Introduction
The hypothalamic–pituitary–adrenal (HPA) axis fulfills important functions during fetal development and the transition to extrauterine life. However, alterations to the HPA axis during fetal life, for example following pharmacological treatment with corticosteroids, may affect development and future health of the individual. In early pregnancy, glucocorticoid treatments may be used in cases of suspected congenital adrenal hyperplasia to prevent masculinization of the fetus, but there are indications that these treatments may affect fetal growth and alter neurological development.Reference Dunlop, Archer, Quinlivan, Beazley and Newnham1, Reference Abbasi, Hirsch and Davis2 In late gestation, glucocorticoids are given to enhance maturation of the fetus in cases identified as being at high risk of preterm birth. However, the diagnosis of probable preterm birth is imprecise and many cases in which the treatment is given actually proceed to a term delivery.Reference Walfisch, Hallak and Mazor3–Reference Newnham and Moss5 Previously we reported that late gestation administration of synthetic glucocorticoids reduced fetal weightReference Li, Moss and Nitsos6 and suppressed plasma insulin-like growth factor-I (IGF-I) concentrations.Reference Gatford, Owens and Li7 However, there is little information concerning effects on individual tissues, especially brain regions, nor on concentrations of other metabolic hormones.
Although the advantages of synthetic glucocorticoid treatment are not questioned, there is continuing debate over potential side effects on growth and neurological development.Reference Jobe, Newnham, Willet, Sly and Ikegami8–Reference Li, Nitsos and Polglase11 In this study, we have attempted to mimic clinical usage to examine the effects of treatment protocols involving dexamethasone (DEX) in early pregnancy and repeated betamethasone (BET) in late pregnancy on weights of individual fetal organs and metabolic hormones. Results presented in this manuscript extend, but do not overlap, information from our previous reports on related outcomes of these dosing regimens.Reference Li, Moss and Nitsos6, Reference Gatford, Owens and Li7, Reference Li, Nitsos and Polglase11, Reference Braun, Li and Sloboda12
Methods
All experimental procedures were approved by the Animal Experimentation Ethics Committee of The University of Western Australia and/or the Department of Agriculture and Food, Western Australia.
Steroid treatments
Details of steroid treatments have been published previously,Reference Li, Moss and Nitsos6, Reference Li, Nitsos and Polglase11–Reference Sloboda, Newnham and Challis14 and are provided here only in summary.
Glucocorticoid treatment in early pregnancy
Pregnant Merino ewes (Ovis aries) bearing singleton fetuses were selected at random to receive maternal intramuscular injections of either saline (control group) or DEX [0.14 mg/kg ewe weight (Mayne Pharma, Victoria, Australia); treatment group] given as four injections at 12-hourly intervals over 48 h on 40–42 days of gestation (dG). All pregnant ewes were maintained in a field environment with minimal human contact. No attempt was made to determine the sex of the fetus before delivery.
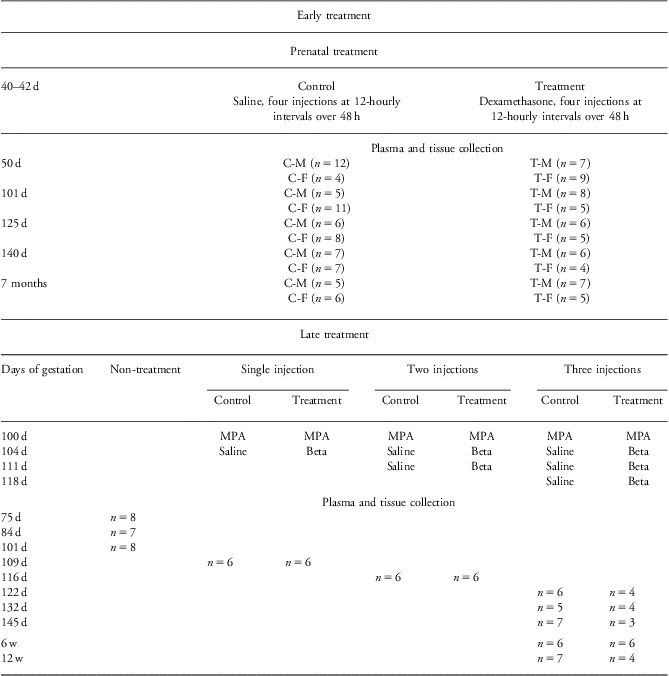
Ewes were killed and measurements made at different time points before or after delivery. For measurements made before term gestation, pregnant ewes were euthanized at 49–51 (50), 101–103 (101), 125–127 (125) and 140–142 (140) dG with a captive bolt. The lambs were immediately delivered by cesarean section, killed with an overdose of pentobarbitone (100 mg/kg, Valabarb, Jurox Pty Ltd, Silverwater, Australia), and weighed. Maternal (jugular) and fetal (cardiac at 50 dG or umbilical arterial collection thereafter) blood samples and tissues were collected. The blood samples were centrifuged at 1800 g for 10 min at 4°C, and plasma was collected. Plasma and the tissues were stored at −80°C.
Other ewes were allowed to deliver their lambs (n = 23) spontaneously and were not disturbed until the lamb was able to stand. The time of birth was recorded and gender determined. Lambs were weighed within 12 h of birth. At 7 months postnatal age, lambs were weighed and then euthanized by pentobarbitone infusion into the jugular vein.Reference Li, Nitsos and Polglase11 Jugular blood samples and tissues were collected and stored as described previouslyReference Li, Nitsos and Polglase11 (Table 1).
Table 1 Protocol of prenatal treatment and sample collection

d, days of gestation age; 7 months, postnatal age; C-M, control male; C-F, control female; T-M, treatment male; T-F, treatment female; MPA, medroxyprogesterone acetate; beta, betamethasone; w, weeks of postnatal age.
Glucocorticoid treatment in late pregnancy
A second cohort of sheep, selected for male fetuses, was treated as describedReference Li, Moss and Nitsos6, Reference Moss, Doherty and Nitsos13, Reference Sloboda, Newnham and Challis14 with BET (0.5 mg/kg body weight; Celestone Chronodose, Schering-Plough, NSW, Australia) at 104 dG (one injection), 104 and 111 dG (two injections) or at 104, 111 and 118 dG (three injections) or saline (control). The pregnant ewes were killed by captive bolt after BET at days 109, 116, 122, 132 and 145. Fetuses were weighed and killed with an overdose of pentobarbitone. Maternal (jugular), fetal (umbilical arterial) blood samples and tissues were collected. Other animals delivered their lambs spontaneously, and tissues were collected at 6 or 12 weeks of postnatal age. At that time, body weights were recorded and the lambs were euthanized by pentobarbitone infusion into the jugular vein. Venous blood samples were taken and tissues were collected and stored as describedReference Li, Moss and Nitsos6 (Table 1).
We collected and weighed the whole brain including cerebrum, brain stem (midbrain, medulla oblongata and pons) and cerebellum; the right and left hemispheres of the cerebellum together with the median vermis; both hippocampi; the pituitary gland using defined landmarks;Reference Winikor, Schlaerth and Rabaglino15 both adrenals; the heart; both kidneys after removal of the adipose capsule, renal blood vessels and ureter; perirenal fat; the liver; both lungs; and the pancreas.
Analytical measures
Plasma IGF-I
Plasma IGF-I in the early treatment group was assayed in duplicate by double-antibody radioimmunoassay (RIA) with human recombinant IGF-I (ARM4050, Amersham Pharmacia Biotech UK Ltd, Buckinghamshire; English-Pharmacia Biotech, Buckinghamshire, UK) and anti-human IGF-I antiserum (AFP4892898, National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NHPP-NIDDK; final dilution; 1:360,000) following acid–ethanol extraction and cryoprecipitation.Reference Gluckman, Johnson-Barrett, Butler, Edgar and Gunn16 The assay method has previously been validated for bovine plasma samples. The intra and inter-assay coefficient of variation were 5.3% and 5.7%, respectively.
Plasma IGF-I measurements in the fetuses and lambs from the late treatment group have been published elsewhere.Reference Gatford, Owens and Li7
Plasma insulin
Plasma insulin levels were measured by using highly purified porcine insulin (26.8 U/mg; Eli Lilly, USA) as a reference preparation and an antiserum that was raised in guinea pigs. Cross-reactions were determined for ovine (100%), bovine (100%) and porcine (56%). The limit of detection was 1.1 ng/ml. The intra-assay coefficients of variation were 10.1% (early treatment) and 8.0% (late treatment).
Plasma leptin
Plasma leptin levels were determined with RIA using an antibody raised in emu against bovine recombinant leptin (School of Animal Biology, The University of Western Australia) and followed by addition of sheep anti-emu immunoglobulin serum.Reference Blache, Tellam and Chagas17 The intra-assay coefficients of variation were 7.8% (early treatment) and 10.4% (late treatment).
Plasma triiodothyronine (T3) and thyroxine (T4)
Plasma T3 and T4 levels were measured using a double-antibody RIAReference Dawson, Deeming, Dick and Sharp18 and validated for sheep plasma.Reference Zhang, Blache, Blackberry and Martin19 The T3 intra-assay coefficients of variation were 6.3% (early treatment) and 7.1% (late treatment). The T4 intra-assay coefficients of variation were 9.1% (early treatment) and 3.7% (late treatment).
Plasma glucose
Plasma glucose was measured with a modification of the method of Bergmeyer and Bernt.Reference Bergmeyer and Bernt20 Plasma was deproteinized by adding 50 μl of sample into 0.5 ml of 0.34 M perchloric acid, mixed on a vortex mixer and then centrifuged at 18,000 g, for 10 min at room temperature. Fresh glucose enzyme buffer (2.5 ml) was added to duplicate supernatant fractions (100 μl) and incubated at 37°C for 20 min. Then 1.5 ml of stop solution (H2SO4 diluted 2:1 with distilled water) was added and the optical density read at 546 nm with a SmartSpec™ 3000 spectrophotometer (BIO-RAD Laboratory Inc., NSW, Australia). The intra-assay coefficients of variation were 9.1% (early treatment) and 11.0% (late treatment).
Statistical analysis
Data are presented as mean ± standard error of the mean (s.e.m.). Outcome data that were not normally distributed were log-transformed before analysis. Two-way analysis of variance (ANOVA) was conducted to determine effects of steroid treatment; where serial measurements were collected on individual fetuses, the results were analyzed using ANOVA with repeated measures. In the late treatment with male fetuses only, two-way ANOVA with treatment and age as fixed factors was used to compare outcomes. When the ANOVA indicated group differences, comparisons between individual groups were performed using the Dunnett's post-hoc test. Data analysis was conducted using SigmaPlot (version 11.0, Systat Software Inc., Chicago, IL, USA) and SAS (version 9.2, SAS Institute Inc., Cary, NC, USA) statistical software. All hypothesis tests were two-sided and statistical significance was accepted at P < 0.05.
Results
Early glucocorticoid treatment
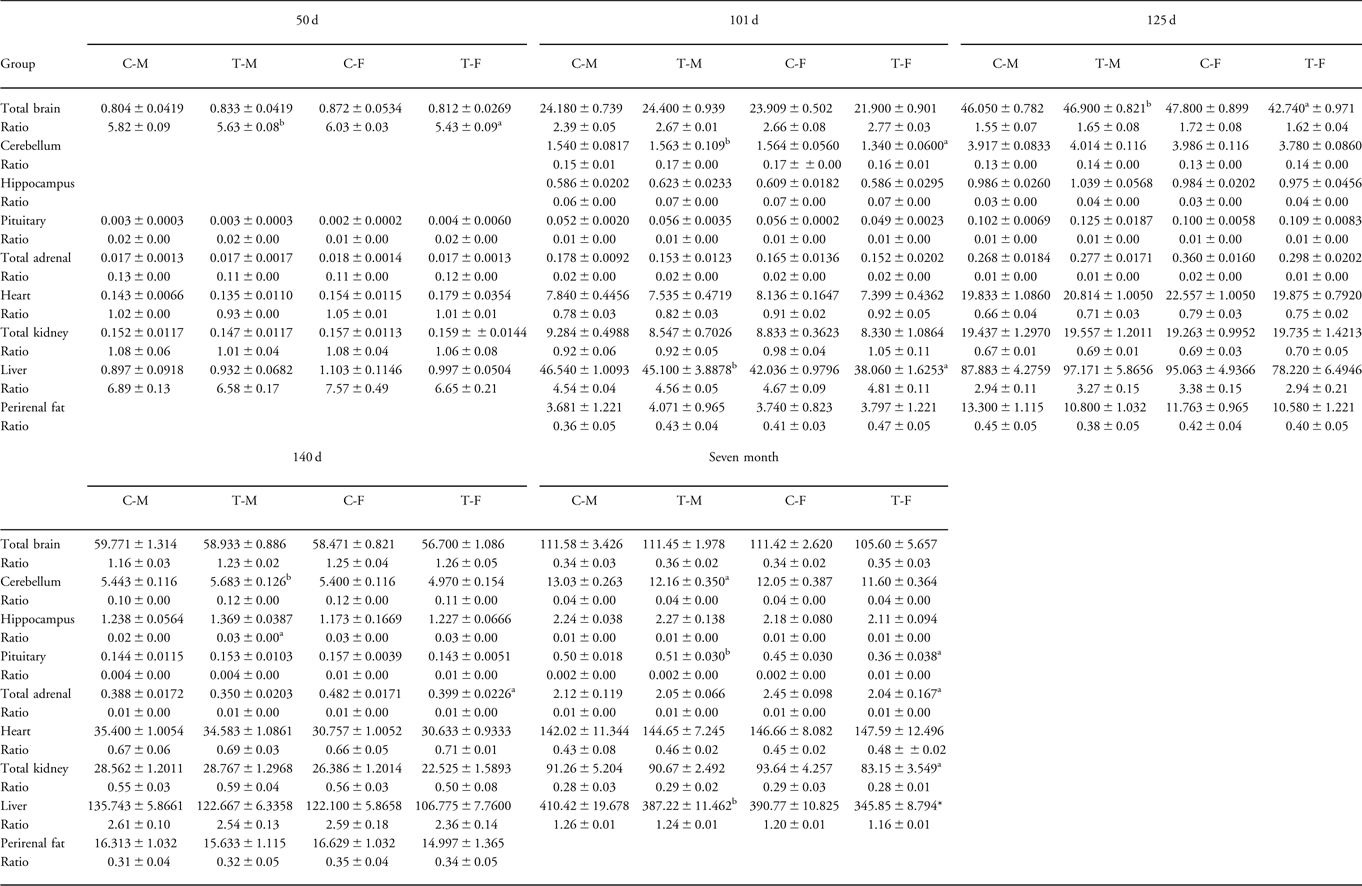
We have previously reportedReference Braun, Li and Sloboda12 that treatment with DEX in early pregnancy had no significant effects on fetal or newborn weights, except at 101 dG when the weights of treatment female fetuses were reduced compared with control female animals. There were no consistent effects on individual organ weights in fetuses at any gestational age, although in lambs at 7 months of age, weights of pituitary, adrenal, kidney and liver were reduced in DEX-treated female animals (Table 2).
Table 2 Absolute and relative fetal and lamb organ weights in the early treatment group

C-M, control male; C-F, control female; T-M, dexamethasone-treated male; T-F, dexamethasone-treated female; BW, body weight; d, days of gestation; 7 months, postnatal age.
Organ weight unit, grams; ratio = normalized organs presented as percentage of total BW.
aTreatment v. control.
bTreatment male v. treatment female.
P < 0.05.
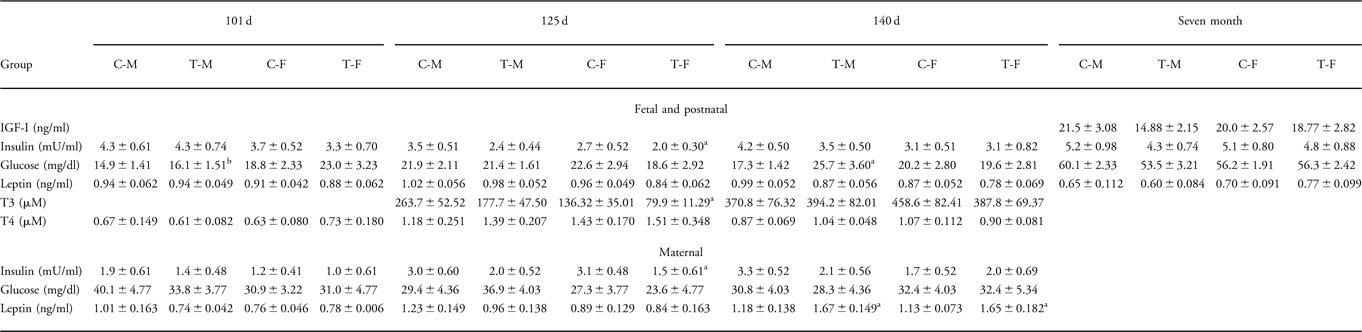
There were no consistent significant differences in fetal and/or postnatal plasma glucose, insulin, IGF-I, leptin, T3 or T4 after maternal DEX treatment in early pregnancy. However, fetal insulin levels at 125 dG were lower in DEX-treated females than in control females; at 101 dG, glucose levels in DEX males were lower than in treatment females and at 140 dG glucose levels in DEX males were higher than in control males (Table 3).
Table 3 Plasma IGF-1, insulin, glucose, leptin, T3 and T4 after DEX treatment in early pregnancy

IGF-I, insulin-like growth factor-I; T3, triiodothyronine; T4, thyroxine; DEX, dexamethasone; C-M, control male; C-F, control female; T-M, dexamethasone-treated male; T-F, dexamethasone-treated female; d, days of gestation; 7 months, postnatal age.
Fetal plasma cortisol concentrations have been published previously.Reference Sloboda, Moss and Li10
aTreatment v. control.
bTreatment male v. treatment female.
P < 0.05.
Maternal glucose was not altered by early DEX treatment. Maternal insulin tended to be lower, and maternal plasma leptin was significantly higher at 140 dG after early DEX treatment in the presence of either male or female fetuses (Table 3).
Late glucocorticoid treatment
After BET treatment in late pregnancy, fetal brain weights were significantly reduced from 109 to 145 dG and at 6 weeks postnatal age. The differences in mean values at 12 weeks of age were not statistically significant (Fig. 1b). Cerebellar weights were significantly lower in BET fetuses from 109 dG to term, but weights of the cerebellum at 6 and 12 postnatal weeks were similar to those in controls (Fig. 1c). Weights of the hippocampus were generally unaffected by BET treatment (Fig. 1d). Pituitary weights were significantly reduced in BET fetuses at 122 and 132 dG (Fig. 1e). Adrenal weights tended to be lower in BET fetuses than in controls at all ages from 122 dG, but the differences were significant only at 132 dG (Fig. 1f). Weights of the heart (Fig. 1g), kidneys (Fig. 1h), liver (Fig. 1i), lungs (Fig. 1j) and pancreas (Fig. 1k) all tended to be lower in BET fetuses with the largest reductions in liver weights (10.7% at 145 dG to 55.7% at 116 and 122 dG). There were no significant reductions in these organ weights in postnatal lambs except for the lung at 12 weeks postnatal age. Total weight of the pancreas was similar in all groups.

Fig. 1 Histogram representing in late treatment (a) fetal and lamb body weights, (b) fetal and lamb brain weights, (c) fetal and lamb cerebellum weights, (d) fetal and lamb hippocampal weights, (e) fetal and lamb pituitary weights, (f) fetal and lamb adrenal weights, (g) fetal and lamb heart weights, (h) fetal and lamb kidney weights, (i) fetal and lamb liver weights, (j) fetal and lamb lung weights and (k) fetal and lamb pancreatic weights. Saline control ![]() $$\[--><$>\raster="fx1"$$$, received one dose betamethasone (BET)
$$\[--><$>\raster="fx1"$$$, received one dose betamethasone (BET) ![]() $$\[--><$>\raster="fx2"$$$, received two doses BET
$$\[--><$>\raster="fx2"$$$, received two doses BET ![]() $$\[--><$>\raster="fx3"$$$, received three doses BET
$$\[--><$>\raster="fx3"$$$, received three doses BET ![]() $$\[--><$>\raster="fx4"$$$. 1: one dose, 2: two doses and 3: three doses. w: weeks of postnatal age. *Significant difference, P < 0.05. Values for fetal weight changes after BET treatment have been published previouslyReference Li, Nitsos and Polglase11, Reference Braun, Li and Sloboda12 and are shown here for completeness.
$$\[--><$>\raster="fx4"$$$. 1: one dose, 2: two doses and 3: three doses. w: weeks of postnatal age. *Significant difference, P < 0.05. Values for fetal weight changes after BET treatment have been published previouslyReference Li, Nitsos and Polglase11, Reference Braun, Li and Sloboda12 and are shown here for completeness.
After expressing each organ weight as a percentage of total body weight, differences previously observed no longer achieved statistical significance except the ratio of liver to body weight at 109 and 122 dG.
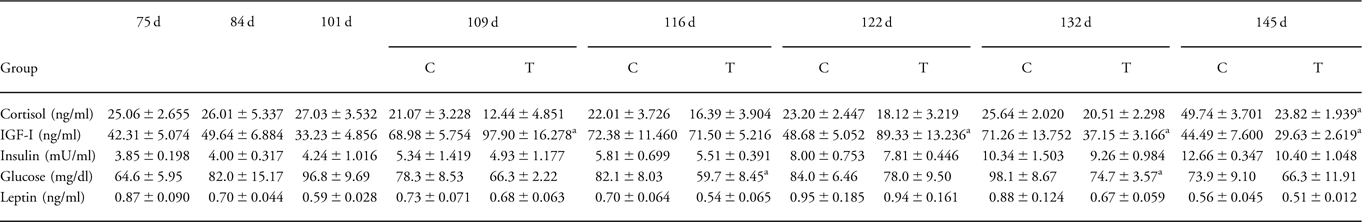
Plasma insulin levels were reduced significantly in BET-treated fetuses at each gestational age studied. Insulin levels at 6 and 12 weeks’ postnatal life were similar in treated and control lambs (Fig. 2b). Glucose levels were significantly lower in fetuses at 109 dG and in lambs at 12 weeks’ postnatal age in BET-treated animals (Fig. 2c). Fetal leptin levels were significantly reduced in BET-treated fetuses at 109, 116, 122 and 132 dG but not at other time points (Fig. 2d). Fetal T3 levels were reduced in BET-treated fetuses at 109, 132 and 145 dG and at postnatal time points (Fig. 2e), but fetal T4 levels were unaffected by the BET treatment (Fig. 2f).

Fig. 2 Histogram representing in late treatment (a) fetal and lamb cortisol levels, (b) fetal and lamb insulin levels, (c) fetal and lamb glucose levels, (d) fetal and lamb leptin levels, (e) fetal and lamb T3 levels and (f) fetal and lamb T4 levels. Saline control ![]() $$\[--><$>\raster="fx1"$$$, received one dose betamethasone (BET)
$$\[--><$>\raster="fx1"$$$, received one dose betamethasone (BET) ![]() $$\[--><$>\raster="fx2"$$$, received two doses BET
$$\[--><$>\raster="fx2"$$$, received two doses BET ![]() $$\[--><$>\raster="fx3"$$$, received three doses BET
$$\[--><$>\raster="fx3"$$$, received three doses BET ![]() $$\[--><$>\raster="fx4"$$$. 1: one dose, 2: two doses and 3: three doses. w: weeks of postnatal age. *Significant difference, P < 0.05. Values for fetal plasma cortisol have been published previouslyReference Jobe, Newnham, Willet, Sly and Ikegami8, Reference Braun, Li and Sloboda12 and are shown here for comparison.
$$\[--><$>\raster="fx4"$$$. 1: one dose, 2: two doses and 3: three doses. w: weeks of postnatal age. *Significant difference, P < 0.05. Values for fetal plasma cortisol have been published previouslyReference Jobe, Newnham, Willet, Sly and Ikegami8, Reference Braun, Li and Sloboda12 and are shown here for comparison.
Maternal cortisol was suppressed at 145 dG and maternal IGF-I was suppressed at 132 and 145 dG after BET treatment in late gestation. Maternal insulin levels were unaffected by BET treatment. Maternal glucose levels were significantly reduced at 116 and 132 dG, but were not different from control animals at term and after birth. Maternal leptin levels were similar in the BET treated and control animals (Table 4).
Table 4 Maternal plasma cortisol, IGF-I, insulin, glucose and leptin levels in the late treatment group

IGF-I, insulin-like growth factor-I; C, control; T, betamethasone treatment; d, days of gestation; w, weeks of postnatal age.
aTreatment v. control.
P < 0.05.
Discussion
The purpose of this study was to evaluate the effects of corticosteroid administration during early or late pregnancy on fetal and lamb body and organ weights and on levels of some key metabolic hormones in the fetus and newborn. We attempted to mimic clinical protocols that involved DEX treatment in early pregnancy and single or repeated BET treatment in late pregnancy. Clearly, the differences in synthetic corticosteroid and the times and duration of administration do not allow us to make direct comparison of their effects, and any different outcomes that we report may simply reflect the protocols we have used. However, in general, early pregnancy treatment with DEX had inconsistent effects on fetal and neonatal organ weight and plasma hormone values, whereas late gestation administration of BET, in single or multiple doses reduced organ weights and some plasma hormone values. We have previously reported the extension of pregnancy length after maternal corticosteroid treatment in either early or late gestationReference Li, Moss and Nitsos6, Reference Li, Nitsos and Polglase11 observations that replicate findings in other species.Reference Liggins and Howie21–Reference Novy and Walsh24
Treatment with synthetic corticosteroid in early pregnancy did not have any consistent effects on body weight at each of the gestational ages at measurement,Reference Li, Nitsos and Polglase11 nor weights of individual organs or their percentages of whole body weight. We report now that the early treatment protocol was, in general, not associated with significant or consistent changes in individual organ weights of fetuses or lambs nor in any of the metabolic hormones measured in this study. It is possible that the occasional significant change in weight has resulted from chance effects. At 7 months postnatal age, treated females had significant reductions in weights of the pituitary, adrenals, kidney and liver. In these animals at term, fetal plasma cortisol values were significantly raised over controls and key fetal adrenal steroidogenic enzymes were altered.Reference Braun, Li and Sloboda12 These results indicate clearly that the DEX given was biologically active, but at the dose and time of gestation chosen, has relatively little effect on fetal or lamb organ weights or development.
In apparent contrast, corticosteroid treatments later in pregnancy had marked effects on fetal growth, with significant reductions at each gestational age that was studied. In our original experiments, we reported reductions in brain weight of 11% after single dose treatment and 17% after repeated steroid injections.Reference Huang, Beazley and Quinlivan25 Single and repeated BET injections were associated with a significant reduction of 7–8% in brain weight in sheep at ∼3.5 years of age.Reference Moss, Doherty and Nitsos13 The present study has substantiated these observations for total fetal brain weights and shown that the growth reducing effects of BET extend to several key brain areas. It is of some concern that the reduction in total brain weight persisted after birth and that the average weights of other brain areas, such as cerebellum and hippocampus were still reduced, although not significantly so, in animals up to 12 weeks of age. These findings are consistent with earlier data in the sheep,Reference Huang, Beazley and Quinlivan25 guinea pigReference Dean, Yu, Lingas and Matthews22 and rhesus monkey,Reference Novy and Walsh24 and with our own studies showing that after prenatal BET there is retarded myelination of the optic nerve.Reference Dunlop, Archer, Quinlivan, Beazley and Newnham1 French et al. Reference French, Hagan, Evans, Mullan and Newnham26 have reported reduced head circumference and increased attention deficit disorder in children of mothers treated with multiple courses of prenatal steroid. The current results are consistent with the possibility that multiple courses of corticosteroids in late pregnancy can affect neurological development of the offspring.
We determined the effects of prenatal corticosteroids on circulating values for several hormones involved in metabolism in the fetus and postnatal period. Previously we had shown that plasma IGF-I and insulin-like growth factor binding protein (IGFBP) were reduced in late pregnancy after prenatal BET treatment.Reference Gatford, Owens and Li7 In the current study, we showed that plasma insulin, leptin and T3 (Fig. 2e) were all reduced after BET and these changes preceded any change in fetal cortisol. It is of interest that the fall in fetal plasma insulin occurred without significant change in fetal glucose concentrations, which remained similar between control and treated animals. Maternal glucose concentrations were significantly reduced at 116, 132 and 145 dG, with maternal insulin levels that were unchanged. We might speculate that the lower fetal insulin levels reflect the lower maternal glucose concentrations and altered placental transfer, with potential long-term implications for growth and development and glucose tolerance in later life. However, these studies would first need substantiating in chronically undisturbed animals. The placental GLUT transporter has variously been reported to be up- or down-regulated by corticosteroid,Reference Challier, Hauguel and Desmaizieres27–Reference Hahn, Barth, Weiss, Mosgoeller and Desoye29 but this was not a subject of investigation in the present study.
Maternal IGF-I concentrations were reduced on 132 and 145 dG, coincident with the reported fall in fetal IGF-I and IGFBP.Reference Gatford, Owens and Li7 In that study, fetal, placental and/or postnatal weights correlated positively with plasma IGF-I, and total IGFBP and the lowered IGF-I persisted into postnatal life, potentially contributing to adverse growth patterns in that period.Reference Gatford, Owens and Li7 We found that fetal leptin was also reduced after prenatal BET treatment, consistent with earlier reports.Reference Smith and Waddell30, Reference Sugden, Langdown, Munns and Holness31 Leptin can be produced in the placenta and by adipocytes.Reference Amico, Thomas, Crowley and Burmeister32, Reference Kawai, Yamaguchi and Murakami33 Enhanced transplacental passage of 125I-leptin in vivo occurs with inhibition of endogenous glucocorticoidReference Smith and Waddell30 and conversely, exogenous maternal DEX reduces fetal leptin levels in rats.Reference Smith and Waddell30, Reference Sugden, Langdown, Munns and Holness31 We are unaware of any comparable data in sheep. Expression of the placental leptin receptor correlates positively with the capacity of the placenta to transport maternal leptin to the fetus and is suppressed by endogenous glucocorticoids, emphasizing the close relationship between glucocorticoid, leptin and regulation of fetal growth. Fetal T3 values were also significantly reduced after prenatal BET treatment, without significant change in T4. T3 is generally regarded as the most important thyroid hormone during fetal lifeReference Blazer, Moreh-Waterman, Miller-Lotan, Tamir and Hochberg34, Reference Chattergoon, Giraud and Thornburg35 with changes in iodination patterns of thyroid hormonesReference Meaney, Diorio and Francis36 occurring in response to the prenatal surge in endogenous cortisol.Reference Challis, Matthews, Gibb and Lye37 Brain growth factors are especially sensitive to thyroid statusReference de Escobar, Obregon and del Rey38 and these changes may contribute to the reduction in brain weights that we report. Areas of the developing brain, for example the hippocampus, express abundant corticosteroid receptors [glucocorticoid receptor (GR) and mineralocorticoid receptor (MR)] and are sensitive to glucocorticoid effectsReference Jacobson and Sapolsky39, Reference Matthews40 with later life implication for cognition, behavior, memory and co-ordination of autonomic activity.Reference Jacobson and Sapolsky39–Reference De Kloet, Vreugdenhil, Oitzl and Joels41 Collectively, the present results suggest that as a consequence of prenatal BET treatment, there are falls in fetal IGF, T3 and glucose, which may persist into the postnatal period and should be regarded with concern in the context of both pre- and postnatal development.
It is of interest that maternal cortisol concentrations tended to be lower after late gestation treatment with BET than in controls, a difference that was significant in animals immediately prior to delivery (Table 4). We are not aware that this effect has been observed previously. However, basal cortisol in maternal sheep plasma does show substantial minute-to-minute variation,Reference Challis, Patrick and Cross42 particularly in late pregnancy, and although the mean values reported here are similar to those in Challis et al.Reference Challis, Patrick and Cross42 and Liggins et al.Reference Liggins, Fairclough, Grieves, Kendall and Knox43 they are somewhat higher than in other reports.Reference Bloomfield, Oliver and Giannoulias44 The present study design unfortunately does not allow us to separate effects of exogenous synthetic glucocorticoid from subtle differences in endogenous cortisol either on placental function or on fetal growth parameters.
Previously we have reported that both early and late gestation treatment with synthetic glucocorticoids altered the pattern of gene expression within the developing HPA axis and for several genes these responses carried into postnatal life.Reference Li, Moss and Nitsos6, Reference Sloboda, Moss and Li10–Reference Braun, Li and Sloboda12, Reference Sloboda, Moss and Li45, Reference Sloboda, Newnham and Challis46 Together, our results have shown that synthetic glucocorticoid given to the pregnant ewe can alter in the fetal, young lamb and adult offspring gene expressions such as GR and MR in the hippocampus; corticotropin-releasing hormone (CRH), arginine vasopressin (AVP) and GR in the hypothalamus; proopiomelanocortin (POMC), prohormone convertase 1 (PC1), prohormone convertase 2 (PC2) and GR in the pituitary; adrenocorticotropic hormone receptor (ACTHr), steroidogenic acute regulatory (StAR), steroid 17 alpha-hydroxylase (P450c17), 3 beta hydroxysteroid dehydrogenase (3β HSD), 11β hydroxysteroid dehydrogenase type 2 (11β HSD2) and GR in the adrenal; and corticosteroid binding globulin (CBG) in the liver. The results of these studies suggest that many of the gene responses to glucocorticoid treatment persist well into subsequent adult life, and work from others has shown that similar effects may also be transmitted into future generations.Reference Pena and Champagne47, Reference Wright48
The beneficial actions of glucocorticoid treatment in early human pregnancy to prevent prenatal virilization or in late pregnancy to promote lung maturity are not challenged by this study. However, it is clear that glucocorticoid-induced organ programming may contribute to fetal growth restriction and endocrine changes in sheep; effects that persist into postnatal life. At the doses and injection regimens employed in this study we suggest that the fetus in late gestation has greater adverse responsiveness to exogenous glucocorticoid, in terms of overall birth weight, brain and cerebellar weights and weights of particular organs. In part, these responses are associated with alterations in the activity of the glucose–insulin–IGF axis and in part with an altered thyroid axis. Organ weights are, of course, only a crude measure of organ development. Future studies will need to determine more subtle tissue specific biomarkers of altered development that will indicate the long-term risks of these treatments, administration of which in human pregnancy undoubtedly confer immediate survival advantage.
Acknowledgments
This work was supported by the Canadian Institutes for Health Research Group in Fetal and Neonatal Health and Development, the National Health and Medical Research Council of Australia (grants 254502, 303261), the Raine Medical Research Foundation of Western Australia and Women and Infants Research Foundation, Western Australia.