Background
Parents contribute in many ways to the development of their children. It is well documented that maternal lifestyle and exposures before and during gestation influences health and development of the next generation.Reference Painter, Roseboom and Bleker 1 In recent years, a significant number of studies on various environmental exposures (nutrition, pesticides, lead, bisphenol A) have also reported an influence of paternal exposures on offspring’s future health. Anderson et al.Reference Anderson, Riffle, Wilson, Travlos, Lubomirski and Alvord 2 reported that food deprivation of male mice before conception leads to an impaired glucose metabolism in offspring. Besides genomic effects (DNA mutations), epigenetic modifications have been suggested to explain these paternally transmitted effects.Reference Soubry, Hoyo, Jirtle and Murphy 3 Epigenetic changes, such as DNA methylation alterations, can occur in the male germ line due to environmental exposures, such as diet, and can be further passed on to the offspring.Reference Marques, João Pinho, Carvalho, Bièche, Barros and Sousa 4 In humans, DNA methylation may result in changes in gene expression and phenotype without altering the DNA sequence itself by adding a methyl-group (CH3) to the carbon-5 position of the base cytosine in CpG dinucleotides, catalyzed by the enzyme DNA methyltransferase (Dnmt).Reference Chen and Riggs 5
The One-Carbon (I-C) metabolism plays a central role in DNA methylation as it determines the flux of methyl-groups toward methylation of DNA. Folate, betaine, choline and methionine are the main sources of methyl-groups in the I-C metabolism. All of them enter the I-C metabolism at different sites and are, in the end, all converted to the universal methyl-group donor S-adenosylmethionine (SAM).Reference McKay and Mathers 6 So far, the effect of methyl donor intake (e.g. folic acid supplementation) on offspring DNA methylation has been mainly studied through maternal intake.Reference Boeke, Baccarelli, Kleinman, Burris, Litonjua and Rifas-Shiman 7 , Reference Steegers-Theunissen, Obermann-Borst, Kremer, Lindemans, Siebel and Steegers 8 However, Mejos et al.Reference Mejos, Kim, Lim and Chang 9 have shown that both maternal and paternal folate deficiency (4-week folate deficient diet) can decrease hepatic global DNA methylation in rat offspring. Carone et al.Reference Carone, Fauquier, Habib, Shea, Hart and Li 10 found that male mice consuming a low-protein diet fathered offspring with altered DNA methylation at specific liver CpG islands [including a potential enhancer for the key lipid regulator peroxisome proliferator-activated receptor alpha (PPARα)] affecting cholesterol and lipid metabolism.
Besides DNA methylation, the DNA can be demethylated by oxidizing 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC) by the Ten-eleven translocation (TET) enzymes and further to 5-formylcytosine (5-fC) and 5-carboxycytosine (5-caC).Reference Dao, Cheng, Revelo, Mitzner and Tang 11 Increased levels of 5-hmC may inhibit the binding of methyl-CpG binding proteins and thereby counteract transcriptional repression of 5-mC.Reference Langie, Achterfeldt, Gorniak, Halley-Hogg, Oxley and van Schooten 12 Changes in DNA methylation have been related to nutritional exposures such as folic acid supplementation.Reference Bae, Ulrich, Bailey, Malysheva, Brown and Maneval 13 – Reference Axume, Smith, Pogribny, Moriarty and Caudill 16 To our very best knowledge, no human studies have evaluated the effect of the parental nutrition on global DNA hydroxymethylation. Most studies on hydroxymethylation were focused on prenatal development, especially stem cell differentiation and lineage. For example, some recent studies have examined the influence of dietary factors (e.g. vitamin C) on 5-hmC. Vitamin C not only induces increased levels of 5-hmC, but also of 5-fC and 5-caC in mouse embryonic stem cells.Reference Yin, Mao, Zhao, Chong, Yang and Zhao 17
First human evidence of epigenetic changes in the offspring being paternally induced came from the Newborn Epigenetics Study. Soubry et al. observed that paternal periconceptional obesity (over-nutrition) was significantly associated with offspring DNA methylation at differentially methylated regions (DMRs) of several imprinted genes. Hypomethylation at the IGF2 DMR,Reference Soubry, Schildkraut, Murtha, Wang, Huang and Bernal 18 MEST, PEG3 and NNAT DMRsReference Soubry, Murphy, Wang, Huang, Vidal and Fuemmeler 19 were associated with paternal obesity. In order to affect offspring methylation through paternal environmental exposures, the exposure needs to be transferred to the male gametes and be sustained through developmental processes. During gametogenesis, from primordial germ cells to spermatozoa, epigenetic marks are established in a sex-specific way. This seems to be the only window of susceptibility during the lifespan of the father (from puberty to adulthood) where paternal environmental exposures can affect epigenetic marks in the gametes. Shortly after fertilization the embryo undergoes genome wide demethylation, except for imprinted marks and repeat sequences which retain their methylation status, making the overall epigenome hypomethylated.Reference Chen and Riggs 5 Imprinted genes are therefore perfect candidate genes to capture and keep the paternal environmental exposure, as they withstand reprogramming.Reference Soubry, Guo, Huang, Hoyo, Romanus and Price 20 Our study focuses on the paternally expressed imprinted insulin-like growth factor 2 (IGF2) which plays a critical role in embryogenesis and fetal growth. Its imprinting is regulated by two DMRs: H19 and IGF2 DMR. The imprint marks at these DMRs are established during spermatogenesis, so methylation is only present on the paternally inherited allele in the offspring.Reference Chao and D’Amore 21 To date, a handful of animal studies suggest an effect of paternal nutrition on offspring DNA methylation.Reference Mejos, Kim, Lim and Chang 9 , Reference Carone, Fauquier, Habib, Shea, Hart and Li 10 In humans however, the impact of paternal diet on offspring DNA methylation and demethylation has not yet been studied.
In this study, we first aimed to determine the effect of paternal dietary methyl-group donor intake (methionine, folate, choline and betaine) on paternal whole blood global DNA methylation and hydroxymethylation. Next, we assessed the effect of paternal methyl donor intake on cord blood global DNA methylation and hydroxymethylation, IGF2 DMR methylation, and investigated a possible link with offspring birth weight.
Methods
Study subjects
The Maternal Nutrition and Offspring’s Epigenome (MANOE) study is an ongoing prospective, observational study at the Department of Obstetrics and Gynecology of the University Hospital Leuven (Belgium) that investigates the link between parental methyl-group donor intake and offspring DNA methylation. Pregnant women were followed-up at their scheduled ultrasounds and at these time points fathers were asked to participate (Fig. 1). Of the 178 women included in the MANOE study, 115 Caucasian fathers provided detailed socio-demographic information (e.g. age, marital status, education), as well as multiple lifestyle or health characteristics (smoking behavior, physical activity, allergies). From these 115 fathers, 41 were excluded from analysis due to missing data (no nutritional information), which resulted in 74 fathers for statistical analysis. We were not able to collect a cord blood sample from 16 newborns, which gives a total of 58 father–infant pairs. Further, two children were excluded because the mother developed gestational diabetes, four due to pre-term delivery (<37 weeks of gestation), and one mother had a high risk of neural tube defects and was therefore given an extreme high dose of folic acid (4 mg/day). A total of 51 father–infant pairs were included in the statistical analysis. A screening for gestational diabetes was performed at 24–28 weeks using a 50 g glucose challenge test. When the test showed a glycemia ⩾140 mg/dl (⩾7.8 mmol/l) a 75 g oral glucose tolerance test was also performed. Based on this test two women were diagnosed with gestational diabetes mellitus (153–199 mg/dl or 8.5–11 mmol/l glucose). 22
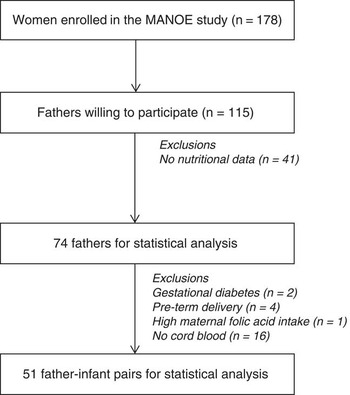
Fig. 1 Flowchart of fathers enrolled in the Maternal Nutrition and Offspring’s Epigenome (MANOE) study and included in the statistical analysis.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the UZ Leuven-Committee for Medical Ethics (reference number: ML7975). At the start of the study, all participants signed an informed consent.
Paternal dietary information
All 74 fathers were seen once at the Department of Obstetrics and Gynecology at the day of a scheduled ultrasound. To assess the paternal intake of dietary methyl-group donors (methionine, folate, betaine and choline) fathers were asked to complete a 7-day estimated dietary record (EDR). The participants were given guidelines to fill out their diary. This food record is an open-entry diary categorized into six eating occasions (breakfast, morning snacks, lunch, afternoon snacks, dinner and evening snacks) and involves reporting all foods and drinks consumed over seven consecutive days. It is often considered the most accurate measure of intake and has been referred to as the gold standard.Reference Willett 23 Detailed information on the type including brand names, the food type (e.g. use of whole, semi-skimmed, or skimmed milk, the type of bread used, etc.) and portion size [expressed as household measures, standard units (e.g. a medium sized apple) or units like grams or liters] of the foods consumed was collected using an open entry format. Only complete food diaries, including seven completed record days and containing sufficiently detailed descriptions of the food products and portion sizes consumed, were taken into consideration. The complete EDRs were encoded and entered into a Diet Entry and Storage program (NUBEL Voedingsplanner) 24 using a manual on food portions and household measures. 25 Methionine, choline, betaine and folate are not included in the Belgian food composition table Nubel, 26 so the diet records were linked to food composition databases from other countries. The Dutch NEVO food composition database 27 was used for folate, the USDA database for the Choline Content of Common FoodsReference Kristine, Seema and Juhi 28 for choline and betaine, and the German BLS Nutrient databaseReference Dehne, Klemm, Henseler and Hermann-Kunz 29 for methionine. The nutritional values of the food products in the four databases were quantified in mg/100 g (methionine, choline and betaine) or μg/100 g (folate). The methyl-group donor intake was calculated by multiplying these nutritional values of each consumed product during the 7-recorded days with the portion size (grams) of the product and dividing it by 100. For each methyl-group donor, the intakes of the products consumed in 1 day were added up. Finally, the average methyl-group donor intake of the 7-recorded days was calculated.
Paternal and neonatal measurements
Through an interview, we collected information about a range of socio-demographic factors, lifestyle habits (e.g. smoking: never smoked/past smoker/current smoker), and physical activity (yes/no). Body mass index (BMI) was calculated from the father’s height and weight. Fathers were weighed at the consultation on a standard weighing scale (SECA Alpha model 888 or 877, Teleflex, Belgium) with indoor clothes (no shoes) to the nearest 0.1 kg. The height was measured with a microtoise to the nearest 0.5 cm (SECA model 206; Leicester Height Measure, Burmingham, UK) without shoes.
Gestational age was determined by measuring crown rump length between 7 and 14 weeks of gestation.Reference Pexsters, Daemen, Bottomley, Van Schoubroeck, De Catte and De Moor 30 At delivery, we collected umbilical cord blood in 4.5 ml tubes containing ethylenediaminetetraacetic acid (EDTA; BD Vacutainer Systems). We obtained birth weight and length from the hospital clinical records. Gender-specific z-scores for birth weight-for-gestational age were generated using the INTERGROWTH-21st tool.Reference Villar, Cheikh Ismail, Victora, Ohuma, Bertino and Altman 31
Sample collection and DNA extraction
Blood samples from fathers were collected using 4.5 ml tubes with EDTA (BD Vacutainer® Blood Collection System). Blood samples were put in the freezer (−20°C) immediately after collection. At delivery, umbilical cord blood was collected via umbilical vein puncture into 4.5 ml tubes containing EDTA (BD Vacutainer® Blood Collection System), followed by storage at −20°C. DNA extraction from whole blood samples was done using the Salting out method.Reference Sambrook and Russell 32 The quantity and purity of DNA was determined by a Nano Drop spectrophotometer. Extracted DNA was further stored in TE-buffer at −80°C until further analysis.
Global DNA (hydroxy)methylation measurements
Paternal and cord blood DNA was analyzed by a fast and sensitive liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of DNA 5-mC and 5-hmC as described previously.Reference Godderis, Schouteden, Tabish, Poels, Hoet and Baccarelli 33 Briefly, isolated genomic DNA samples (10 µg) were hydrolyzed to individual deoxyribonucleosides by a simple one-step DNA hydrolysis procedure. For this, a digest mix was prepared by adding phosphodiesterase I, alkaline phosphatase and benzonase® Nuclease to Tris-HCl buffer; 10 µl of digest mix was added to the extracted DNA and incubated at 37°C for at least 8 h. After hydrolysis, 490 µl of acetonitrile/water was added to each sample. Global DNA methylation and hydroxymethylation was obtained by quantifying 5-mdC, 5-hmdC and dC using ultra-pressure liquid chromatography, in combination with tandem mass spectrometry (MS-MS). Global DNA methylation was expressed as a percentage of 5-mdC v. the sum of 5-mdC, 5-hmdC and dC [%global DNA methylation=5-mdC/(5-mdC+5-hmdC+dC)], while global DNA hydroxymethylation was expressed as a percentage of 5-hmdC v. the sum of 5-mdC, 5-hmdC and dC [%global DNA hydroxymethylation=5-hmdC/(5-mdC+5-hmdC+dC)].
IGF2 DMR methylation measurements
Bisulfite conversion and polymerase chain reaction (PCR)
Genomic DNA (200 ng) was bisulfite converted using the EZ-96 DNA Methylation-GoldTM Kit (#D5008; Zymo Research). Converted DNA was eluted with 30 μl of M-elution buffer. Subsequently, 1 μl of converted DNA was amplified by PCR in a total volume of 25 μl containing 0.2 μM of primers and 2× Qiagen PyroMark PCR Master Mix (#978703; Qiagen). Primer sequences for IGF2 DMR were taken from the original paper. The IGF2 DMR is one of the two DMRs that are involved in the imprinting of the IGF2/H19 domain on chromosome 11p15.5. This DMR is located upstream of the imprinted promoters of IGF2.Reference Murphy, Huang and Hoyo 34 PCR reactions for IGF2 DMR consisted of an initial hold at 5°C for 15 min followed by five cycles of 30 s at 94°C, 30 s at 68°C and 30 s at 72°C. This was followed by 50 cycles of 30 s at 94°C, 30 s at 64°C and 30 s at 72°C and ended with a final extension step at 72°C for 10 min.
Pyrosequencing
In order to assess CpG methylation levels, 20 μl of biotinylated PCR product was immobilized to Streptavidin Sepharose High Performance beads (#17-5113-01, GE Healthcare) followed by annealing to 25 μl of 0.3 μM sequencing primer at 80°C for 2 min with a subsequent 10 min cooling down period. Pyrosequencing was performed using Pyro Gold reagents (#970802, Qiagen) on the PyroMark Q24 instrument (Qiagen) following the manufacturer’s instructions. Pyrosequencing results were analyzed using the PyroMark analysis 2.0.7 software (Qiagen).
Statistical analysis
First, an independent t-test was used to compare the characteristics of fathers with and without dietary data. Next, Pearson’s correlations were used to display the association between paternal global DNA methylation and global DNA hydroxymethylation. To determine the effect of paternal methyl-group donor intake on paternal global DNA (hydroxy)methylation, cord blood global DNA (hydroxy)methylation, cord blood IGF2 DMR methylation, and birth weight linear regression models were used. Multivariable models were used to correct for possible confounders. Potential confounders were selected based on the association with paternal nutrition and paternal methylation: paternal age, paternal physical activity (yes/no), paternal smoking (never/past/current), and paternal BMI. When assessing the effect of paternal nutrition on offspring methylation; maternal smoking (did not smoke during pregnancy/smoked during pregnancy), maternal BMI, and maternal methyl-group donor intake (methionine, betaine, choline and folate) were also selected as potential confounders. Model selection was based on the Akaike Information Criterion (AIC): the model with the lowest AIC (indicating the best model fit) was selected among all tested models (every possible combination of the four methyl-group donors together with the pairwise interactions). All tests were two-sided, a 5% significance level was assumed for all tests. Analyses were performed using SAS software (version 9.4 of the SAS System for Windows).
Results
Paternal characteristics and methyl-group donor intake
Characteristics of the fathers are presented in Table 1. From the 115 included fathers, mean paternal age was 31.8 years (range: 2–48). BMI of the participating fathers averaged 24.7±2.9 kg/m2. Most men (53.9%, n=62) never smoked cigarettes and 32 men (27.8%) smoked in the past; 67% (n=77) of the fathers were physically active (yes/no). From the included fathers with dietary data (n=74) mean paternal age was 32 years (range: 25–48). BMI of these fathers averaged 24.6±2.9 kg/m2. Most men (55.4%, n=41) never smoked cigarettes and 22 men (29.7%) smoked in the past; 67.6% (n=50) were physically active (yes/no). From the excluded fathers without dietary data (n=41) mean paternal age was 31.2y (range: 24–38). BMI of these fathers averaged 24.9±3.3 kg/m2. Most men (56%, n=23) never smoked cigarettes and nine men (22%) smoked in the past; 68.3% (n=28) were physically active (yes/no). No significant differences between fathers with and without dietary data were observed.
Table 1 Paternal characteristics

BMI, body mass index.
Independent sample t-test was performed to compare characteristics of fathers with and without dietary data.
The average daily intake of methyl-group donors of the 74 fathers is shown in Table 2. The average intake of choline and folate corresponded with the average requirements for these nutrients.Reference Burris, Braun, Byun, Tarantini, Mercado and Wright 35 , Reference Hoyo, Fortner, Murtha, Schildkraut, Soubry and Demark-Wahnefried 36 A total of 55.4% of the fathers had intake below the dietary guideline for folate and 79.7% for choline. The dietary guideline for methionine is 10.4 mg/kg. 37 Mean weight of the fathers with dietary data was 81.3±12.0 kg, resulting in a recommended daily intake 845.2±124.8 mg for methionine. The father’s intake of methionine was much higher than the dietary guideline (range: 1234.4–3602.1 mg). For betaine no guideline for dietary intake exists.
Table 2 Paternal average daily intake of methyl-group donors (n=74)

The effect of methyl-group donor intake on paternal DNA methylation
The 74 fathers had a mean global DNA methylation level of 5.92±1.45% and a mean global DNA hydroxymethylation level of 0.12±0.08%. The inter-individual variability of the global DNA methylation and global DNA hydroxymethylation levels ranged 3.17–8.37% and 0.02–0.28%, respectively. These results are in line with some studies that reported similar inter-individual variability.Reference Janssen, Byun, Gyselaers, Lefebvre, Baccarelli and Nawrot 38 , Reference Li and Franke 39 Nevertheless, lower variability were previously reported while studying a limited number of volunteersReference Godderis, Schouteden, Tabish, Poels, Hoet and Baccarelli 33 or on other tissue then whole bloodReference Dwi Putra, Neuber, Reichetzeder, Hocher and Kleuser 40 Global DNA methylation and global DNA hydroxymethylation were highly correlated (r=0.88, P<0.0001) (Fig. 2).

Fig. 2 Relationship between paternal global DNA methylation and global DNA hydroxymethylation percentages in blood.
The best model explaining paternal hydroxymethylation via paternal methyl-group donor intake was a model with betaine as the only predictive value. Higher intakes of betaine was associated with higher levels of paternal global DNA hydroxymethylation in a model adjusted for age, BMI, smoking status, and physical activity (0.028% per 100 mg betaine increase, 95% CI: 0.003, 0.053, P=0.03). There was no evidence that paternal methyl-group donor intake had any predictive value for paternal global DNA methylation, although the association between paternal betaine intake and paternal global DNA methylation was borderline significant (P=0.08) (Table 3).
Table 3 Associations between paternal methyl-group donor intake and paternal global DNA (hydroxy)methylation (n=74)

CI, confidence interval.
β-estimate is an absolute change in percentage of global DNA (hydroxy)methylation; slope >(<) 0 means positive (negative) association.
Bold values are the statistically significant results (p < 0.05).
The effect of paternal methyl-group donor intake on offspring
Besides the effect of dietary methyl-group donors consumed by the father on paternal methylation, we were also interested in its effect on offspring methylation and growth. This analysis was performed on 51 father–infant pairs. Newborn characteristics and methylation profiles are described in Table 4. The newborns, 26 of which were girls (51%), had a mean birth weight of 3.472±0.392 kg, and mean gestational age of 39.75±0.92 weeks. Birth weight-for-gestational age z-score was calculated and a mean z-score of 0.39±0.95 was obtained (range: −1.38 to 2.45). The 51 newborns had a mean global DNA methylation level of 6.61±1.66% and a mean global DNA hydroxymethylation level of 0.24±0.15%. The mean methylation percentage of the three CpGs of the IGF2 DMR was 51.04±3.93%. Offspring cord blood global DNA methylation and global DNA hydroxymethylation were highly correlated (r=0.86, P<0.0001) (Fig. 3).

Fig. 3 Relationship between offspring global DNA methylation and global DNA hydroxymethylation percentages in cord blood.
Table 4 Newborn characteristics and methylation profiles (n=51)

IGF2, insulin-like growth factor 2; DMR, differentially methylated region.
To assess the effects of paternal methyl-group donor intake on offspring global DNA methylation, the best model was the model with betaine as the only predictive value. Higher intakes of betaine was linked with higher levels of offspring global DNA methylation (0.679% per 100 mg betaine increase, 95% CI: 0.057, 1.302, P=0.03) in a model adjusted for paternal age, paternal BMI, paternal smoking status, and paternal physical activity. We also included maternal BMI, maternal smoking status and maternal methyl-group donor intake as possible confounders. There was no evidence that paternal methyl-group donor intake had any predictive value for offspring global DNA hydroxymethylation (Table 5).
Table 5 Associations between paternal methyl-group donor intake and offspring global DNA (hydroxy)methylation (n=51)

CI, confidence interval.
β-estimate is an absolute change in percentage of global DNA (hydroxymethylation); slope >(<) 0 means positive (negative) association.
Bold values are the statistically significant results (p < 0.05).
We also determined the effect of paternal methyl-group donor intake on offspring IGF2 DMR methylation. We assessed the effect on each CpG separately (CpG1, CpG2 and CpG3) and on the mean methylation of the three CpGs. Only significant results are shown in Table 6. The best model to test the effects of paternal methyl-group donor intake on IGF2 DMR CpG1 and mean CpG methylation was a model with methionine as the only predictive value. Higher intakes of methionine correlated with higher levels at CpG1 of IGF2 DMR (0.336% per 100 mg methionine increase, 95% CI: 0.103, 0.569, P=0.006) and mean CpG methylation (0.201% per 100 mg methionine increase, 95% CI: 0.001, 0.402, P=0.049). There was no evidence that paternal methyl-group donor intake has any predictive value for IGF2 DMR CpG2 and CpG3 methylation.
Table 6 Associations between paternal methyl-group donor intake and offspring IGF2 DMR methylation in cord blood (n=51)

IGF2, insulin-like growth factor 2; DMR, differentially methylated region; CI, confidence interval.
β-estimate is an absolute change in percentage of IGF2 DMR methylation; slope >0 means positive association.
At last, we determined the effect of paternal methyl-group donor intake on fetal growth, using birth weight (kg) and birth weight-for-gestational age z-scores. For the effects of paternal methyl-group donor intake on birth weight and birth weight-for-gestational age z-score the best model, was a model with betaine, choline and methionine as the predictive values. Table 7 shows the results for the three methyl-group donors in the multivariable model. The results show a negative association between birth weight/birth weight-for-gestational age z-score and betaine/methionine. In addition, a positive association between choline and birth weight/birth weight-for-gestational age z-score was found.
Table 7 Associations between paternal methyl-group donor intake and offspring birth weight (kg) and birth weight-for-gestational age z-score (n=51)

CI, confidence interval.
β-estimate is an absolute change in z-score of birth weight; slope >(<) 0 means positive (negative) association.
Discussion
Combining paternal dietary and methylation data, we were able to assess the effect of methyl-group donor intake on global DNA methylation and global DNA hydroxymethylation. Although our sample size was limited, we found a statistically significant positive association between betaine intake and global DNA hydroxymethylation. Betaine, present in foods like wheat, shellfish, spinach and sugar beets, is the immediate substrate providing methyl-groups to remethylate homocysteine and form methionine.Reference Drummond and Gibney 41 In 30 Gambian women of reproductive age, the methyl-group donor intake was measured through dietary records and blood biomarkers related to the I-C metabolism were determined. Positive correlations between dietary intakes and I-C blood biomarkers (homocysteine and dimethylglycine concentrations) were also found for betaine only.Reference Dominguez-Salas, Moore, Cole, da Costa, Cox and Dyer 42 We observed a positive association between betaine and global DNA hydroxymethylation. No associations between methyl-group donor intake and global DNA methylation was found, however the (positive) association between betaine intake and global DNA methylation was borderline significant (P=0.08); 5-hmC has been shown to be a relatively stable epigenetic mark, likely to play an independent regulatory function.Reference Iurlaro, Ficz, Oxley, Raiber, Bachman and Booth 43 – Reference Bachman, Uribe-Lewis, Yang, Williams, Murrell and Balasubramanian 45 DNA methylation is a mechanism that is essential to maintain genomic stability and for the regulation of gene expression, so the methylation level may be more strictly regulated.Reference Tellez-Plaza, Tang, Shang, Umans, Francesconi and Goessler 46 It is shown that even when dietary methyl-group donors are restricted, there is still an increased gene expression of DNMT1 and DNMT3A.Reference Takumi, Okamura, Yanagisawa, Sano, Kobayashi and Nohara 47 Although little is known about the effect of methyl-group donor intake on hydroxymethylation, a recent study by Takumi et al.Reference Takumi, Okamura, Yanagisawa, Sano, Kobayashi and Nohara 47 found that a methionine-choline-deficient diet for 1 week activated the active DNA demethylation pathway starting with oxidation of 5-mC by TET enzymes (TET 2 and TET 3 gene expression was significantly upregulated). Based on ours and other results,Reference Dao, Cheng, Revelo, Mitzner and Tang 11 , Reference Tellez-Plaza, Tang, Shang, Umans, Francesconi and Goessler 46 , Reference Sanchez-Guerra, Zheng, Osorio-Yanez, Zhong, Chervona and Wang 48 we could hypothesize that 5-hmC might be a more sensitive biomarker of environmental exposures. We did not observe an association between betaine intake and global DNA methylation, which was borderline significant, but the direction of the association between betaine and global DNA methylation/global DNA hydroxymethylation was both positive. The same direction in association of both epigenetic markers was also found by Tellez-PlazaReference Tellez-Plaza, Tang, Shang, Umans, Francesconi and Goessler 46 who investigated the relationship between metal exposure and global DNA methylation and hydroxymethylation in 48 participants at two different visits about 10 years apart. They found a correlation of 0.32 (P=0.03) at visit 1 and 0.54 (P<0.001) at visit 2 between global DNA methylation and global DNA hydroxymethylation, which lies in line with our findings (r=0.88, P<0.0001 for whole blood paternal samples and r=0.86, P<0.0001 for cord blood samples).
We hypothesized that not only in utero exposures through the mother, but also preconceptional exposures through the father may induce epigenetic shifts in global DNA (hydroxy)methylation and at the DMR of IGF2 in the offspring. These epigenetic alterations may provide a plausible link between paternal diet and adverse birth outcomes. We only found a significant positive association between paternal betaine intake and offspring global DNA methylation. To our very best knowledge, this is the first study that examines the association between paternal methyl-group donor intake and global DNA (hydroxy)methylation in the offspring of humans. The association between maternal methyl-group donor intake and offspring LINE-1 methylation has been studied. Boeke et al.Reference Boeke, Baccarelli, Kleinman, Burris, Litonjua and Rifas-Shiman 7 did not find associations between intake of methyl donor nutrients during pregnancy and LINE-1 methylation. However, in a post-hoc sex-specific analysis, they found lower cord blood methylation with higher periconceptional intakes of choline and betaine in male offspring only. We confirmed this in a parallel study where we also did not find an association between maternal dietary methyl-group donor intake and offspring global DNA (hydroxy)methylation in the MANOE study,Reference Pauwels, Ghosh, Duca, Bekaert, Freson and Huybrechts 49 suggesting that parental dietary methyl-group donor intake does not affect offspring global DNA (hydroxy)methylation. However, several studies have shown the possibility that parental methyl-group donor intake could induce changes in offspring gene specific DNA methylation.Reference Mejos, Kim, Lim and Chang 9 , Reference Carone, Fauquier, Habib, Shea, Hart and Li 10 , Reference Hoyo, Murtha, Schildkraut, Jirtle, Demark-Wahnefried and Forman 50 – Reference Jiang, Yan, West, Perry, Malysheva and Devapatla 52
In this study we selected the paternally expressed IGF2 DMR gene which is important during embryogenesis and fetal growth.Reference Chao and D’Amore 21 Higher intakes of paternal methionine suggested higher levels of IGF2 DMR CpG1 and mean of the three CpGs. Methionine, an essential amino acid, is in the end converted to SAM, which is the universal methyl-group donor. High dietary intake of methionine can influence the I-C metabolism and can therefore induce epigenetic changes.Reference Steegers-Theunissen, Obermann-Borst, Kremer, Lindemans, Siebel and Steegers 8 , Reference Waterland 53 Carone et al.Reference Carone, Fauquier, Habib, Shea, Hart and Li 10 demonstrated that male mice consuming a low-protein diet fathered offspring with altered DNA methylation at gene specific CpG islands in the liver (e.g. an increase in methylation at a CpG island upstream of PPARα). In humans, Soubry et al. Reference Soubry, Schildkraut, Murtha, Wang, Huang and Bernal 18 , Reference Soubry, Murphy, Wang, Huang, Vidal and Fuemmeler 19 showed that paternal obesity (poor/over-nutrition during spermatogenesis) is associated with altered DNA methylation patterns at imprinted genes (hypomethylation at IGF2 DMR, MEST, PEG3 and NNAT DMRs). Based on these results we could conclude that the availability of paternal dietary methyl-group donors after conception, which stands as a proxy for intake before conception, may affect offspring IGF2 DMR methylation.
We also investigated the paternal contribution through the diet on offspring birth weight. Paternal as well as maternal factors can influence offspring birth weight, although maternal factors make bigger contributions.Reference Fan, Huang, Cui, Gao, Song and Wang 54 In this study however, we did find a negative association between paternal betaine/methionine intake and birth weight/birth weight-for-gestational age z-score. In addition, choline was positively associated with birth weight and birth weight-for-gestational age z-score. The possible mechanism behind this could be that methyl-group donor intake alters the level of DNA methylation in spermatogenesis with consequences for the sperm epigenome and pregnancy outcomes. As there is no simple correlation between methyl-group donor intake and DNA methylation, the paternal intake of methyl-group donors seems to differently influence offspring DNA methylation, the metabolic status of the offspring, and affect phenotypic outcomes.Reference Schagdarsurengin and Steger 55 Lambrot et al.Reference Lambrot, Xu, Saint-Phar, Chountalos, Cohen and Paquet 56 showed that folate status of male mice alters gene specific sperm DNA methylation and was associated with birth defects (e.g. musculoskeletal malformations). Genes affected were implicated in development and chronic disease (Aff3, Nkx2-2 and Uts2, which are implicated in diabetes).
Some strengths and limitations need to be addressed. Good inclusion and exclusion criteria were set up. One of the strengths is that only Caucasian men were enrolled in the study as there can be biogeographic differences in DNA methylation levels.Reference Wu, Dyer, King, Richardson and Innis 57 Furthermore, infants from mothers who developed pregnancy complications (gestational diabetes and preeclampsia) or delivered pre-term were excluded because these disorders can cause differences in offspring DNA methylation levels.Reference Zeisel 58 , Reference Finer, Mathews, Lowe, Smart, Hillman and Foo 59 A 7-day EDR was used instead of a food-frequency questionnaire to calculate methyl-group donor intake, as there is no validated questionnaire available to assess methyl-group donor intake in men. A 7-day EDR is completed in a prospective manner, so it does not depend on memory, is open-ended, and involves a direct estimation of portion size.Reference Hochberg, Feil, Constancia, Fraga, Junien and Carel 60 The 7-day EDR also takes into account the within-person variability in food intake, which is necessary because there is a strong day-of-the-week effect.Reference Lillycrop, Slater-Jefferies, Hanson, Godfrey, Jackson and Burdge 61 Estimated diet records (instead of weighed diet records) were used because they have the same order of accuracy when ranking subjects and the respondent burden is lower.Reference Hall, Lingenfelter, Adams, Lasser, Hansen and Bean 62 Lastly, we selected the imprinted IGF2 gene, as it is paternally expressed, so methylation is only present on the paternally inherited allele in the offspring. Isolated leukocytes from cord blood were used as a marker for the newborn’s epigenetic status. The use of cord blood, which has different cell types, could be a potential limitation; however, the epigenetic profile of imprinted genes is expected to be similar across all cell types, given the establishment of the epigenetic profile before conception.Reference Faulk and Dolinoy 63 , Reference Kravetz and Federman 64 Murphy et al.Reference Murphy, Huang and Hoyo 34 found no difference in IGF2 DMR methylation profiles in DNA from different cell fractions from cord blood.
The main limitation of our study is its small sample size. However Soubry et al.Reference Soubry, Schildkraut, Murtha, Wang, Huang and Bernal 18 also described an effect of paternal obesity on IGF2 DMR methylation in offspring from a small sample size (n=79), suggesting that the paternal impact may be strong enough to be detected in a small population. Another potential concern is proof of paternity. Paternal methyl-group donor intake information was collected after conception. However, Pauwels et al.Reference Pauwels, Duca, Devlieger, Freson, Straetmans and Van Herck 65 showed that the maternal intake of methyl-group donors during pregnancy is stable, except the folate intake was significantly higher before conception. These results give us an indication that paternal methyl-group intake at the moment of conception is similar to the intake at the contact moment, assuming that also the paternal intake is stable over time. It should also be noted that food composition data for methyl-group donors is still scarce (mainly for betaine and choline as the database has only recently became available), therefore a direct match with the foods consumed was not always possible as no local (Belgian) data were available for these methyl-group donors. Finally, a multitude of statistical tests were performed without correction for multiple testing. Therefore, the results of the linear regression model should be considered exploratory and considered hypothesis generating.
Conclusion
We found a positive association between paternal betaine intake and paternal global DNA hydroxymethylation and offspring global DNA methylation, and a negative association with birth weight-for-gestational age z-score. A positive association was also found between paternal methionine intake and offspring IGF2 DMR methylation. These results suggest that preconceptional paternal methyl-group donor intake may cause epigenetic effects in the next generation. The MANOE children will be followed-up to see if paternally induced epigenetic changes may increase the susceptibility for chronic diseases, like obesity, at a later age.
Acknowledgments
The authors acknowledge the men who volunteered to take part in this study. Also the Unit Leuven Biostatistics and Statistical Bioinformatics Centre (L-BioStat) and in particular Annouschka Laenen who did the statistical analysis. Sabine Langie is the beneficiary of the Cefic-LRI Innovative Science Award 2013 and of a post-doctoral fellowship (12L5216N; http://www.fwo.be/) provided by The Research Foundation-Flanders (FWO) and the Flemish Institute of Technological Research (VITO).
Financial Support
Funding for the present study was provided by a PhD grant (grant number 11B1812N) from The Research Foundation-Flanders (FWO) and the Flemish Institute of Technological Research (VITO).
Conflicts of Interest
None.
Ethical Standards
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the UZ Leuven-Committee for Medical Ethics (reference number: ML7975). At the start of the study, all participants signed an informed consent.













