Introduction
Placental insufficiency (PI) is the leading cause of intrauterine growth restriction (IUGR),Reference Hendrix and Berghella1 and affected individuals have an 18-fold greater incidence of complications associated with the metabolic syndrome in childhood, adolescence and adulthood.Reference Reinehr, Kleber and Toschke2–Reference Barker, Hales and Fall5 PI is caused by reduced placental mass,Reference Regnault, Galan, Parker and Anthony6 abnormal placental microvasculatureReference Regnault, Orbus and de Vrijer7 and decreased transport abundance and activity,Reference Limesand, Regnault and Hay8, Reference de Vrijer, Regnault, Wilkening, Meschia and Battaglia9 all of which limit nutrient and oxygen transport to the fetus. As fetal nutrient requirements increase with exponential growth in the third trimester, fetal hypoglycemia and hypoxemia progressively worsen in fetuses with PI.Reference Leos, Anderson and Chen10 In response, fetal plasma norepinephrine (NE) concentrations are elevated, which, along with hypoglycemia, act to lower plasma insulin concentrations.Reference Leos, Anderson and Chen10–Reference Harwell, Padbury and Anand13 In addition, fetal plasma insulin-like growth factor-1 (IGF-1) is lower in PI fetuses near term.Reference de, Davidsen, Wilkening, Anthony and Regnault14 Together, these metabolic and endocrine modifications promote glucose and oxygen sparing to preserve necessary fetal functions but also result in stunted and asymmetrical fetal growth.Reference Galan, Hussey and Barbera15, Reference Fowden16, Reference Limesand, Rozance, Smith and Hay17
The fetal adrenal medulla responds to hypoglycemia and hypoxemia by secreting catecholamines, predominantly NE.Reference Sperling, Christensen, Ganguli and Anand18–Reference Yates, Macko and Chen20 NE suppresses insulin secretion from the fetal β cells via α2 adrenergic receptors (ADRα2).Reference Jackson, Piasecki, Cohn and Cohen12, Reference Bassett21–Reference Sperling, Ganguli, Leslie and Landt23 Chronic NE exposure has also been implicated in fetal growth suppression and adrenergic desensitization in skeletal muscle and adipose tissue by acting via adrenergic receptor β2 (ADRβ2).Reference Yates, Macko and Chen20, Reference Yates, Macko and Nearing24, Reference Chen, Fahy and Green25 Moreover, chronic exposure to high-plasma NE in utero produces adaptive responses in metabolism, growth and β-cell function.Reference Chen, Fahy and Green25–Reference Green, Rozance and Limesand27
In an ovine model of PI-induced IUGR, fetuses at 0.9 gestation are severely hypoxemic and hypercatecholaminemicReference Limesand, Rozance, Zerbe, Hutton and Hay28, which parallels human IUGR fetuses.Reference Greenough, Nicolaides and Lagercrantz29–Reference Pardi, Cetin and Marconi32 PI-IUGR fetal sheep also have lower basal and glucose-stimulated insulin concentrations, which are negatively associated with greater plasma NE concentrations.Reference Leos, Anderson and Chen10 Acute pharmacological adrenergic blockade (ADR-block) near term had no effect on plasma insulin concentrations in control fetuses but increased basal and glucose-stimulated plasma insulin concentrations and improved insulin secretion responsiveness to glucose in PI-IUGR fetuses, despite a 56% reduction in β-cell mass.Reference Leos, Anderson and Chen10 Together, these findings indicate a compensatory insulin stimulus-secretion adaptation that is intrinsic to PI β cells and is mediated by chronic adrenergic exposure.
At the onset of the third trimester, PI fetuses are hypoxemic (30%) and hypoglycemic (23%), and have three-fold greater plasma NE concentrations compared with age-matched controls.Reference Limesand, Rozance and Macko33 However, these NE concentrations do not exceed the normal range in near-term fetuses that were unaffected by an adrenergic antagonists.Reference Leos, Anderson and Chen10 Therefore, the objective of this study was to determine whether elevations in fetal plasma NE concentrations detected in PI fetuses at 0.7 gestation inhibit fetal insulin concentrations and identify compensatory β-cell responsiveness. To test our objective, fetal insulin concentrations were measured during basal, glucose-stimulated and arginine-stimulated conditions in the absence and presence of an ADR-block.
Materials and methods
Fetal sheep preparations
Columbia-Rambouillet crossbred ewes were purchased from Nebeker Ranch, Lancaster, CA, USA. Ewes were received at 35 days of gestation (dGA), and singleton pregnancies were confirmed by ultrasound. All animal care and use were carried out with institutional approval at the Agricultural Research Complex at The University of Arizona (Tucson, AZ, USA,) which is accredited by the American Association for Accreditation of Laboratory Animal Care, National Institutes of Health and US Department of Agriculture. PI fetuses (n = 10) were created by exposing pregnant ewes to elevated ambient temperatures (40°C for 12 h; 35°C for 12 h; relative humidity of 40 ± 5%) for 55 days (40–95 dGA, term = 148 dGA) as previously described.Reference Regnault, Orbus, Battaglia, Wilkening and Anthony34, Reference Limesand, Jensen, Hutton and Hay35 Control fetuses (n = 8) were from healthy pregnant ewes that were maintained at thermoneutral conditions (25°C). To eliminate dietary effects, control ewes were pair-fed to the average feed intake of ewes exposed to hyperthermic conditions.
Surgical preparation
At 99 ± 1 dGA, indwelling fetal catheters (Tygon Microbore Tubing formulation 5-54-HL; 1.1 mm outer diameter; Norton Performance Plastics, Akron, OH, USA) were surgically placed in the abdominal aorta via the femoral arteries and in the inferior vena cava via the saphenous veins as described previously.Reference Limesand, Rozance, Zerbe, Hutton and Hay28, Reference Limesand, Rozance and Macko33 Catheters were tunneled subcutaneously to the ewe's flank, exteriorized through a skin incision and kept in a plastic mesh pouch sutured to the ewe's skin. Ampicillin (500 mg; Claris Life Science, North Burnswick, NJ, USA) was administered into the amniotic fluid at surgery, and ewes were given phenylbutazone (0.5 g/45 kg, i.v.; Butler Schein Animal Health, Dublin, OH, USA) for 48 h, following surgery. Ewes were allowed to recover for 4 days before studies were conducted.
Study design
Two square-wave hyperglycemic clamp studies were conducted on separate days between 103 and 105 dGA to evaluate glucose-stimulated insulin secretion (GSIS) and glucose-potentiated arginine-induced insulin secretion (GPAIS) in each fetus as previously described.Reference Limesand, Rozance and Macko33, Reference Green, Chen and Macko36 In brief, after three basal samples were collected, the hyperglycemic clamp was initiated with an intravenous glucose bolus (383 ± 31 mg/kg), followed by a continuous glucose infusion (13.0 ± 1.8 mg/min/kg) to increase and maintain arterial plasma glucose concentration at a steady state of ∼2.5 mmol/l. Three hyperglycemic steady-state blood and plasma samples were collected, beginning 40 min after the glucose bolus. Afterwards, an arginine bolus (0.5 mmol/kg estimated fetal) was administered intravenously over 4 min, and three samples were collected to assess GPAIS. In the saline vehicle-control study, fetuses received an initial 2 ml bolus and constant infusion of saline (vehicle; 2 ml/h) that began 1 h before basal samples were collected and continued throughout the study. In the ADR-block study, fetuses received an initial bolus of the adrenergic antagonists, propranolol (420 μg/kg; Sigma-Aldrich, St. Louis, MO, USA) and Antisedan (150 μg/kg atipamezole HCl; Pfizer Animal Health, New York, NY, USA), followed by constant infusion at a rate of 7 μg/min/kg and 2.5 μg/min/kg, respectively, as previously described.Reference Jackson, Piasecki, Cohn and Cohen12 Saline vehicle-control and ADR-block studies were conducted in each fetus. To eliminate potential confounding side effects, ADR-block studies were conducted at least 24 h but not more than 48 h following saline vehicle-control studies.
After completing in vivo studies, fetuses were recovered to pre-study conditions for >20 h. Ewes and fetuses were euthanized on 107 ± 1 dGA with an intravenous lethal dose of sodium pentobarbital (86 mg/kg) and phenytoin sodium (11 mg/kg; Euthasol, Virbac Animal Health, Fort Worth, TX, USA). Fetal body weights and organ weights were measured, and fetal pancreatic islets were isolated to determine insulin content as previously described.Reference Limesand, Rozance, Zerbe, Hutton and Hay28 In brief, sufficient islet numbers were obtained from five controls and eight PI fetuses to provide 10 replicates of 10 hand-picked islets for the analysis of islet insulin content.
Biochemical analysis
Blood gases, pH and oximetry were measured with an ABL 720 (Radiometer, Copenhagen, Denmark), and values were temperature-corrected to 39.1°C, the average core body temperature for sheep. Plasma glucose and lactate concentrations were measured with a YSI model 2700 SELECT Biochemistry Analyzer (Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma insulin concentrations and fetal islet insulin contents were measured with an ovine insulin ELISA (ALPCO Diagnostics, Windham, NH; intra- and inter-assay coefficients of variation were <10%). Plasma NE was measured with a noradrenaline ELISA (Labor Diagnostika Nord GmbH & Co.KG, Germany; intra- and inter-assay coefficient of variation were <14%). Plasma IGF-I was measured via ELISA (Alpco Diagnostic; intra- and inter-assay coefficients of variation were <10%) as previously described.Reference de, Davidsen, Wilkening, Anthony and Regnault14
Statistical analysis
All data are expressed as mean ± s.e. Samples were averaged across basal and hyperglycemic periods for each animal, and period means were used for biochemical and hematological value comparisons. For the GPAIS period, individual samples were compared. Statistical analyses for biochemical, hematological, NE, IGF-1 and fetal weight values were performed by one-way ANOVA using the general linear means procedure of SAS (SAS Institute Inc., Cary, NC, USA), and differences were determined with a post hoc Fisher's least significant difference test. Insulin and glucose concentrations during basal and hyperglycemic periods were analyzed by repeated measures ANOVA, using the mixed procedure of SAS, with fixed effects for treatment, study and period (draw time), and with random effects for sheep. Correlations were determined with SAS on observations pooled across treatments in the basal period of the saline GSIS study, and the best-fit equation for plasma insulin was determined with linear regression.
Results
Maternal core body temperature
Mean core body temperature in control ewes averaged 38.7 ± 0.2°C during the treatment period. PI treatment elevated (P < 0.05) mean core body temperatures in ewes to 39.7 ± 0.3°C, which was similar to values previously published for ewes exposed to this treatment.Reference Regnault, Orbus, Battaglia, Wilkening and Anthony37 After PI treatment, mean core body temperature in PI ewes returned to 38.6 ± 0.3°C, which was not different from control ewes.
Fetal and placental weights
Placental weights were reduced (P < 0.05) by 38% in the PI group compared with controls (222.1 ± 22.5 g v. 356.5 ± 28.8 g). Fetal weights were not different (P > 0.05) between PI fetuses and controls (1.17 ± 0.09 and 1.21 ± 0.06 kg, respectively). Similarly, brain (28.1 ± 1.5 g in PI and 28.9 ± 1.7 g in controls) and liver (64.2 ± 2.6 g in PI and 58.4 ± 4.2 g in controls) weights were not different between treatments.
Fetal hematological values
Fetal arterial lactate, pH, blood gas and oximetry variables are presented for the basal period (Table 1). Basal blood oxygen content and saturation were 21% and 24% lower (P < 0.05), respectively, in PI fetuses compared with controls. PI fetuses had increased pH, arterial carbon dioxide tension and bicarbonate compared with control fetuses. In control fetuses, no differences were found in any measured basal parameters between saline and ADR-block studies. However, in PI fetuses, ADR-block decreased (P < 0.05) basal arterial oxygen content, arterial oxygen saturation and pH compared with the saline study.
Table 1 Fetal hematology parameters for the baseline period
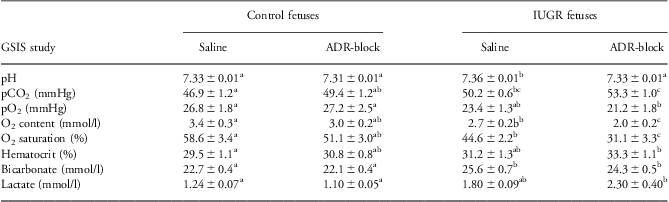
ADR-block, acute pharmacological adrenergic blockade; GSIS, glucose-stimulated insulin secretion; IUGR, intrauterine growth restriction.
a,b,c,ab,Superscripts denote differences (P < 0.05) within rows.
Similar patterns were observed between control and PI fetuses during the hyperglycemic steady-state period of the saline studies (Table 2). No differences were identified between saline and ADR-block studies in control fetuses for any variables during the hyperglycemic steady-state period. However, oxygen content, oxygen saturation, pH and bicarbonate values were decreased in PI fetuses during the hyperglycemic period, and arterial carbon dioxide tension and plasma lactate concentrations were increased with the ADR-block study.
Table 2 Fetal hematology parameters for the hyperglycemic period
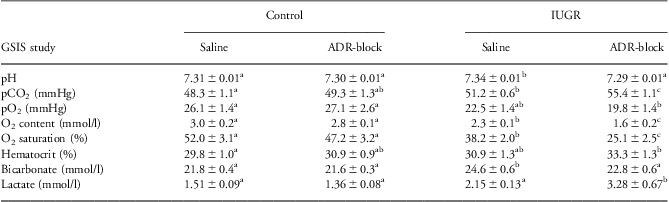
ADR-block, acute pharmacological adrenergic blockade; GSIS, glucose-stimulated insulin secretion; IUGR, intrauterine growth restriction.
a,b,c,abSuperscripts denote differences (P < 0.05) within rows.
Plasma NE and IGF-1 concentrations
Plasma NE concentrations were three-fold greater (P < 0.05) in PI fetuses compared with control fetuses during the saline study (Fig. 1). No differences were found between basal and hyperglycemic steady-state periods in the saline study.

Fig. 1 Fetal plasma norepinephrine (NE) concentrations. Plasma NE concentrations (±s.e.m.) are presented for control (n = 8) and PI (n = 10) fetuses. NE concentrations were not different between basal and hyperglycemic steady-state periods and are averaged for the study. The asterisks denote a difference (P < 0.05) between treatments. PI, placental insufficiency.
Plasma IGF-1 concentrations were lower (P < 0.05) in PI fetuses (50.4 ± 6.8 ng/ml) compared with control fetuses (70.2 ± 5.8 ng/ml) in the basal period. ADR-block did not affect plasma IGF-1 concentrations in either PI or control fetuses.
GSIS response to an adrenergic blockade
Plasma glucose concentrations during basal and hyperglycemic periods were lower (P < 0.05) in PI fetuses than in control fetuses for both studies (Fig. 2). ADR-block reduced (P < 0.05) basal plasma glucose concentrations 18% in PI fetuses and 12% in controls compared with the saline study. In accordance with the GSIS experimental design, plasma glucose concentrations increased (P < 0.05) during the hyperglycemic period from basal in all studies. Hyperglycemic glucose concentrations were not different between studies in control fetuses and were 4% greater (P < 0.05) in PI fetuses during the ADR-block study compared with the saline study, but were within the margin of error for maximum stimulatory glucose concentrations in both studies.Reference Green, Macko and Rozance38

Fig. 2 Fetal plasma glucose concentrations. Plasma glucose concentrations (±s.e.m.) are presented for basal and hyperglycemic steady-state periods of saline and pharmacological adrenergic blockade (block) glucose-stimulated insulin secretion (GSIS) studies. Means are for control (n = 8) and PI (n = 10) fetuses. Differences (P < 0.05) among means are indicated by different letters. PI, placental insufficiency.
In the saline study, plasma insulin concentrations were 47% lower (P < 0.05) during the basal period and 36% lower (P < 0.05) during the hyperglycemic period in PI fetuses compared with control fetuses (Fig. 3). Basal plasma insulin concentrations were not affected by ADR-block in PI or control fetuses. In controls, plasma insulin concentrations were reduced by 20% (P < 0.05) during the hyperglycemic period with the ADR-block. Conversely, hyperglycemic plasma insulin concentration in PI fetuses increased 1.7-fold (P < 0.05) in the ADR-block study compared with the saline study. Consequently, insulin concentrations were greater (P < 0.05) in PI fetuses than controls during the hyperglycemic period of ADR-block study, and were not different from the maximum insulin concentrations observed in controls during hyperglycemic period of the saline study.

Fig. 3 Fetal plasma insulin concentrations at glucose-stimulated insulin secretion steady-state conditions. Plasma insulin concentrations (±s.e.m.) are presented for the basal and hyperglycemic steady-state periods of saline and adrenergic blockade block) GSIS studies. Means are for control (n = 8) and PI (n = 10) fetuses. Differences (P < 0.05) among means are indicated by different letters. PI, placental insufficiency.
In control fetuses, the GSIS responsiveness (difference between insulin concentrations during the basal and hyperglycemic period) was not different between saline and ADR-block studies (0.59 ± 0.07 and 0.49 ± 0.07 ng/ml insulin, respectively). In contrast, GSIS responsiveness was increased 1.9-fold (P < 0.01) in PI fetuses during the ADR-block study compared with the saline study (0.85 ± 0.06 v. 0.44 ± 0.06 ng/ml insulin; P < 0.01). Moreover, GSIS responsiveness was greater (P < 0.01) in PI fetuses compared with control fetuses during the ADR-block studies.
Plasma glucose-to-plasma insulin ratio (G/I), which indicates the sensitivity of fetal glucose clearance to insulin, was greater (P < 0.05) in PI fetuses than control fetuses during the saline study. During the ADR-block study, the G/I ratio did not change in control fetuses but decreased (P < 0.05) in PI fetuses to a value not different from controls (Fig. 4).

Fig. 4 Fetal glucose-to-insulin ratios during the basal period. Mean plasma glucose/insulin concentrations (±s.e.m.) are presented for the basal steady-state period of the saline and adrenergic blockade studies. Means are presented for control (n = 8) and PI (n = 10) fetuses. Differences (P < 0.05) between means are indicated by different letters. PI, placental insufficiency.
GPAIS
Plasma insulin reached maximum concentrations 5 min after the administration of the arginine bolus in PI and control fetuses (Fig. 5). Plasma insulin concentrations were lower (P < 0.05) in PI fetuses compared with controls at 5 and 15 min after the arginine bolus. In control fetuses, plasma insulin concentrations during the GPAIS period were not different between saline and ADR-block studies. Plasma insulin concentrations during the GPAIS period increased (P < 0.05) to a greater extent in the ADR-block study than in the saline study at all-time points in PI fetuses, and were also greater (P < 0.05) than controls during the ADR-block study at 15 and 30 min.

Fig. 5 Fetal plasma insulin concentrations during glucose-potentiated arginine-stimulated insulin secretion (GPAIS). Plasma insulin concentrations (y-axis; ±s.e.m.) are shown for 5-, 15- and 30-min samples, following the arginine bolus initiated at time 0 (x-axis). The GPAIS was determined in control (n = 8) and PI (n = 10) fetuses in the presence (block) or absence (saline) of adrenergic antagonists. Letters within a given collection time denote differences (P < 0.05). PI, placental insufficiency.
Fetal islet insulin content
Fetal pancreatic islet insulin contents were not different (P > 0.05) between PI fetuses and controls (17.5 ± 6.3 and 17.0 ± 5.7 ng/islet, respectively).
Regression analysis for basal parameters
Plasma NE concentrations were inversely correlated with arterial blood oxygen content, plasma glucose and insulin concentrations (Table 3). Plasma NE concentrations positively correlated with G/I, plasma lactate concentrations and arterial carbon dioxide tension. The best-fit equation for insulin concentration predicted plasma NE and glucose concentrations as influential variables. Plasma IGF-1 concentrations were positively correlated with plasma glucose concentrations but were inversely correlated with plasma NE concentrations.
Table 3 Correlation coefficients for fetal parameters and regression equation for insulin concentrations

G/I, Plasma glucose-to-plasma insulin ratio; IGF-1, insulin-like growth factor-1; NE, norepinephrine.
ns, not significant (P > 0.05).
Discussion
In this study, we show that elevated fetal plasma NE concentrations effectively inhibit metabolism-dependent (GSIS) and metabolism-independent (GPAIS) stimulation of insulin secretion and augment insulin sensitivity in PI fetuses at 0.7 gestation, which is before measureable growth restriction. These findings are consistent with previous studies in near-term fetuses (0.9 gestation) that are severely hypoglycemic, hypoxemic and growth restricted. Our findings also indicate that the inhibition of glucose-stimulated insulin secretion is chronically suppressed in PI fetuses by elevated plasma NE throughout the final trimester.Reference Leos, Anderson and Chen10, Reference Limesand, Rozance, Zerbe, Hutton and Hay28, Reference Limesand, Rozance and Macko33 Interestingly, the magnitude to which circulating NE is elevated was less profound in our younger cohort of PI fetuses than in near-term PI-IUGR fetuses,Reference Leos, Anderson and Chen10, Reference Limesand, Rozance, Zerbe, Hutton and Hay28 which may reflect the relative immaturity of fetal adrenal medullary responsiveness or the progressively increasing severity of fetal hypoglycemia and hypoxemia.Reference Cheung39, Reference Comline and Silver40 Although plasma NE concentrations measured in PI fetuses in the current study did not exceed threshold levels previously determined to alter glucose metabolism in near-term fetuses,Reference Cheung39, Reference Padbury, Ludlow, Ervin, Jacobs and Humme41 we did observe reduced GSIS during the saline study and reduced basal glucose concentrations and enhanced GSIS in the presence of an ADR-block in these fetuses, which suggests that the thresholds for NE are lower at earlier gestational ages and that fetal β-cell adaptations to chronic adrenergic stimulation precede IUGR.
Our findings support the hypothesis that compensatory insulin-secretion responsiveness in PI fetuses is intrinsic to fetal β cells, and that underlying functional adaptations are present as early as 0.7 of gestation. The ADR-block increased glucose-stimulated and arginine-stimulated insulin concentrations in PI fetuses but not controls. In fact, the adrenergic blockade decreased hyperglycemic insulin concentrations in control fetuses, which we postulate was because of an interference with tonic β-adrenergic stimulation of the β cell or lower glucagon potentiation of insulin secretion.Reference Jackson, Piasecki, Cohn and Cohen12, Reference Limesand, Rozance and Macko33, Reference Yates, Macko and Chen42, Reference Kawai, Yokota, Ohashi, Watanabe and Yamashita43 In a previous cohort, β-cell mass was 41% lower in PI fetuses compared with controls at 0.7 gestation,Reference Limesand, Rozance and Macko33 which in part explains lower-plasma insulin concentrations. GPAIS causes β-cell membrane depolarization, independent of metabolism, and thus provides an estimate of the readily releasable pool of insulin.Reference Robertson44, Reference Hermans, Schmeer and Henquin45 Reduced GPAIS in PI fetuses during the saline study indicates that NE inhibits vesicle trafficking, docking and fusion in the β cells of PI fetuses, as previously shown in an insulinoma cell line.Reference Schmidt, Bladt and Goedecke46 However, greater augmentation of GPAIS in PI fetuses than in controls during the adrenergic blockade, despite reduced β-cell mass, and similar islet insulin content indicates that the capacity for insulin release is enhanced in the β cells of PI fetuses. From these observations, it is reasonable to postulate that presently undefined mechanisms intrinsic to the fetal β cells of PI fetuses result in greater insulin exocytosis rather than greater insulin biosynthesis or storage.
PI fetuses in this study exhibited indices of greater insulin sensitivity that were abolished by adrenergic blockade. Although insulin sensitivity was not directly tested in this study, G/I ratios indicate that whole-fetus insulin sensitivity for glucose utilization is greater at the start of the third trimester in PI fetuses than in controls, which was similar to previous findings at 0.7 of gestation, as well as findings at 0.9 of gestation.Reference Limesand, Rozance, Smith and Hay17, Reference Limesand, Rozance and Macko33, Reference Thorn, Regnault and Brown47 Moreover, insulin hypersensitivity for glucose utilization was observed in a different fetal sheep model of placental restriction created by carunclectomy.Reference Owens, Falconer and Robinson48, Reference Owens, Gatford and De Blasio49 By comparing outcomes in PI fetuses in the presence and absence of adrenergic blockade, this study uniquely identifies the role that NE plays in enhanced insulin sensitivity, which may include actions that are independent of suppressed insulin secretion.Reference Chen, Fahy and Green25, Reference Yates, Macko and Chen42, Reference Deibert and DeFronzo50 During the basal period, adrenergic blockade reduced glucose concentrations in PI fetuses without increasing insulin concentrations, which indicates greater glucose utilization that is independent of insulin secretion. Furthermore, the positive association between NE and lactate concentrations indicates a shift from oxidative metabolism to anerobic glycolysis in PI fetuses. Together, these data demonstrate a multifaceted role for NE in the regulation of glucose homeostasis in PI fetuses that includes the enhancement of insulin sensitivity for glucose utilization and impaired β-cell function with compensatory insulin secretion responsiveness, which was revealed by ADR-block and independent of enhanced fetal insulin sensitivity.
Plasma IGF-1 concentrations were reduced in PI fetuses compared with controls at 0.7 gestation. Previous studies using models of chronic and progressive PI have demonstrated decreased insulin and IGF-1 concentration near term.Reference Thorn, Regnault and Brown47, Reference Brown, Rozance, Thorn, Friedman and Hay51 We now demonstrate that these influential fetal growth factors are already deficient in PI fetuses at the beginning of the third trimester. Fetal plasma IGF-1 concentrations were not recovered by adrenergic blockade, which indicates that the strong correlation between IGF-1 and NE concentrations are associative and that IGF-1 is not directly or acutely regulated by plasma NE concentrations. Rather, lower IGF-1 concentrations likely result from the reductions in nutrient supply associated with PI or from indirect actions of NE on hepatic IGF-1 production.
In conclusion, NE concentrations were elevated in PI fetuses at 0.7 gestation and act to inhibit insulin secretion and increase insulin sensitivity, albeit at concentrations below previously defined threshold levels in near-term sheep fetuses. The actions of NE did not extend to IGF-1, which was lower in PI fetuses but unaffected by adrenergic blockade. Finally, this study identifies a compensatory response in the β cells of PI fetuses during adrenergic inhibition that resulted in greater insulin-secretion responsiveness to glucose and arginine. These findings allow us to postulate that chronic elevations in fetal plasma NE concentrations in PI fetuses are involved in proactive fetal metabolic programming in anticipation of nutrient conditions that would not support normal rates of fetal growth and metabolism over the final trimester.
Acknowledgments
We thank Miranda J. Anderson and Mandie M. Dunham for technical assistance.
Financial Support
Funding for this work was from the National Institutes of Health R01 DK084842 (S.W. Limesand, Principal Investigator). D.T. Yates and A.R. Macko were supported by T32 HL7249, and D.T. Yates was supported by USDA-NIFA Fellowship, 2012-67012-19855. A.S. Green was supported by F32 DK088514. L.D. Brown was supported by National Institutes of Health Building Interdisciplinary Careers in Women's Health Scholar Award K12 HD057022 and Children's Hospital Colorado Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Statement of Interest
The authors have no conflicts of interest.
Author Contributions
All authors approved the submission of the manuscript and contributed as follows: A.R.M. and S.W.L. conducted the experimental design; A.R.M. wrote the manuscript; D.T.Y., X.C., A.S.G., A.C.K., L.D.B. and S.W.L. carried out experiments, statistical analysis, and proofread the manuscript. All authors read and approved the final manuscript.






