMI cut-off points were used in this study, caution must be exercised when BMI cut-off points derived from other populations are used to define overweight and obesity amongst Indigenous people. Future research should determine the appropriateness of these cut-off points in Indigenous Australians from different communities, since there is a wide variation in body composition between them.Reference Wang, Hoy and McDonald 67 , Reference Adegbija, Hoy and Wang 68
Our study has several strengths and limitations. To our knowledge, this is the largest Indigenous community-based birth cohort study in NSW, with birth records obtained from the hospital. The collection of biochemical and anthropometric data in the pregnancy and follow-up study were undertaken with adherence to a research study protocol. However, this study also has some limitations. A notable one is that the sample size at follow-up was relatively small, and the population only comprised of women from a rural town in NSW, so the conclusions may not be generalizable to all Indigenous women. Several reasons for lack of full retention of all women postpartum include: participants from this cohort moving away from the study locations, the time constraints on the mother and the lack of incentives for continued participation. Given the unpredictable nature of infants and the number of measures to be collected, it is not always possible to obtain all measures at a given time. This project however, provides a useful benchmark for the growth trajectory of Indigenous infants in this community, enhancing our knowledge of where to target public health interventions and policies to address the current gaps.
Conclusion
In conclusion, we identified a statistically significant association between being born preterm and an increased risk of developing obesity in early childhood within the current cohort of Indigenous women and their infants. Given that 12% of Indigenous Australian infants in this cohort are born preterm, this is likely to increase their risk for chronic diseases later in life and action needs to be taken to improve their birth outcomes. This study also shows that maternal non-fasting plasma glucose concentrations are positively associated with birth weight centiles and that Indigenous infants born LGA or from mothers with increased body fat are at increased risk of obesity in early childhood. Future studies should therefore work with Indigenous communities to design appropriate interventions aimed at reducing maternal adiposity and plasma glucose levels before pregnancy to ensure optimal metabolic health for future generations.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174418000302
Financial Support
This work was supported by the National Health and Medical Research Council (grant numbers 569239, APP1026733, APP1063123). Y.Q.L. is supported by a Susan Alberti PhD Scholarship, K.G.P. is supported by an ARC Future Fellowship (FT150100179) and C.E.C. is supported by an NHMRC Senior Research Fellowship and a Faculty of Health and Medicine Gladys M Brawn Senior Fellowship.
Conflicts of Interest
None.
Acknowledgments
The authors wish to pay their respects to Elders past and present, and extend that respect to any Indigenous readers. The authors of the paper would like to acknowledge the Gomeroi gaaynggal Aboriginal Steering committee for their ongoing advice for the Gomeroi gaaynggal studies. Shalem Yiner-Lee Leemaqz has assisted the investigators with software issues related to management of the data set. The authors would like to gratefully recognize the women and children who dedicate their time to participate in the Gomeroi gaaynggal study.