Background
Obesity/overweight and related conditions are global concerns increasing exponentially with each generation.Reference Vickers 1 It is projected that 75% of the Australian population will be overweight or obese by 2030.Reference Moodie 2 This will create an unsustainable burden of disease, which will only increase unless the inter-generational cycle of obesity is broken.
Excess weight gain early in pregnancy is independently associated with obesity for the subsequent generation.Reference Gaillard, Durmus, Hofman, Mackenbach, Steegers and Jaddoe 3 – Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams 6 Longitudinal population studies (Child Health and Development Studies,Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams 6 Generation R,Reference Gaillard, Durmus, Hofman, Mackenbach, Steegers and Jaddoe 3 Avon Longitudinal Study of Parents and Children (ALSPAC)Reference Fraser, Tilling and Macdonald-Wallis 5 and the Raine StudyReference Gaillard, Welten and Oddy 4 ) demonstrate that high weight gain in early pregnancy is associated with increased childhood and adolescent adiposity levels and related adverse cardio-metabolic profiles. More specifically, maternal excess weight gain during early (not middle or late) pregnancy has been repeatedly associated with greater childhood and adolescent adiposity.Reference Gaillard, Durmus, Hofman, Mackenbach, Steegers and Jaddoe 3 – Reference Margerison-Zilko, Shrimali, Eskenazi, Lahiff, Lindquist and Abrams 6 Therefore, the first trimester of pregnancy has been identified as a potentially critical and attractive window of opportunity for short-term interventions to break the ‘transmission’ of obesity from one generation to the next.
While multiple studies around the world robustly demonstrate an association between excessive gestational weight gain (GWG) and childhood obesity, individual randomised controlled trials (RCTs) have shown inconsistent results about the capacity of lifestyle trials to modify GWG and alter offspring obesity. Nevertheless, a recent individual patient data meta-analysis of 12,343 participants demonstrated that lifestyle interventions during pregnancy overall prevents excess GWG, although there was no demonstrable effect on neonatal health outcomes.Reference Rogozinska, Marlin and Jackson 7
Lack of consistent results within individual RCTs may lie partially in variable intervention timing. To date, many commence their intervention between 10 and 20 weeks gestation, and the majority have not specifically targeted early pregnancy recruitment. Those RCTs that have successfully recruited participants during the first trimester have until recently been modestly powered.Reference Abdel-Aziz, Hegazy, Mohamed, Abu El Kasem and Hagag 8 – Reference Wang, Wei and Zhang 18 However, recently, Haby et al. Reference Haby, Berg, Gyllensten, Hanas and Premberg 19 recruited 459 women into their intervention arm early in pregnancy (7.9–8.6 weeks) and, furthermore, showed significantly lower birthweight and rates of macrosomia compared to the intervention group.
The use of technology in health care is increasing because of increased uptake in the general population and recognition of its potential to reach a wide audience. A recent trial compared efficacy of obesity treatment delivered via smartphone, a more intensive-group-based program or control.Reference Thomas, Bond, Raynor, Papandonatos and Wing 20 The mobile online delivery achieved weight loss outcomes that were at least as good as those obtained via the more intensive-group-based approach. Similarly, in pregnancy care, the use of e-health has been increasing. A recent meta-analysis identified 15 RCTs using e-health in pregnancy. Overall, they showed moderate-to-large effect sizes in promoting maternal health, mental health and health knowledge.Reference Chan and Chen 21
The established evidence from population studies and emerging RCT evidence suggest that the early pregnancy period is critical in affecting obesity outcomes in the next generation. To ascertain if modifying factors at this time can change obesity outcomes in the offspring, RCTs targeting this period are required. Furthermore, a web-based delivery of such an intervention is likely to be effective and potentially scalable. Therefore, we developed a web-supported 12-week diet and physical activity intervention in early pregnancy, which aimed to promote healthy GWG. This paper reports on the feasibility of testing this web-based app in an RCT. The primary pilot objectives are to test feasibility of recruitment in early pregnancy from 6 to 10 weeks of gestation and feasibility of delivering an intervention using technology. We report some preliminary outcome data to provide an indication of potential efficacy of an e-health delivered intervention, to inform future sample size calculations and decisions on primary outcome selection. These outcome data relate to GWG, maternal diet, physical activity and psychological parameters, and infant outcomes.
Methods
Study design
The study was an unblinded RCT.
Study participants
Pregnant women were recruited from Joondalup Health Campus (JHC; Site 1) and St John of God Subiaco Hospital (SJOG; Site 2) through advertising (posters and postcards in waiting rooms), self-referral and obstetric staff. The study was promoted as a means to improve lifestyle during pregnancy. Postcards were posted with the welcome package sent by obstetricians to new patients. Eligible participants included pregnant women aged over 18 years, at less than 11 weeks gestation, with a body mass index (BMI) ≥20 kg/m2, who were able to speak and understand conversational English. Women were excluded if they used donor egg or sperm to conceive, had a pre-existing medical condition (including pre-pregnancy type 1 or 2 diabetes, eating disorder, psychosis, polycystic ovarian syndrome treated with medication or thyroid condition) or were not having a singleton pregnancy.
Participants were randomised at recruitment to receive (1) the Pregnancy Lifestyle Activity Nutrition (PLAN) intervention (intervention group) or (2) routine antenatal care (control group). Randomisation was performed using a computer-generated program that stratified by the BMI category (BMI < 25 kg/m2; BMI ≥ 25 kg/m2) to the intervention group or the control group.
PLAN intervention
In addition to routine antenatal care, the PLAN intervention group received a web-based program providing diet, physical activity and well-being advice over a period of 12 weeks (Fig. 1a). The PLAN intervention involved:
Personalised advice regarding GWG and Institute of Medicine (IOM) guidelines. The web-based app provided a closed loop, real-time feedback of a participant’s weight compared to IOM guidelines for optimal weight gain, synchronised to stage of pregnancy.
Dietary education on low glycaemic index, low saturated fat, increased omega-3 fatty acid, increased fibre, healthy portion sizes, take-out options and snack substitution was provided via the web-based app.
Cognitive behavioural materials to encourage goal setting, self-monitoring and problem solving.
One private session with a dietician who delivered tailored feedback on dietary intake and accelerometer data.
Weekly contact by SMS
The PLAN project website with information on respective topics released on a week-by-week basis.
Formation of a short, measured, achievable, relevant and timely goal to work towards.Reference Bovend’Eerdt, Botell and Wade 22

Fig. 1. Timeline of PLAN intervention and data collection. (a) Outlines the 12-week intervention components and (b) shows details of timing and specifics of data collected.
The intervention was designed to target behavioural change techniquesReference Michie, Wood, Johnston, Abraham, Francis and Hardeman 23 with emphasis on social support, self-monitoring of behaviour and outcomes, goal setting of behaviours, instruction on how to perform the behaviour, problem solving and action planning.
Data collection
Participants were asked to attend study appointments at the start of the intervention (up to 11 weeks gestation), 2 weeks into the intervention (dietician consult only, no assessments), at the end of the intervention (12 weeks after first appointment, 18–23 weeks gestation), and then at 28 weeks gestation (Site 1 only), 36 weeks gestation and 3 months post-delivery (Site 2 only) (Fig. 1). Data were recorded in hard copy and stored on REDCap (version 6.10.12) online data management program.
Basic demographic information was collected at baseline including date of birth, smoking status and ethnicity. Anthropometric measurements were performed at all visits and included weight (calibrated Seca digital scales), height (calibrated Seca Rod Stadiometer) and girth (calibrated Seca tape measure) at the waist, hip and mid-upper arm. Skin fold thickness was also measured with Harpenden calipers at the triceps, biceps, subscapular, suprailiac and mid-anterior thigh. Enrolment BMI was calculated based on weight and height obtained at the start of intervention appointment. The IOM BMI ranges were used to classify the women in respective groups: normal weight (<25 kg/m2), overweight (25–30 kg/m2) and obese (>30 kg/m2). Total GWG was determined by subtracting the last recorded weight (36 weeks gestation) from the first recorded weight (8–10 weeks gestation).
Dietary intake was assessed using a food diary and physical activity with an accelerometer at the start and end of the intervention, and then at 36 weeks gestation and 3 months post-delivery. Participants were asked to record food and beverage consumption over a 3-day period in the food diary, which was analysed using the FoodWorks v8 Professional program. To measure energy expenditure and physical activity intensity and duration, participants were asked to wear an accelerometer (Actigraph Bluetooth Smart wGT3X-BT wireless activity monitor) for a 7-day period. Participants were asked to wear the device during waking hours but to remove it when showering or swimming (as it is not waterproof). Participants were also given an instruction sheet to take home. The accelerometer was set up with an epoch of 5 s, measuring on one axis with normal filter and step count selected. Valid wear time was ascertained from a diary. The percentage of total daily time spent in categories of different intensities of physical activity (low, medium and high intensity and sedentary) was calculated.
Lifestyle changes were assessed using two lifestyle questionnaires: the readiness to change 6-item questionnaire adapted by Hill et al. Reference Hill, Skouteris, Fuller-Tyszkiewicz, Kothe and McPhie 24 modelled from Mason and ButlerReference Mason and Butler 25 and the 26-item World Health Organisation’s Abbreviated Quality of Life (WHOQOL-BREF).Reference Webster, Nicholas, Velacott, Cridland and Fawcett 26 These questionnaires were administered before and at the end of the intervention during study appointments.
After the birth, mothers at JHC were approached while still in hospital to perform a PEA POD (Cosmed, Italy) analysis on the baby. The PEA POD determines body composition by air displacement plethysmography. Delivery records were obtained from the hospitals.
Data analysis
Means, standard deviations, percentages and 95% confidence interval (CI) were used to describe the distributions of continuous variables in the sample, with T-tests used to compare groups. Chi-square test was used to analyse differences between treatment groups for categorical variables. Treatment effect was estimated by a repeated measures analysis and analysis of covariance (ANCOVA).
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS version 23.0). The outcomes were assessed on an intention-to-treat basis. All available data were assessed for validity. An alpha value of 0.05 was used for statistical significance.
Ethics and trial registration
Study approval was obtained from JHC and SJOG. The trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617000725369).
Results
Recruitment
A total of 329 potential participants were contacted over a period of 17 months (15 December 2015–6 April 2017). The majority (81.2%, 267/329)of contacts were referrals from obstetricians from two sites. Of these, 173 were still interested, eligible and contactable on second screening. They underwent full eligibility screening and 57 were enrolled in the study (Fig. 2). Final recruitment from Site 1 (JHC) was 43 from 280 and from Site 2 (SJOG) was 14 from 49 screened.

Fig. 2. Summary of recruitment process.
Study participant characteristics
Mean age at recruitment was 33.26 ± 4.16 years (intervention 32.47 ± 4.18 years; control 34.15 ± 4.02 years; p = 0.128). Mean gestational age at recruitment was 9.21 ± 1.21 weeks (intervention 9.06 ± 1.29 weeks; control 9.38 ± 1.12 weeks; p = 0.327). Mean BMI at enrolment was 25.3 ± 5.25 and 26.0 ± 1.3 kg/m2 within the control and intervention groups, respectively. Within the control group, 41% (11/27) were overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥30 kg/m2). Within the intervention group, 47% (14/30) were overweight or obese (Table 1). Overall, within both the control and intervention participants, 44% of participants were either overweight or obese according to their reported pre-pregnancy weight and height. The majority of women enrolled were Caucasians, educated from medium- to high-income households (Census 2016) and attending private obstetric practices. At enrolment, the control and intervention groups were comparable, with no difference in the baseline characteristics (Table 1).
Table 1. Description of maternal baseline characteristics by the randomised treatment group

Values expressed as mean (95% CI) or n (%).
ˆ Other ethnicities (n = 3) were Chinese and Portuguese.
# p-value comparing ex-smoker versus non-smoker since none of the women smoked during pregnancy.
† p-values from Fisher’s exact test as the expected count is <5 in one or more cell.
Participant retention
During the study, 12.3% (7/57) uncomplicated early pregnancy miscarriages occurred and a further 11 participants withdrew (Table 2). Reasons for withdrawal included health problems (n = 4), time commitments (n = 6) or the study was not what was expected (n = 1). Two participants opted to stay in the study as inactive participants, due to time commitments, following the intervention period to allow birth records to be collected.
Table 2. Summary of withdrawals, miscarriage and retention

ˆ %those retained voluntarily.
† Inactive participants opted to participate in the study until birth but not attend any more study appointments.
‡ Only participants at Site 1 were scheduled a 3-month follow-up.
Retention past the 12-week intervention period dropped to 64.9% (37/57) at birth and 55.8% (24/43) at 3 months.
A total of 42 participants (19 control and 23 intervention) had full data collection completed for both the pre- and post-intervention (12 week) assessments. Of those participants who remained in the study (n = 39), 90.2% (156/173) of all scheduled appointments were attended. The 28-week appointment had 75.0% (21/28) attendance, the 36-week appointment had 87.2% (34/39) attendance and the 3-month appointment had 92.3% attendance (24/26).
GWG and its antecedents of dietary intake, physical activity and psychological parameter
Over the intervention period, no statistically significant differences in GWG were detected (Table 3).
Table 3. Effect of the intervention on gestational weight gain

Values expressed as mean (95% CI) or n (%). Weight gain over the 12-week intervention period = difference in weight between the start and end of the intervention period. Total GWG = weight gain from first recorded weight (6–11 weeks gestation) to last recorded weight in pregnancy (36 weeks gestation).
Values expressed as mean (95% CI) or n (%).
Energy intake was significantly lower in the PLAN intervention group (7497 kJ/day, 95% CI 6799–8196) compared to the control group (8992 kJ/day, 95% CI 8112–9873) at baseline (p = 0.008) (Table 4). Fat (68.27 versus 90.77 g, p = 0.001), saturated fat (25.85 versus 35.26 g, p = 0.001) and trans fat (1.21 versus 1.58 g, p = 0.008) were also lower in the intervention group compared to the standard care group at baseline. After the 12-week intervention, % calories due to total (37% versus 34%, p = 0.039) and saturated (15% versus 13%, p = 0.026) fat were lower in the intervention compared to the control group. After the intervention, there was also a trend for lower fat (78.94 g, 95% CI 68.80–89.07 versus 103.08 g, 95% CI 87.94–107.59), saturated fat (30.90 g, 95% CI 26.63–35.17 versus 37.54 g, 95% CI 30.87–44.21) and trans fat (1.48 g, 95% CI 1.22–1.73 versus 1.82 g, 95% CI 1.51–2.13) in the intervention group compared to the control group remained. Fruit intake was higher in the intervention compared with the control group (2.3 serves/day, 95% CI 1.60–3.09 versus 1.3 serves/day, 95% CI 0.90–1.74) after intervention.
Table 4. Dietary intake and activity level at baseline and post-intervention in the control and intervention groups. Results are in bold font (p<0.05) where difference between control and intervention is statistically significant (p-value < 0.05)
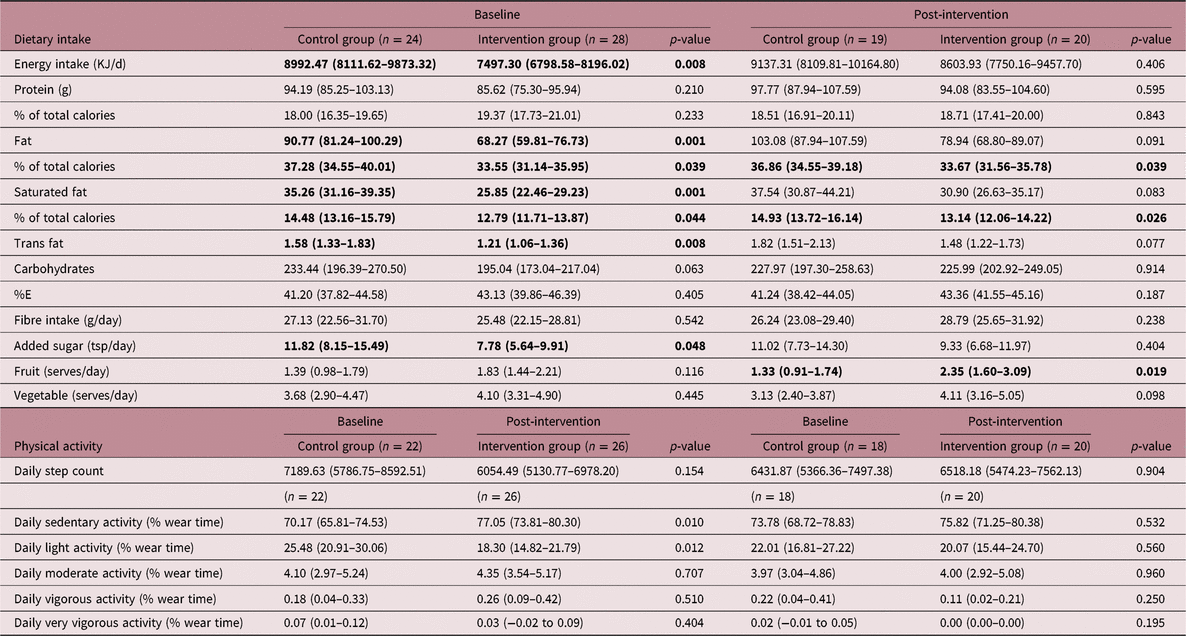
The control group had lower percentage of total daily time spent sedentary and higher daily light activity percentage at baseline. By the end of the intervention, these differences disappeared (Table 4).
At baseline, there were differences in six readiness to change parameters (Table 5). Diet (8.47, 95% CI 7.75–9.20 versus 7.27, 95% CI 6.50–8.05) and physical activity (8.11, 95% CI 7.27–8.94 versus 6.41, 95% CI 5.53–7.29) were rated as more important in the intervention group. Confidence in their ability to make lasting changes to diet (7.84, 95% CI 7.19–8.49 versus 6.45, 95% CI 5.70–7.21) and physical activity (7.53, 95% CI 6.86–8.20 versus 6.41, 95% CI 5.59–7.23) were also higher in the intervention group. Readiness to change diet (8.32, 95% CI 7.60–9.04 versus 7.23, 95% CI 6.52–7.94) and physical activity (8.32, 95% CI 7.61–9.02 versus 6.91, 95% CI 6.15–7.67) were higher in the intervention group at baseline. By the end of intervention, three of these parameters maintained greater scores in the intervention group, namely, importance, confidence and readiness to change diet. No differences in WHOQOL measures were detected.
Table 5. Psychological parameters including the eight-item readiness to change responses and four domains of WHOQOL-BREF at start and end of intervention period compared between the control and intervention groups. Results are in bold font where the difference between intervention and control group on T-test is statistically significant (p<0.05)

Baseline differences in dietary and physical activity parameters make interpretation challenging. Nevertheless, treatment effect on dietary and psychological parameters over the 12-week intervention period accounting for baseline differences was estimated by a repeated measures analysis and ANCOVA (Supplementary Figure 1). No significant interaction between the treatment group and the time was seen except with the outcome of average energy intake. By analysis with ANCOVA, there was significant higher confidence in diet score (β = 1.22 ± 0.46, p = 0.009) and readiness for exercise score (β = 1.21 ± 0.51, p = 0.016) with adjustment for baseline score.
Infant outcomes
No difference in size or body composition at birth (Table 6) was noted; however, by 3 months old, the intervention babies (n = 12) were significantly lighter than controls (n = 12), respectively (5404.8 g (95% CI 4913.9–5895.8) versus 6192.9 g (95% CI 5862.6–6523.3), and had lower ponderal index (25.5 ± 3.0 versus 28.8 ± 4.0 kg/m3)(Fig. 3).
Table 6. Infant outcomes at birth and at 3 months old
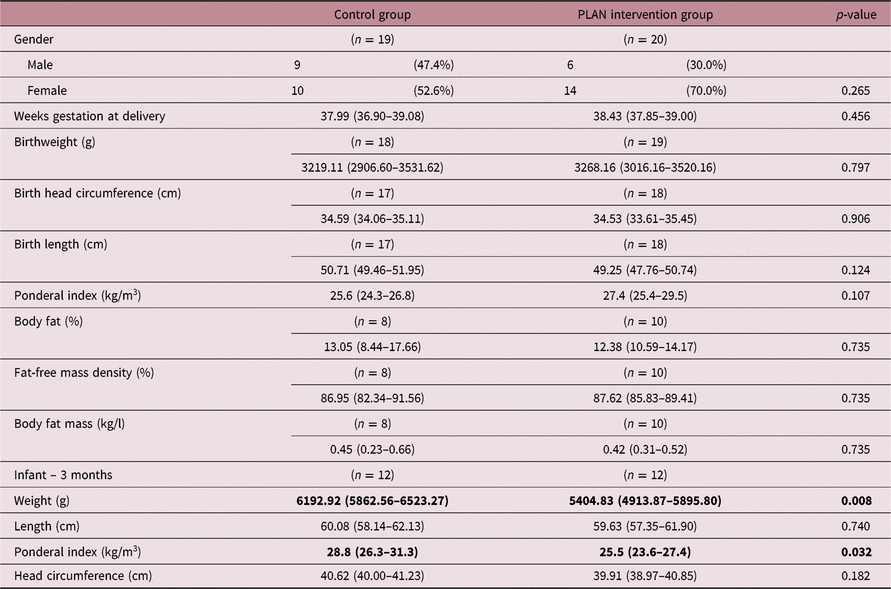

Fig. 3. Differences in infant weight and ponderal index at 3 months old.
Other outcomes
Maternal adiposity
Over the intervention period, no statistically significant differences in measures of maternal adiposity were detected (Supplementary Table 2).
Pregnancy and birth outcomes
The rates of pregnancy and birth complications are listed in Supplementary Table 3. No differences were detected between control and intervention for any of the pregnancy or birth complications. In the intervention, 30% underwent non-elective caesarean section versus 10% in the control group. Gestation age at delivery for controls was 38.0 (95% CI 36.9–39.1) weeks and 38.4 (37.9–39.0) weeks for the intervention group. There was no difference in APGAR (Appearance, Pulse, Grimace, Activity and Respiration) scores or delivery complications.
Discussion
This pilot study has demonstrated the feasibility of early pregnancy (first trimester) recruitment and intervention delivery via electronic means of a lifestyle program targeting early GWG. Our recruitment strategy resulted in a moderate recruitment rate of a representative proportion of overweight/obese women. This was accompanied by a relatively high retention rate over the 3 months of the intervention, but poorer long-term retention for assessments of birth and early infancy outcomes. Potential drawbacks (differences in self-reporting of lifestyle factors at baseline and loss to follow-up after 12 weeks) were identified. These lessons from the feasibility study will inform the design and conduct of a larger trial to reduce threats to the validity of a larger trial.
Ancillary outcomes reported include changes in diet and well-being during the 12 weeks of intervention, an absence of differences in significant obstetrical and birth outcomes and a lower body weight and ponderal index among the smaller numbers of babies available for assessment 3 months after birth.
Demonstrating feasibility
This study demonstrates that it is feasible to recruit and enrol participants in the first trimester. The mean gestational age for recruitment into the PLAN study was 9.21 ± 1.21 weeks of gestation. Recruitment at such an early gestation has inherent challenges including miscarriages that occur during this early period. A total of seven (12.3%) women enrolled in the study miscarried during their first trimester. These were equally distributed across both groups (Table 2). This is consistent with general rates of background pregnancy miscarriage reported of around 13%.Reference Nybo Andersen, Wohlfahrt, Christens, Olsen and Melbye 27 , Reference Risch, Weiss, Clarke and Miller 28 Within this feasibility study, we did not observe a higher rate of early miscarriage; however, it does mean that power calculations for recruitment need to factor in this loss to the study. To date, the majority of studies including the larger of these RCTs, examining the effect of dietary and lifestyle interventions in pregnancy, have recruited during the second trimester. Recently, Haby et al. Reference Haby, Berg, Gyllensten, Hanas and Premberg 19 recruited early in pregnancy prior to 9 weeks gestation. Our study concurs that, in the Australian urban context, recruitment early in pregnancy while challenging is feasible. Demonstrating the ability to recruit early in pregnancy is important as there is biological plausibility that early pregnancy is a critical period for foetal programming. A potential underlying mechanism could lie in epigenetic alterations that appear to be sensitive to environmental and dietary influences around the time of conception.
While early recruitment was feasible, those recruited into the study were predominantly attending private obstetric practices and of higher socio-economic status than the background general community. This bias towards recruitment among those with higher socio-economic status may have also played a role in high initial retention rates. A future larger RCT needs to employ specific strategies to enhance recruitment of public patients.
In this study, we show that delivering a lifestyle program via technology is feasible. As technology becomes more advanced and available, it is being used increasingly to attempt to improve health outcomes.Reference Chaudhry, Wang and Wu 29 , Reference Head, Noar, Iannarino and Harrington 30 To date, the number of studies in the field of lifestyle intervention in pregnant women is still limited,Reference O’Brien, McCarthy, Gibney and McAuliffe 31 but pilot studies show promise.Reference Soltani, Duxbury, Arden, Dearden, Furness and Garland 32 , Reference Knight-Agarwal, Davis, Williams, Davey, Cox and Clarke 33 Pilot studies including-Text4twoReference Willcox, Wilkinson and Lappas 34 and a trial by Herring et al. Reference Herring, Cruice, Bennett, Rose, Davey and Foster 35 demonstrate the ability to modify GWG using technology. Herring et al. showed that with n = 66, an intervention, which included text messaging and coaching through Facebook, lowered the prevalence of excessive GWG. Willcox et al. (n = 91) demonstrated significantly lower GWG in the intervention group (7.8 kg, 4.7 versus 9.7 kg, 3.9; p = 0.041) compared with the control group. Our current study has demonstrated that a technology-supported intervention can not only modify lifestyle contributing to GWG but also potentially assist in lowering offspring weight. Although observed in a small study, these results are promising given the limited effect of traditional lifestyle interventions thus far upon neonatal health outcomes.Reference Rogozinska, Marlin and Jackson 7
From those initially screened, we observed a recruitment rate of 17.3% (57/329). This was due to the inclusion and exclusion criteria (23.4% ineligible, 77/329), and personal factors which meant some women were not interested in participating (28.6%, 94/329). The overall rate of eventual enrolment after screening potential participants (17.3%) is in line with UPBEAT (UK Pregnancies Better Eating and Activity Trial) reporting 17.6% (1555 women recruited of 8820 assessed).Reference Poston, Bell and Croker 36 Recruitment rates in other studies with first trimester commencement were variable. One study reported a 6% recruitment rate (445/7605, Ronnberg et al. Reference Ronnberg, Ostlund, Fadl, Gottvall and Nilsson 16 ), another study by Garnaes et al. Reference Garnaes, Morkved, Salvesen, Salvesen and Moholdt 37 acknowledged under-recruitment as a significant challenge, while a further study reported a 78% recruitment rate (154/197).Reference Di Carlo, Iannotti and Sparice 9 These rates of enrolment are important for others designing such studies who will need to plan staffing and realistic timelines to achieve adequate participants. Unfortunately, not all RCTs report these figures, hence providing no guidance for future studies.
Participant retention for the duration of the 12-week intervention was relatively high at 82.6% and 75.9% for the control and intervention groups, respectively, but dropped to 65% at birth and 56% at 3 months. Participants reported their need to drop out due to having busy lives, work commitments and other children. This is consistent with studies by Guelinckx et al.,Reference Guelinckx, Devlieger, Mullie and Vansant 10 Krukowski et al. Reference Krukowski, West and DiCarlo 13 and Wang et al.,Reference Wang, Wei and Zhang 18 where the most common reasons for withdrawal were time commitments, miscarriage, premature delivery or unwillingness to continue to participate.
In this study, 44% were either overweight or obese according to their reported pre-pregnancy weight and height. This is similar, but higher than previous reportedReference Callaway, Prins, Chang and McIntyre 38 of overweight/obesity (34%) during pregnancy in the Australian population. This may reflect ongoing rising prevalence of obesity over the last decade. It is notable that maternal pre-pregnancy overweight/obesity is not only associated with increased risk of offspring obesity,Reference Gaillard, Welten and Oddy 4 but is also a predicting factor in exceeding GWG.Reference Gaillard, Welten and Oddy 4, Reference Gaillard, Felix, Duijts and Jaddoe 39 In general, pregnant Australian women have poor knowledge about obesity, GWG and its consequences on childhood obesity.Reference Willcox, Ball, Campbell, Crawford and Wilkinson 40 – Reference Bookari, Yeatman and Williamson 42 The PLAN intervention was designed to increase knowledge and provide tools to manage weight gain during pregnancy starting from the first trimester.
Lessons learnt from feasibility study
Three main potential drawbacks were identified in the current feasibility study: differences in self-reporting of lifestyle factors at baseline, loss to follow-up after 12 weeks and biased recruitment from private practice. These will inform the design and conduct of a larger trial. Understanding these drawbacks and incorporation into future design will reduce threats to the validity of a larger trial.
To achieve the enrolment of participants prior to 10 weeks gestation, a decision was made to randomise participants on their first study visit. As the food diary and accelerometers are collected over 3–5 days, this would have delayed introduction of the app for a week. This decision, in effect, resulted in the non-blinding of the participants, while ongoing dietary, accelerometry and psychological data were collected. This resulted in differences at baseline of these parameters. We acknowledge this as a limitation of the pilot and that such baseline differences in a larger, definitive trial would make interpretation of conventional analysis of the effectiveness of the 12-week intervention difficult. For optimum design of a future larger RCT, any attempt to facilitate early recruitment needs to be secondary to participant delay blinding until baseline data are fully collected.
Nevertheless, this ‘placebo’-like effect and motivation boost by being selected into the intervention were marked and worth reporting. This might be an underrecognised phenomenon, potentially occurring in most lifestyle RCTs, where it is almost impossible to blind participants. It would not be measured in what would have been deemed conventionally a more optimal design. This is consistent with knowledge that short-term placebo effects occur with response expectancy, conditioning and goal activation. The amelioration of some of these beneficial changes is also consistent with the fact that long-term therapeutic placebo change is achieved through other effects such as goal satisfaction and effects on the hypothalamic–pituitary–adrenal axis.Reference Hyland 43
Further investigation is warranted about whether this phenomenon can be leveraged to motivate lifestyle change. In the current study, consistent results across related domains were observed. As the effect sizes observed are larger than usually achieved with lifestyle interventions, the usefulness of immediately enhancing early motivation is worthwhile exploring further. Of note, a delay to maintain blinding of the participant until after baseline parameters were collected would not be necessary if it were not part of an intervention trial. Consequently, if offered to the general public, there would be no issue with needing to collect unbiased baseline data and one could still maximise early engagement as soon as a woman is aware she has become pregnant.
A further lesson learnt is that long-term follow-up into the offspring generation declined to almost 50% by 3 months post-partum. This reinforces the need for such trials that attempt to modify risk into the next generation, to have sufficient resources to maintain long-term engagement. Sufficient duration of offspring follow-up is essential if such trials are to answer the question as to whether offspring outcomes can be modified.Reference Toledano, Smith, Brook, Douglass and Elliott 44
In this study, recruitment was predominantly of private, not public obstetric patients. Hence, a future larger RCT will need to employ greater resources and targeted strategies to engage those in the public health system.
Differences detected in outcomes
This study was designed to demonstrate feasibility and therefore not powered to detect outcome differences. Nevertheless, between-group differences were detected in dietary intake and psychological readiness to change, both requisite antecedents to modifying weight gain.
Significant differences between the intervention and control groups were noted in dietary and psychological parameters at the start (9 weeks gestation) and end (22 weeks gestation). Accounting for baseline values, there was an increase in score for confidence to implement dietary changes and score for readiness to exercise.
Power calculations should ensure that a future study has sufficient power to investigate if an interaction between the BMI category and the intervention group was present. This would indicate if the efficacy of intervention was different between normal weight, overweight and obese mothers.
At the end of the intervention, the differences between groups were of similar or larger magnitude than other pregnancy lifestyle RCTs. For example, % total fat intake at the end of intervention in the intervention group versus controls was (30.5% ± 5.2 versus 31.5% ± 5.1) in UPBEAT,Reference Poston, Bell and Croker 36 while within the PLAN study, it was (33.7% ± 1.1 versus 36.9% ± 1.2). Percentage saturated fat intake at the end of intervention in the intervention group versus controls was (12.1% ± 2.8 versus 13.1% ± 3.0) in UPBEAT,Reference Poston, Bell and Croker 36 while within the PLAN study, it was (13.1% ± 0.3 versus 14.9% ± 0.6).
Our current study detected a significant difference on infants. Children in the intervention group were lighter by on average 789 g and had lower ponderal index (25.5 ± 3.0 versus 28.8 ± 4.0 kg/m3, p = 0.032) at 3 months of age, compared to the control group. While large-scale observational studies have consistently observed positive associations between GWG, maternal fat deposition and future childhood cardiometabolic risk, causality from observational studies cannot be inferred. Potential confounders such as breastfeeding practices and selective attrition could influence findings of observational studies. Establishing causation requires evidence from RCTs. The UPBEAT RCT follow-up reported reduced infant subscapular skin thickness z score (0.26 standard deviation (SD) lower in the intervention arm). This was achieved in larger numbers (698 infants) and with modification of maternal antenatal diet throughout the pregnancy, using a more intensive intervention. Haby et al. show that after recruiting to a lifestyle modification program very early in pregnancy, birthweight was significantly lower, and macrosomia (i.e., birthweight > 4500 g) significantly less in the intervention group. Our study has several key differences. Haby et al. focused on obese women with BMI >30. We included all women within all weight categories (normal, overweight and obese). We utilised technology and automation, hence relying on a lower intensity of face-to-face counselling. As well, our intervention relied on a shorter period, limited to 3 months of the pregnancy. Despite being a pilot, and insufficiently powered to detect difference in outcomes, our study has found multiple infant effects in the same direction as these two larger studies. Our findings need confirmation in future larger studies focusing on two key factors: technology-based interventions and targeting a limited time in pregnancy. If differences in infant adiposity are confirmed, it would suggest that an equal effect on infants might be achieved targeting specific parts of pregnancy, rather than needing to sustain changes throughout a 9-month pregnancy. This might be easier to maintain and require less manpower
A limitation of this pilot is in adequate power to assess outcomes. Nevertheless, we detected some differences between the intervention and control groups. These significant findings may still be valid and are certainly worthy of replication in a larger study. However, no conclusions can be drawn from the absence of detected difference in outcomes.
A further limitation of this study is the lack of blinding. As with all lifestyle RCTs, double blinding is inherently difficult. The larger randomised studies in this field have also not blinded participants.Reference Haby, Berg, Gyllensten, Hanas and Premberg 19 , Reference Poston, Bell and Croker 36 , Reference Dodd, Turnbull and McPhee 45 Blinding of assessors has been done in someReference Dodd, Turnbull and McPhee 45 and not others.Reference Poston, Bell and Croker 36 As a pilot study, there were insufficient staff to allow blinding of assessors. Blinding would be undertaken in subsequent larger studies. Alternate study designs may address this, such as cluster randomised trials, which alleviate issues around blinding in lifestyle trials.
Conclusion
Findings suggest that a technology supported dietary and lifestyle intervention starting in the first trimester of pregnancy is feasible in an Australian urban context. Recruitment before 10 weeks was feasible; however, a delay in group allocation should be implemented in future studies, to maintain blinding during baseline dietary, psychosocial and physical activity data collection. A further, serendipitous finding was the ‘placebo’-like effect of randomisation to receive extra support in the intervention group, which resulted in immediate positive changes in lifestyle. This warrants future study as both a factor that may be underestimated in lifestyle trials and potential phenomenon that could be leveraged to motivate lifestyle change.
In conclusion, this study highlights that it may be possible to change obesity trajectories in the next generation, through targeting a limited time in early pregnancy and utilising technology. This warrants a further, larger RCT. If effects of the intervention were confirmed in a larger RCT, roll out to all eligible obstetric patients could be instituted with minimal cost and effort, potentially leading to a lifetime of cost savings.
Author ORCIDs
Rae-Chi Huang, 0000-0002-8464-6639
Acknowledgements
We acknowledge PLAN Study participants and their families. We thank Rachel Pearce (dietician), Alana McNamara (research assistant), Alexander Shivarev (medical student) and Brittany Phillips (medical student) for their help. We thank Western Pathology for use of their facilities and ORIGINS for their kind support. R-CH is supported by a National Health and Medical Research Council (NHMRC) Fellowship (grant number 1053384). HC is supported by an Australian National Heart Foundation Future Leader Fellowship (#100794).
Conflicts of Interest
Authors have no conflicts of interest to declare.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174419000400











