Introduction
Bisphenol-A (BPA) is a weak estrogen that is present as a ubiquitous contaminant in our environment.Reference Michalowicz 1 It is used in the manufacture of a variety of plastic products and can leach from plastics when they are subjected to heat or when plastics are degraded.Reference Hoekstra and Simoneau 2 , Reference Ranjit, Siefert and Padmanabhan 3 It has also been found in air, dust and water sources.Reference Vandenberg, Hauser, Marcus, Olea and Welshons 4 Pregnant women are likely to be exposed to BPA through dermal contact,Reference Liao and Kannan 5 consumption of canned beverages and foods, and inhalation.Reference Geens, Roosens, Neels and Covaci 6 As a result, BPA is present in maternal circulation,Reference Padmanabhan, Siefert and Ransom 7 and is known to cross the placenta to reach the fetus and impact fetal growth.Reference Rochester 8 It is also excreted through breast milk.Reference Sun, Irie and Kishikawa 9 Therefore, exposure to BPA can occur in utero and during early postnatal life,Reference Golub, Wu and Kaufman 10 when organ systems are differentiating. Prenatal BPA exposure is known to affect a number of organs, however, its effects on the heart have not been studied in detail.
Most studies examining the effects of BPA on the heart or cardiac myocytes have been in rodents. These studies have found that BPA exposure induces arrhythmogenicity in ventricular myocytes most probably because of altered calcium mobility across the sarcoplasmic reticulum.Reference Yan, Chen and Dong 11 In vitro studies have shown that acute BPA treatment can affect cardiac impulse propagation and increase the risk for complete heart block.Reference Posnack, Jaimes and Asfour 12 Moreover, BPA treatment sustains ventricular arrhythmias in female rats that are subjected to ischemia and reperfusion, and there appears to be a synergistic effect between BPA and estradiol-17β in inducing arrhythmias.Reference Yan, Song and Chen 13 This effect is probably mediated through estrogen receptors.Reference Yan, Song and Chen 13 , Reference Belcher, Chen, Yan and Wang 14 In another study involving male mice, BPA exposure following ischemia induced inflammatory changes and reduced the ability of the heart to undergo remodeling.Reference Patel, Kasneci and Bolt 15 Although these studies indicate that BPA can affect the heart, we are not clear if prenatal exposure to BPA can affect the heart as well.
In the present study, we used a sheep model that has a similar developmental trajectory as humans.Reference Padmanabhan and Veiga-Lopez 16 Moreover, cardiac development in the ovine fetus parallels that of the human fetus.Reference Burrell, Boyn and Kumarasamy 17 Sheep are also good models for studying cardiovascular changes after intrauterine compromise.Reference Thompson, Piorkowska, Gagnon, Richardson and Regnault 18 Ovine heart subjected to intrauterine hypoxia has higher levels of inflammatory markers, collagen deposition and matrix metalloproteinases,Reference Thompson, Piorkowska, Gagnon, Richardson and Regnault 18 very similar to what is seen with BPA exposure after ischemia.Reference Patel, Kasneci and Bolt 15 Therefore, we hypothesized that prenatal BPA exposure in sheep might compromise cardiac function in the offspring resulting in altered remodeling parameters. More recent evidence suggests that outcomes resulting from early developmental insults can be exacerbated by the postnatal environment.Reference Prins, Tang, Belmonte and Ho 19 We have previously demonstrated that prenatal BPA exposure followed by overfeeding of the offspring in adulthood can affect insulin sensitivity and increase adipose tissue deposition in sheep.Reference Veiga-Lopez, Moeller and Sreedharan 20 We hypothesized that overfeeding in adulthood can further impair cardiac function and that this effect would be accompanied by molecular changes that reflect hypertrophy and reduced compliance.
Materials and methods
Animals and treatment
Mature Suffolk ewes 2–3 years of age were purchased from local farmers and maintained at the Sheep Research Facility, University of Michigan, Ann Arbor, MI, USA. They were maintained under natural photoperiod and fed 0.5 kg of shelled corn and 1.5–2 kg of alfalfa hay/animal/day. Animals were bred during the breeding season using protocols approved by the Animal Care and Use Committee, University of Michigan. Experiments were performed in accordance with the Guidelines for the Care and Use of Agricultural Animals in Research and Teaching. The experimental protocol for this experiment was recently published.Reference Veiga-Lopez, Moeller and Sreedharan 20 In brief, pregnant ewes were given daily subcutaneous injections of corn oil (control) or BPA (0.5 mg/kg/day in corn oil; Sigma, St. Louis, MO, USA) from day 30 to day 90 of gestation. This window was chosen based on a previous study where pregnant sheep were exposed to excess testosterone, an estrogen precursor, and this caused hypertension in the female offspring.Reference Veiga-Lopez, Luense, Christenson and Padmanabhan 21 The circulating concentrations of BPA achieved with this dose in the umbilical artery averages 2.62±0.52 ng/ml at gestation day 90Reference Veiga-Lopez, Luense, Christenson and Padmanabhan 21 and is close to that observed in the maternal circulation of U.S. women.Reference Padmanabhan, Siefert and Ransom 7 Only female offsprings of these animals were used in this study making sure that only one female offspring from each dam was used in the experiment. When the lambs were 14 weeks old, a subset of female offspring of these dams were fed ad libitum [overfed group (OF group)]. Diet for the OF group included additional corn and followed a previously published overfeeding regimen from 14 weeks of age to the end of the experiment.Reference Steckler, Herkimer, Dumesic and Padmanabhan 22 This resulted in animals increasing their body weight (BW) to ~30% over that of controls. The remaining animals were fed a normal diet as described above [normal fed (NF group)]. This resulted in four treatment groups: control-NF (n=6), control-OF (n=7), BPA-NF (n=7) and BPA-OF (n=7).
Assessing cardiovascular function
Cardiovascular function of adult females was assessed using non-invasive echocardiography at 21 months of age. Blood pressure was measured using a cuff placed on the thigh and a digital blood pressure monitor with the animal in standing position. Animals were restrained manually and a small (3×3') area of skin was clipped and cleaned with alcohol on the thoracic wall on either side, behind the elbow region. Ultrasound gel was applied over the clipped area. The forelimbs were pulled forward to visualize the heart on the echocardiogram. A 5 mHz ultrasonic probe was used in conjunction with the echo device (Vivid-I; GE Healthcare, Little Chalfont, UK) and the EchoPac software. The probe was placed on the fourth or fifth intercostal space with the animal in standing position to examine the heart. Besides two-dimensional echocardiography, Doppler studies (color Doppler and spectral Doppler) were also performed to assess valve function, blood flow velocity and direction. An electrocardiogram was connected simultaneously with the leads placed in all the four limbs to measure electrical changes within the heart. The parameters measured included systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean blood pressure (MBP), aortic diameter (AoD) and aortic circumference, pulmonary arterial diameter, left ventricular posterior wall thickness (LVPW), internal diameter and area, left atrial diameter (LAD), interventricular septal thickness (IVS), cardiac output, ejection fraction, stroke volume, fractional shortening, end systolic and end diastolic volume, ejection fraction and heart rate.
Organ weights
Animals were sacrificed at 22 months of age using Fatal plus (Vortech Pharmaceuticals, Dearborn, MI, USA). We collected various organs including the heart, lung, liver, adrenals, kidneys and spleen and obtained their wet weights. Heart was dissected and flash frozen in a dry ice bath and stored at −80°C until further processing.
Quantitative real-time polymerase chain reaction (RT-PCR) for atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), collagen-1 (COL1) and collagen-3a1 (COL3A1)
RNA from ventricular tissue was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and purified with deoxyribonuclease treatment. To access the quality of extracted RNA, a Nanodrop Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) was used. Samples with low-quality RNA (assessed by OD (optical density) ratio of absorbance 260/280 nm 260/280 ratio) were excluded from further analysis. First strand complementary DNA (cDNA) was synthesized from RNA using SuperScript III First-Strand Synthesis System (Invitrogen Cat. No. 18080-051). cDNA was prepared from 1 μg of total RNA. Concentrations of cDNA over 1000-fold range was amplified using each target gene and the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-specific primers to determine the final concentration at which the each primer sequence for both housekeeping gene and gene of interest were equally and >90% efficient. The primer sequences were obtained from Aitken et al.Reference Aitken, Raizis and Yandle 23 for ANP and BNP and primers for COL1 and COL3A1 were designed in our lab using BLAST (Table 1).
Table 1 Primer sequences for primers used in quantitative polymerase chain reaction assay

ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; COL1A1, collagen-1a1; COL3A1, collagen-3a1.
The SYBR Green RT-PCR assay was performed using the cDNA generated. A negative reverse transcriptase control was run for each sample to rule out genomic DNA contamination of isolated RNA. Quantitative RT-PCR reactions were run on a 7200 Real-Time PCR Instrument (Applied Biosystems, Foster City, CA, USA) and were performed in triplicates. Power SYBR Green (Thermo Fisher Scientific, Waltham, MA, USA) was used to detect the sheep-specific primer sequences of genes. The efficiency of the primers used was determined by generating a standard curve for each. Melting curves were performed to validate the outcomes of the PCR product. Results of the assay were quantified using the cycle threshold (C
T
) values of each sample. The C
T
values of each gene of interest were compared against GAPDH. Only values <35 were considered positive. The fold change was calculated by the
![]() $$2^{{\Delta \Delta C_{T} }} $$
method.Reference Livak and Schmittgen
24
$$2^{{\Delta \Delta C_{T} }} $$
method.Reference Livak and Schmittgen
24
Statistical analysis
All parameters were analyzed by two-way ANOVA followed by Tukey–Kramer post-hoc test. All data were expressed as mean±s.e.m. P<0.05 was considered to be significant.
Results
BW
BWs (kg; mean±s.e.) of female lambs from control-NF (5.47±0.23), BPA-NF (4.65±0.48), control-OF (5.55±0.29) and BPA-OF groups (5.06±0.29) were not different from each other. BW (kg; mean±s.e.) of control-NF and BPA-NF groups at the end of the study were 78.5±1.5 and 81.2±3.5, respectively, and did not differ significantly (Fig. 1A). There was a significant diet effect with overfeeding producing a marked increase in BW both in the control (108.5±2.6) and prenatal BPA-treated sheep (103.5±1.9; P<0.0001).

Fig. 1 (A) Body weight in sheep from different treatment groups. Control+NF and BPA+NF groups were subjected to prenatal exposure to vehicle (control) or BPA and placed on NF diet. Control+OF and BPA+OF groups were subjected to prenatal exposure as above and overfed into adulthood. NF groups demonstrate the effect of prenatal exposures alone, and the OF groups demonstrate the effects of prenatal exposure+postnatal overfeeding. (B) Heart weight in sheep from the different treatment groups. (C) Heart weight to body weight ratio in the different treatment groups. a,bSignificantly different from each other (P<0.05). NF, normal fed group; BPA, bisphenol-A; OF, overfed group.
Organ weight
Although there were significant, albeit modest, effects on heart weight with overfeeding (Fig. 1B), there were no changes when heart weight was normalized to BW (Fig. 1C). There was a significant reduction in kidney weight to BW ratio with prenatal BPA exposure. Although postnatal overfeeding decreased this ratio, it could not completely reverse the effect of prenatal BPA exposure. Prenatal BPA exposure also reduced lung weight, but these changes were masked by the effects of overfeeding (Table 4).
Functional parameters: heart rate and blood pressure
Neither prenatal BPA treatment nor postnatal overfeeding had an effect on the heart rate (Fig. 2A). However, a significant diet (F=7.79) and diet×BPA treatment interaction (F=8.056) was evident relative to SBP (Fig. 2B). Post-hoc analyses found SBP (mmHg; mean±s.e.) increased significantly in the control-OF group (144.8±11.1) relative to control-NF (93.8±9.6; P<0.05) but not in the BPA-OF group.

Fig. 2 (A) Effects of prenatal vehicle or BPA exposure in combination with postnatal normal feeding or overfeeding on heart rate. (B–D) Effects on systolic, diastolic and mean blood pressure. Blood pressure was measured using the tail cuff method. a,bSignificant differences between groups (P<0.05). Bars with a,b indicate that they are not different from bars with a and b. NF, normal fed group; OF, overfed group; BPA, bisphenol-A.
A modest overall BPA effect (F=4.533), pronounced overfeeding effect (F=10.78) and a significant diet×BPA interaction (F=14.55) was evident relative to DBP (Fig. 2C). Post-hoc analyses found DBP (mmHg; mean±s.e.) was significantly elevated in the control-OF group (97.8±7.9) compared with the control-NF (53.6±4.2) and BPA-NF groups (63.8±2.9). Interestingly, DBP in the BPA-OF (60.8±7.9) group did not differ from control or BPA-NF groups. Relative to MBP, ANOVA showed a significant overfeeding (F=10.034; P=0.004) and overfeeding×BPA interaction (F=8.584; P=0.0078) (Fig. 2D). Post-hoc analyses found overfeeding increased MBP (mmHg) in the control-OF (120.8±5.7) compared with control-NF (72.5±7.8) and BPA-NF (87.2±8.7) groups. The obesity-related increase in MBP seen in control-OF group was not evident in the BPA-OF group (88.8±10.3).
Structural changes associated with hypertension
A significant effect of overfeeding and not prenatal BPA was evident in LAD, AoD, aortic area and aortic circumference (Table 2). A significant diet×BPA treatment interaction was also evident in left ventricular internal diameter during systole (LVID during systole).
Table 2 Effect of prenatal vehicle or bisphenol-A (BPA) exposure in combination with postnatal normal feeding or overfeeding on morphometric measurements of the heart that are associated with hypertension
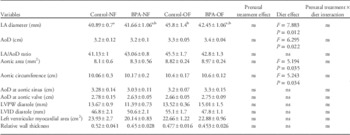
NF, normal fed group; OF, overfed group; LA, left atrial; AoD, aortic diameter; LVPW, left ventricular posterior wall thickness; LVID, left ventricular internal diameter; ns, no significant differences.
a,bGroups with different alphabetic notations are different from each other. a,b indicates no change from a and b. If there are no notations, it indicates that there are no significant changes between groups.
Other functional parameters
Effects of prenatal BPA and overfeeding were not evident in functional parameters such as end diastolic volume, end systolic volume, cardiac output, fractional shortening, ejection fraction and stroke volume (Table 3).
Table 3 Effect of prenatal vehicle or bisphenol-A (BPA) exposure in combination with postnatal normal feeding or overfeeding on functional parameters of the heart

NF, normal fed group; OF, overfed group; ns, non significant.
Morphometric measurements: left ventricular changes
ANOVA revealed a marked diet×prenatal treatment interaction (F=10.734) in left ventricular area (LVA) (cm2; mean±s.e.) during diastole (Fig. 3A). Post-hoc analyses found LVA in diastole to be significantly higher in the control-OF group compared with the control-NF group. Interestingly, prenatal exposure to BPA blocked this overfeeding-induced increase. During systole, a significant overfeeding×BPA interaction (F=11.853; P=0.0022) (Fig. 3B) was evident in LVA. Post-hoc analyses found that the BPA-NF group had significantly increased LVA compared with the BPA-OF group, which was similar to the LVA in the control group.

Fig. 3 Effect of prenatal vehicle or BPA exposure in combination with postnatal normal feeding or overfeeding on left ventricular area during diastole (A) and during systole (B), and in IVS thickness in diastole (C) and systole (D). a,bSignificant differences between groups (P<0.05). Bars with a,b indicate that they are not different from bars with a and b. NF, normal fed group; OF, overfed group; BPA, bisphenol-A; IVS thickness, interventricular septal thickness.
A significant diet effect was apparent in IVS in both diastole and systole (Fig. 3C and 3D). However, post-hoc analyses revealed significant group differences only during systole with the BPA-OF group being thicker than the BPA-NF group (Fig. 3D). Except for modest interaction effects in LVID during systole, there were no effects in LVID during diastole. There were no changes in LVPW with BPA treatment or overfeeding (Table 2).
Molecular changes
Impact of prenatal BPA on ventricular ANP and BNP expression (fold change relative to control; mean±s.e.) are shown in Fig. 4. ANP expression was significantly elevated in the BPA-NF group compared with control-NF in the left ventricle (Fig. 4A). ANP expression was also elevated in the right ventricle of BPA-NF group compared with the control-NF and control-OF groups (Fig. 4B). There were no significant changes in BNP expression in both right and left ventricular tissue (Fig. 4C and 4D). In the left ventricle, there was modest increase in collagen-1a1 (COL1A1) expression (P=0.05) and COL3A1 expression with BPA treatment (P=0.0737; Fig. 5A and 5C). However, a marked reduction in COL1A1 and COL3A1 expression were apparent in the right ventricle with BPA treatment (P<0.01; Fig. 5B and 5D).

Fig. 4 Effect of prenatal vehicle or BPA exposure in combination with postnatal normal feeding or overfeeding on ANP gene expression on LV and RV (A and B, respectively) and on BNP gene expression on LV and RV (C and D, respectively). a,bSignificant differences between groups (P<0.05). Bars with a,b indicate that they are not different from bars with a and b. Graphs that have no notations indicate that there are no significant differences between groups. NF, normal fed group; OF, overfed group; BPA, bisphenol-A; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; LV, left ventricle; RV, right ventricle.

Fig. 5 Effect of prenatal vehicle or BPA exposure in combination with postnatal normal feeding or overfeeding on COL1A1 gene expression on LV and RV (A and B, respectively) and on COL3A1 gene expression on LV and RV (C and D, respectively). a,bSignificant differences between groups (P<0.05). NF, normal fed group; OF, overfed group; BPA, bisphenol-A; COL1A1, collagen-1a1; COL3A1, collagen-3a1; LV, left ventricle; RV, right ventricle.
Discussion
Results from this study involving a large animal model with developmental trajectory similar to human demonstrate that overfeeding leads to marked increases in SBP, DBP and MBP. Paradoxically, while prenatal BPA had little effect on the cardiovascular function in the maintenance-fed animals, overfeeding as adults did not increase their DBP and MBP to the level seen in the control-OF group. This makes it appear as if prenatal BPA exposure may in fact be beneficial in preventing overfeeding-induced increases in blood pressure. On the other hand, it could merely indicate that there were no additive effects on blood pressure due to overfeeding in BPA-exposed sheep. It could also indicate that prenatal exposure to BPA induces changes in cardiac structure and function that makes the heart less resilient to the effects of overfeeding.
Impact of prenatal BPA
Prenatal BPA exposure by itself produced significant increases in ANP gene expression in both ventricles compared with the control group. It did not affect other cardiac structural and functional parameters significantly. ANP and BNP are known to increase in response to elevated atrial and ventricular transmural pressureReference Rademaker and Richards 25 and can be upregulated in heart failure and myocardial infarction.Reference Charles, Prickett and Espiner 26 – Reference Cameron, Rademaker and Ellmers 28 ANP and BNP are also markers of ventricular hypertrophy.Reference Gardner 29 The significant increase in ANP in left ventricles of prenatal BPA-treated offspring compared with control animals could be suggestive of adverse left ventricular programming with BPA, but further studies are needed to characterize this. Available evidence suggests that ANP is produced by cardiac myocytes in response to increased blood pressureReference Nishimura, Mizukawa and Nakao 30 and could play a role in preventing cardiac hypertrophy.Reference Horio, Nishikimi and Yoshihara 31 ANP expression in the ventricles of control animals were comparable with a previously published study by Cameron et al.Reference Cameron, Rademaker and Ellmers 28 The finding that increased ANP expression was also seen in the right ventricle of BPA-treated animals suggests the possibility that prenatal BPA treatment adversely programs pulmonary structure and function as reported in rodent and human studies,Reference Hijazi, Guan, Cernea and Yang 32 , Reference Spanier, Kahn and Kunselman 33 an aspect not investigated in this study.
Besides affecting ANP expression in the ventricles, prenatal BPA exposure also affected collagen expression in these tissues. COL1 and COL3 are fibrillar collagens that are present in the heart. Fibrillar collagen is an important contributing factor to ventricular compliance during diastole and affects systolic function by modulating myocyte shortening.Reference Graham and Trafford 34 Intrauterine hypoxia is known to increase COL1 and COL3 expression in the ventricles of ovine fetuses and this is believed to compromise systolic and diastolic function.Reference Thompson, Piorkowska, Gagnon, Richardson and Regnault 18 In the present study, although COL1A1 and COL3A1 expression were increased modestly in the left ventricle, their expression was reduced in the right ventricle with prenatal BPA exposure. These changes could suggest compensatory mechanisms, nevertheless, and could be indicators of altered ventricular compliance and need further investigation.
Impact of postnatal overfeeding
It is well known that excess food intake and high-fat diet are detrimental to cardiovascular function due to the associated hemodynamic overload.Reference Alpert 35 In this model, there is insulin resistance and increased adipose tissue deposition as a result of overfeeding. There are also marked changes in organ weights, especially the kidneys and lungs (Table 4).Reference Veiga-Lopez, Moeller and Sreedharan 20 The marked increase in DBP and the parallel increase in MBP in the control-OF group could be a function of the weight gain in these animals (Fig. 1). These increases resulted in the expected compensatory changes in LAD (Table 1) that matches with what is observed in obese patients.Reference Gottdiener, Reda, Williams and Materson 36 Moreover, there are marked increases in LVA during diastole (Fig. 3A) but not in LVID (Table 1) in the control-OF group that suggest ventricular remodeling as observed in obese people.Reference de Simone, Devereux, Roman, Alderman and Laragh 37 The hemodynamic load due to obesity is likely to increase both ventricular preload and afterload that could have pronounced effects on blood pressure.Reference Vasan 38 Moreover, peripheral resistance and stiffness of larger blood vessels that are frequently observed in obese individuals are believed to contribute to the increase in blood pressure as well.Reference Matthews, Kuller, Sutton-Tyrrell and Chang 39 Furthermore, obese patients generally have increased heart rate, higher stroke volume and cardiac output;Reference Alexander 40 parameters that did not change significantly in our model with obesity. The combination of obesity and hypertension leads to a variety of compensatory changes in the circulatory system. The modest increases in aortic area and circumference that were observed in the OF groups in this study are likely to be compensatory changes induced by obesity and associated hypertension.
Table 4 Changes in organ weight:body weight (BW) ratios with prenatal treatment and postnatal overfeeding

NF, normal fed group; OF, overfed group; BPA, bisphenol-A; ns, no significant differences.
a,b,c,dGroups with different alphabetic notations are different from each other. a,b indicates no change from a and b. If there are no notations, it indicates that there are no significant changes between groups.
Interaction between prenatal BPA and postnatal overfeeding
Contrary to our expectation that postnatal obesity would exaggerate the detrimental effects of prenatal BPA, prenatal BPA treatment prevented the increase in DBP, SBP and MBP caused by overfeeding. Moreover, it decreased LVA in diastole and reduced COL expression in the right ventricle. It is likely that the molecular effects seen with prenatal BPA exposure could have induced structural alterations that interfere with ventricular compliance. Further studies are needed to investigate the changes in cardiac myocytes at the ultrastructural and molecular level.
Relevance of BPA dose used in the study
The BPA dose used in this study produces circulating levels of 2.62±0.52 ng/ml in the umbilical artery on day 90 of gestation.Reference Veiga-Lopez, Luense, Christenson and Padmanabhan 21 These levels are comparable with what is seen in pregnant women both at delivery and during the first trimester.Reference Veiga-Lopez, Kannan and Liao 41 In addition, at the time of study, the BWs of control-NF and BPA-NF were similar as were the control-OF and BPA-OF, thus eliminating differences in adiposity between groups contributing to the differences.
Conclusions
Taken together, the findings from this study indicate that prenatal BPA exposure alone, while having no effects on cardiac structure, may be inducing changes at the molecular level that could reduce compliance and prevent compensatory responses to postnatal overfeeding. Alternatively, the findings may suggest that BPA pre-treatment alters the trajectory of cardiac structure/function differentially from overfeeding alone that may be adaptive v. maladaptive. This needs to be further investigated at the molecular level.
Acknowledgments
The authors are grateful to Douglas Doop and Gary McCalla for their valuable assistance in breeding, lambing and careful animal care; Jacob Moeller and Carol Herkimer for the assistance provided during prenatal treatment, echocardiography and tissue harvest; Coral Hahn-Townsend for editorial support; and Dr Soyeon Ahn for statistical help.
Financial Support
The authors would like to thank funding support from MSU AgBioResearch and NIH R01AG027697 to P.S.M., and NIH R01ES016541 to V.P. T.D.R.’s effort was supported by CVM, MSU.
Conflicts of Interest
None.












