Introduction
Maternal iron deficiency during pregnancy, which is common in human populations worldwide, is associated with a range of adverse outcomes in the offspring.Reference Lozoff, Klein and Nelson 1 – Reference Lozoff, Beard and Connor 4 Evidence from experimental animal models shows that iron deficiency during pregnancy causes fetal growth restriction, and this is associated with the offspring developing obesity and high blood pressure later in life.Reference Alwan and Hamamy 5 The mitochondria play a central role in iron metabolism. Iron is essential for the synthesis of haem and the assembly of iron–sulphur proteins, which are vital for the function of the electron transport chain (ETC).Reference Lill, Hoffmann and Molik 6 Evidence from several animal models suggests that mitochondrial dysfunction initiated during fetal development may be part of the underlying mechanisms associated with long-term changes in metabolism. For example, the activity of the ETC complexes are reduced in the offspring of mice and rats fed high fat (HF) diets during gestation and lactationReference Borengasser, Lau and Kang 7 , Reference Borengasser, Faske and Kang 8 introducing a range of metabolic abnormalities, which include the development of a fatty liver in adult life.Reference Gregorio, Souza-Mello, Carvalho, Mandarim-de-Lacerda and Aguila 9 – Reference Bruce, Cagampang and Argenton 12 Furthermore, maternal obesity results in fewer copies of the mitochondrial genome (mtDNA) per cell in the liver of the offspring.Reference Wu, Russell and Wong 13 Mitochondria are unique in that they have their own DNA which is inherited through the maternal line. Animal studies show that feeding of iron-deficient diets destabilizes mtDNA and increases the proportion of damaged molecules within the cell.Reference Walter, Knutson and Paler-Martinez 14 Damage to mtDNA introduced by iron deficiency during fetal development and propagated into adult tissues may therefore introduce metabolic changes in the offspring of iron-deficient mothers.
Under normal conditions, the cell has a considerable reserve of metabolic capacity which limits the impact of short-term changes in the diet. As a result perturbations of mitochondrial function initiated during fetal life, such as changes in mitochondrial dynamics, ETC activity or mutations in D-loop region may have relatively modest effects in young animals fed a complete or balanced diet. However, mitochondrial capacity declines with age,Reference Petersen, Morino and Alves 15 so that the full impact of early mitochondrial damage may only become apparent when aged animals are exposed to a more pronounced and prolonged metabolic stress. One such stressor is the consumption of a HF diet which impairs mitochondrial biogenesis in the liverReference Nadal-Casellas, Amengual-Cladera, Proenza, Llado and Gianotti 16 and increases damage to mtDNA.Reference Yuzefovych, Musiyenko, Wilson and Rachek 17 To test the possibility that pre-natal iron deficiency has altered mitochondrial activity and damaged mtDNA we have examined the livers of 64-week-old offspring from control and iron-deficient dams, maintained on complete (stock) laboratory chow or challenged with a HF diet before necroscopy.
Methods
Animals
All experimental procedures were approved by the ethical review committee of the Rowett Research Institute and conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986. Animals were group housed in cages, with a 12 h light–dark cycle and conditions of constant temperature and humidity. All animals were fed ad libitum and provided with distilled water.
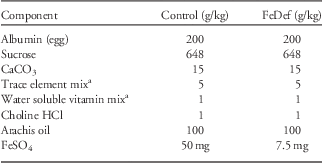
Weanling rats of the Rowett Hooded Lister strain were fed stock diets for 2 weeks before being randomly split into two groups of 10 animals. The animals were then fed semi-synthetic diets (Table 1), with FeSO4 providing either 50 mg Fe/kg diet (control) or 7.5 mg Fe/kg diet (FeDef), as described previously.Reference Gambling, Danzeisen and Gair 18 The animals were fed the experimental diets for 4 weeks before mating and for the duration of gestation. Within 24 h of birth the litters were culled to eight pups which were then cross-fostered onto dams fed the stock laboratory chow diet (CRM; Special Diets Services, Witham, UK). On day19 after birth the male offspring were weaned onto the stock CRM diet and were group housed by litter. All offspring were weighed three times weekly. At 52 weeks after birth, where possible at least one male offspring from each litter was fed ad libitum a HF diet providing 45% of calories from fat (TD.06415; Harlan Laboratories, Bicester, UK). A control group of male littermates continued to receive the stock chow diet (Stock). Animals were weighed every 3 days. The body composition of the animals was measured by magnetic resonance imaging (MRI) (EchoMRI, Houston, TX, USA) as described previously.Reference Lobley, Bremner, Holtrop, Johnstone and Maloney 19 After feeding for 12 weeks animals from the Con (Con-Stock and Con-HF) or FeD prenatal diet groups (FeD-Stock and FeD-HF) were fasted for 8 h, anesthetized with isoflurane and killed by cervical dislocation. Tissues were rapidly removed, frozen in liquid nitrogen and stored at −80°C until required.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Frozen tissue samples (30 mg) were crushed before DNase treated total RNA was isolated using the Qiagen RNeasy mini kit (Qiagen, Manchester, UK) following the manufacturer’s instructions. The RNA was checked for integrity and quantified using an Agilent 2100Bioanalyser (Agilent Technologies, Stockport, Cheshire, UK). Samples of 200 ng total RNA with an RNA integrity number >8 were reverse transcribed with the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems, Warrington, Cheshire, UK) primed with random hexamers following the manufacturer’s instructions. The products were diluted to give a final concentration equivalent to 0.2 ng RNA/ µl and 5 µl aliquots were used for the PCR reactions which were carried out with either the SYBR Green RT-PCR kit or TaqMan® Gene Expression Assays (Applied Biosystems). The qPCR reactions used the standard protocol provided by the manufacturer with the primers shown in Tables 2a and 2b or as described previously.Reference Maloney, Hay and Reid 20 The amplification efficiency was routinely determined from standard curves included with each run. The products of reactions using the SYBR Green chemistry were identified by sequencing and melt curves were routinely carried out to confirm the presence of a single product. The abundance of complementary DNAs (cDNAs) were measured relative to the 18S ribosomal RNA (rRNA) Cat no 4319413E, Eukaryotic 18S rRNA Endogenous Control (VIC®/MGB probe, Primer Limited; Applied Biosystems) and relative target quantity was calculated from a standard curve in order to facilitate comparisons between the SYBR Green and TaqMan chemistries.
Table 2a Primers used for quantitative polymerase chain reaction analysis with SYBR Green chemistry

Table 2b Primers used for quantitative polymerase chain reaction analysis with TaqMan chemistry

Triglyceride analysis
Hepatic triglyceride, choline and phosphocholine concentrations were measured in chloroform and aqueous extracts as detailed previously.Reference McNeil, Hay and Rucklidge 21
Mitochondrial complex activity.
The activities of the ETC enzymes from the offspring’s liver tissue were determined using a spectrophotometric assayReference Spinazzi, Casarin, Pertegato, Salviati and Angelini 22 , Reference Medja, Allouche and Frachon 23 modified for a 96 well format. Briefly; liver was homogenized in ice cold 25 mM potassium phosphate buffer (pH 7.2), (1:6 wt:vol) using a glass–Teflon homogenizer (Braun). After centrifugation at 600 g for 5 min at 4°C, samples of the supernatant were stored at −20°C until required. NADH ubiquinone oxido-reductase (complex I) was measured by following the rotenone sensitive oxidation of NADH at 340 nm in a reaction mixture containing 100 µM NADH, 100 µM decylubiquinone, 50 mM K phosphate (pH 7.5) and 3.75 mg/ml bovine serum albumin (BSA), with or without 12.5 µM rotenone. Succinate ubiquinone oxido-reductase (Complex II) was measured by following the oxidation of 2,6dichlorophenolindophenol (DCPIP) in a reaction mixture containing 25 mM K phosphate (pH 7.5) 20 mM succinate, 100 µM decylubiquinone, 50 µM DCPIP, 1 mM NaN3, 100 µM ATP and 2 mg/ml BSA. Cytochrome c oxidase (complex IV) was assayed by measuring the sodium azide sensitive oxidation of cytochrome c in a mixture containing 50 mM K phosphate (pH 7.0) and 100 µM reduced cytochrome c. Citrate synthase activity was measured by following the formation of 5-thio-2-nitrobenzoate at 412 nm in a reaction mixture containing 100 µM 5,5′-dithio-bis-(2nitrobenzoic acid), 100 mM Tris HCl (pH 8.1), 300 µM acetyl CoA, 500 µM oxaloacetate and 0.1% Triton ×100. All reactions were followed for 10 min after addition of homogenate containing ~50 μg protein as determined by the Lowry method. The rate was calculated from the linear part of the progress curve in nanomoles per minute per milligram protein.
mtDNA analysis
Samples of liver DNA were extracted using the GenElute Mammalian genomic DNA miniprep kit (Product No G1N10; Sigma Chemical Co, Poole Dorset, UK) following the manufacturer’s instructions. Relative mtDNA copy number and the common mtDNA deletion in the rat was measured relative to the genomic β-actin gene by quantitative TaqMan assay using the protocol and primers described previously.Reference Nicklas, Brooks, Hunter, Single and Branda 24
Statistical analysis
Data were analysed using Genstat 13th edition (VSN International Ltd, Hemel Hempstead, UK) and presented as mean±s.e.m. The data were analysed by Student’s t-test, analysis of variance or linear mixed models (restricted maximum likelihood), blocked for dam where necessary. Statistical significance was set at P<0.05.
Results
Offspring characteristics
The offspring characteristics are shown in Table 3. There were no differences in the litter sizes of the control and FeD pups. At weaning, the male FeD pups were ~12% smaller than the controls (P=0.005). This difference in body weight persisted, such that the offspring of the FeD dams remained ~12% smaller than the controls at 52 weeks of age (Table 3). This difference was reflected in the lower lean mass of the animals. There was also a difference in body fat, however, fat as a percentage of body weight or the ratio of fat to lean was not different in the two groups of animals.
Table 3 Growth characteristics of the male offspring to 52 weeks of age

All data are presented as mean values with their standard errors of the mean. Data were analysed by analysis of variance blocked for dam.
ns P>0.1.
The body weight of the offspring fed the HF diet from weeks 52 to 64 increased by ~12% when compared with littermates fed the stock diet over the same period (Table 4). The absolute body weight at 64 weeks was also different with respect to the prenatal diet, with the effect of iron deficiency during gestation continuing to influence the lean mass of the offspring. The absolute amount of fat in the FeD group was as expected, increased by the HF diet, however, there was no effect of prenatal diet when fat was expressed as a percentage of the lean mass.
Table 4 Growth characteristics of the male offspring fed the high fat (HF) diet from 52 to 64 weeks of age

All data are presented as mean values with their standard errors of the mean. Data were analysed by restricted maximum likelihood with Pre+Post+Pre×Post diet as fixed term and Dam No. as the random term.
a,b,cIndicate differences determined by post-hoc testing (P<0.05).
ns P>0.1.
Lipid metabolism and gene expression
The intrahepatic triglyceride concentration in animals fed the HF diet was ~60% higher than in the animals fed the stock diet (Table 5). Prenatal iron deficiency did not change the intrahepatic triglyceride concentrations in either group when compared with its respective control. Whilst there were no differences in intrahepatic choline concentrations between the stock and HF fed animals, prenatal iron deficiency tended to decrease free choline concentrations by ~40% (P=0.07). The storage form of choline, phosphocholine was also unaffected by feeding the HF diet, but was reduced by ~25% (P=0.04) in the animals exposed to prenatal iron deficiency.
Table 5 Intrahepatic lipid concentrations in the male offspring at 64 weeks of age

HF, high fat.
All data are presented as mean values with their standard errors of the mean. Data were analysed by two-way analysis of variance (ANOVA).
ns P>0.05.
The relative abundance of the messenger RNA (mRNA) coding for Acc-1 and PPAR-α in the liver, was unaffected by either the prenatal or postnatal diet (Table 6). The HF diet increased the abundance of mRNAs for L-CPT-1 by ~100%, the mRNA for CD36 by ~50%, the mRNA for SREBP-1c by ~25% and the mRNA for glucocorticoid receptor (Gccr) by 17% when compared with the stock diet. The relative abundance of the mRNA for PPAR-γ was reduced by ~30% in the animals fed the HF diet. Prenatal iron deficiency had no effect on the relative abundance of any of these mRNAs or on the magnitude of the change induced by the HF diet.
Table 6 Relative abundance of messenger RNAs (mRNAs) in the liver of male offspring at 64 weeks

HF, high fat.
The relative abundance of mRNAs expressed in arbitrary units relative to the 18S ribosomal RNA.
All data are presented as mean values with their standard errors of the mean. Data were analysed by two-way analysis of variance.
ns P>0.05.
Mitochondrial enzyme activities
The citrate synthase activity of liver homogenates was unaffected by either the prenatal or postnatal diets (Table 7). There were no differences in the ratio of activities of the ETC complexes to citrate synthase.
Table 7 Ratios of hepatic mitochondrial electron transport chain activities to citrate synthase (CS) in the liver of male offspring at 64 weeks

HF, high fat.
All data are presented as mean values with their standard errors of the mean. Data were analysed by two-way analysis of variance.
ns P>0.05.
Mitochondrial numbers and D-loop mutation
The relative mtDNA copy number in the livers of male offspring at 64 weeks of age was increased by approximately fourfold in the animals fed the HF diets when compared with littermates fed the stock diet (Fig. 1a). There was no effect of prenatal iron deficiency in either the animals fed the stock diet or those fed the HF diet. The frequency of the common D-loop deletion (Fig. 1b) increased from 1.12±0.16 copies per copy of the genomic β-actin gene in the stock fed animals to 2.16±0.50 copies per copy of the genomic β-actin gene in animals the fed high diet. Prenatal iron deficiency did not change the mtDNA copy number, the response to HF feeding or the prevalence of the D-loop deletion. The ratio of citrate synthase to mtDNA copy number (Fig. 1c) was lower (P<0.001) in the animals fed the HF diet compared with the stock fed controls, but unaffected by prenatal iron deficiency.

Fig. 1 Mitochondrial DNA (mtDNA) in the liver of the male offspring of rats fed control (clear) or iron-deficient diets (hatched bars) during gestation. The male offspring were subsequently fed stock (white bars) or high fat (HF) diets (grey bars). Data are presented as mean + s.e.m. (Con-Stock n=7, Con-HF n=7, FeD-Stock n=6, FeD-HF n=7). a,bBars with different letter superscripts are significantly different Fisher’s least significant difference test (P<0.05). (a) mtDNA copy number (b) D-loop deletion copy number. (c) Ratio of citrate synthase to mtDNA copy number.
Expression of mitochondrial regulators in the offspring
The relative abundance of the mRNAs coding for mitochondrial transcription factor A (TFAM) and PGC-1β were unchanged by either prenatal iron deficiency or postnatal HF diets (Table 8). There was, however, an increase of ~25% in the abundance of the mRNA coding for PPAR-gamma-co-activator-1-alpha (PGC-1α) in the offspring exposed to prenatal iron deficiency, although there was no effect of the postnatal HF diets. The mRNAs associated with mitochondrial fusion were unchanged apart from Mfn1 which showed and interaction between the prenatal and postnatal diets. This was the consequence of an increase of ~15% in the abundance of Mfn1 in the control animals fed the HF diet, whereas there was no change in the FeD animals. There was a decrease of ~15% in the abundance of the mRNA for Oma1 which is associated with mitochondrial fission in the HF diet groups.
Table 8 Expression of mitochondrial regulators in the liver of male offspring at 64 weeks

HF, high fat.
The relative abundance of messenger RNAs expressed in arbitrary units relative to the 18S ribosomal RNA.
All data are presented as mean values with their standard errors of the mean. Data were analysed by two-way analysis of variance.
ns P>0.05.
Discussion
The present study sought to examine the potential for long-term effects on mitochondrial dynamics, ETC activity and D-loop mutation in the aged offspring of iron-deficient dams. In line with previous studies there was a substantial decrease in the birthweight of the FeD offspring which persisted into adult life. However, although the FeD offspring were smaller, the proportions of fat to lean were similar and there was no evidence that the prenatal diet had any influence on adiposity of the offspring fed the stock diet. Feeding the HF diet increased body weight but here also there was no evidence for an effect of the prenatal diet, with the relative proportions of fat to lean remaining unchanged. In this respect the present study differs from previous reports of increased visceral fat in the offspring of iron-deficient dams fed a HF diet in adult life.Reference Bourque, Komolova, McCabe, Adams and Nakatsu 25 , Reference Lewis, Petry, Ozanne and Hales 26
The liver plays a central role in lipid metabolism and the development of hepatic steatosis in the offspring is a common feature of many models for the fetal origins of adult disease. In the present experiment the HF challenge lead to substantial increases in intrahepatic triglyceride concentrations as well as increases in the abundance of mRNAs associated with the oxidation of fatty acids such as L-CPT, CD36 and the transcriptional activators controlling them. However, there was no evidence that these parameters were affected by prenatal iron deficiency, in either the stock or HF fed offspring. The only substantial change in gene expression following prenatal iron deficiency was an increase in the abundance of the mRNA coding for PGC-1α, a factor implicated in other models of fetal programming.Reference Guéant, Elakoum and Ziegler 27 However, in other models the pattern is of a decrease in relative abundance. In the offspring of dams fed a HF diet there is a decrease in mRNA abundance in the liverReference Borengasser, Faske and Kang 8 and the protein abundance is reduced in the liver of methyl-deficient offspring.Reference Pooya, Blaise and Moreno Garcia 28 , Reference Garcia, Guéant-Rodriguez and Pooya 29 Prenatal iron deficiency also reduces the steady state concentration of phosphocholine and tends to reduce free choline in the liver. It is tempting to speculate that these changes may be related to the reduced expression of PGC-1α, reflecting some hitherto unidentified effect of iron deficiency on hepatic function in the offspring.
It is not clear why the present study failed to replicate findings of an increase in visceral adipose tissue in the offspring of iron-deficient dams reported elsewhere.Reference Bourque, Komolova, McCabe, Adams and Nakatsu 25 , Reference Komolova, Bourque, Nakatsu and Adams 30 The degree of maternal iron deficiency, the composition of the experimental diet and strain differences could all be factors. Although the MRI data does not differentiate between visceral and subcutaneous adipose depots, reciprocal changes in distribution are unlikely, suggesting that that there was no differential effect on visceral adiposity. Total body and intrahepatic triglyceride was increased in animals fed the HF diet, however, even in the HF group, fat as a proportion of lean tissue mass was unaffected by prenatal iron deficiency. Increases in the expression of the glucocorticoid receptor have been reported in the offspring of rats fed a low protein diet during gestation,Reference Bertram, Trowern, Copin, Jackson and Whorwood 31 however, although HF feeding increases the abundance of the mRNA, there is no evidence for an effect of prenatal iron deficiency, suggesting that this endocrine system is unaffected. It is possible that subtle differences in the prenatal diet composition may also be a factor. The extent and timing of iron deficiency are similar and unlikely to be a factor, however, other diet components may also be important. The lipid composition of the diet, a factor which has been shown to be particularly important in other models of prenatal programming such as dietary protein restrictionReference Langley-Evans 32 may also influence the metabolic phenotype of iron-deficient offspring. Changes in both glucose tolerance and hepatic gene expression were observed in the offspring of dams fed a low protein diet prepared with soya oil, whereas there was no difference when the diet was prepared with corn oil.Reference Maloney, Lilley, Czopek, Hay and Rees 33 It is possible that these effects are related to increases in maternal plasma triglyceride concentrations which are higher in the low protein soya oil diet compared with the corn oil diet.Reference McNeil, Maloney, Hay and Rees 34 Iron deficiency also increases triglyceride concentrations in the maternal circulation by 20–40%Reference Hay, McArdle, Hayes, Stevens and Rees 35 but this is small when compared with the more than twofold increase seen in animals fed low protein diets. The present study used arachis oil as the lipid source and as this has a different fatty acid profile from corn and soy oil, it would be interesting to see if the fatty acid composition of the maternal diet also modulates the impact of iron deficiency.
Mitochondria within eukaryotic cells are constantly being remodelled by biogenesis, fusion, fission and autophagy. These dynamic processes modify cellular metabolism in response to changes in the environment such as those produced by feeding a HF diet.Reference Otera, Ishihara and Mihara 36 The increase in intrahepatic triglyceride concentrations in the animals fed the HF diet is accompanied by an increase in the expression of L-CPT-1, indicative of more fatty acid being transferred to the mitochondrion for oxidation. The increase in mtDNA relative to genomic DNA shows that there has been substantial mitochondrial biogenesis in the HF animals, however, that there is no evidence for an effect of prenatal iron deficiency on the mtDNA copy number and hence the proliferation of hepatic mitochondria.
In other models of fetal programming imbalances in the maternal diet affect the activities of the mitochondrial ETC complexes in the offspring. For example; maternal diets high in fatReference Bruce, Cagampang and Argenton 12 or deficient in methyl donorsReference Pooya, Blaise and Moreno Garcia 28 are both associated with a decrease of more than 50% in the activity of mitochondrial ETC Complexes I and II in the liver of the offspring. There is no evidence for similar changes in the hepatic ETC activity in the offspring of iron-deficient dams. This is the case even in the animals fed the HF diet which increases the amount of substrate available for oxidation and increases the flow of electrons through the ETC. However, the response of the mitochondria to this additional substrate is complex. Although there was a fourfold increase in mtDNA, citrate synthase activity and the activities of individual ETC complexes were unchanged when compared with the stock fed animals. As a result the citrate synthase and ETC activity per copy of mtDNA was substantially lower in the animals fed the HF diets. These results suggest that the individual mitochondria are working less efficiently and that a reduced yield from oxidative phosphorylation counters the increased number of mitochondria. These results are consistent with previous studies showing an increased number of less differentiated mitochondria in the liver of HF fed animals.Reference Nadal-Casellas, Amengual-Cladera, Proenza, Llado and Gianotti 16 Although an increase in the overall number of mitochondria would be expected to enhance any lasting defect such as mtDNA damage induced by prenatal iron deficiency, this may be masked by a failure to induce additional ETC enzyme expression. However, there is no evidence for an effect of the prenatal diet in either the stock or HF diet groups.
Mitochondrial biogenesis is regulated by nuclear encoded mitochondrial genes which include the transcriptional activators PGC-1α and PGC-1β and TFAM.Reference Archer 37 The lack of change in the HF fed animals is probably explained by the transient changes in the expression of these factors during the first 2 or 3 weeks following the introduction of a HF diet.Reference Flamment, Rieusset and Vidal 38 It is likely that this adaptive phase has passed and a new steady state has been achieved. Transient changes in gene expression probably also account for the lack of change in the mRNAs coding for proteins regulating fusion and fission. The decreases in the efficiency of oxidative phosphorylation in HF fed animals is associated with a shift towards mitochondrial fission (the splitting into two organelles) and is regulated by the dynamin-like proteins (Dnml1) and the mitochondrial metalloproteinase Oma1.Reference Lionetti, Mollica and Donizzetti 39 , Reference Baker, Lampe and Stojanovski 40 The tendency for a small decrease in the abundance of Oma1 in the HF fed animals may reflect the reduced mitochondrial efficiency, however, may also be part of a mechanism to remove damaged mtDNA (see below). It is clear, however, that there is no evidence for long-lasting changes as a result of prenatal iron deficiency on either the abundance of the mRNAs regulating fission or in the ratio of citrate synthase to mtDNA, suggesting that the mitochondrial network is unaffected by the maternal diet.
The mitochondrial genome is a small circular DNA molecule located within the organelle and is susceptible to damage because of its proximity to the machinery of oxidation. A 4834 bp sequence in the D-loop of mtDNA is particularly prone to deletion following oxidative damage. The increased frequency of this deletion in the liver of animals fed the HF diet reflects damage caused by the increase in reactive oxygen species arising from higher rates of fat oxidation. It is likely that the small decrease in the abundance of Oma1 mRNA in the HF group is associated with the removal of these damaged mitochondria by mitophagy. Both excessesReference Gao, Campian, Qian, Sun and Eaton 41 and deficienciesReference Walter, Knutson and Paler-Martinez 14 of iron have been shown to increase the damage to mtDNA and since mtDNA is replicated as cells divide, damage sustained in the prenatal period may still be present later in life. However, there is no evidence for changes in the frequency of the D-loop deletion in the offspring exposed to prenatal iron deficiency. This is the case even when the hepatic mitochondria are induced to proliferate by feeding the HF diet. Measurement of the mtDNA deletion is not a comprehensive analysis and cannot eliminate the possibility of an increased frequency of point mutations. However, studies have shown that damage to mtDNA results in a parallel decline in mitochondrial respiration due to defective synthesis of ETC subunits.Reference Gao, Campian, Qian, Sun and Eaton 41 As there is no effect of the prenatal diet on ETC activity, our data suggest that mitochondrial dysfunction is not caused by iron deficiency during fetal development.
To summarize this study shows that there is no long-term effect on lipid deposition, hepatic lipid metabolism or mitochondrial dynamics, ETC activity and mtDNA mutation in the offspring of iron-deficient dams even when challenged with a HF diet. These results suggest that the mechanism and subsequent impact of maternal iron deficiency on metabolism differs from other models of fetal programming, however, the final phenotype may still be a product of interactions with other components of the maternal diet.
Acknowledgements
The authors wish to express their thanks to staff from Bioresources Unit for animal care.
Financial Support
This work was funded as a part of the Scottish Government Rural and Environment Science and Analytical Services (RESAS) Strategic Research Program.
Conflicts of Interest
None.
Ethical Standards
All experimental procedures were approved by the ethical review committee of the Rowett Research Institute and conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986.