Introduction
Stress experienced early in life exerts a powerful, lasting influence on development. Already during gestation maternal stress is transmitted to the fetus via stress-related hormones such as glucocorticoids and catecholamines. Later on, the developing child faces many potential sources of stress, ranging from physical danger and material deprivation to psychosocial stressors such as family conflict, harsh or neglectful parenting and peer rejection or hostility. Converging empirical findings show that early stress becomes deeply embedded in the child’s neurobiology, with an astonishing range of long-term effects on cognition, emotion and behavior.Reference Flinn 1 – Reference Ellison 10 Most dramatically, stress exposure during early life stages has been linked to increased risk for psychopathology, from depression and conduct disorders to autism and schizophrenia.Reference Glover 2 , Reference Carr, Martins and Stingel 11
Why does stress play such a central role in behavioral development? What biological mechanisms mediate the long-term effects of early stress? Are those effects entirely maladaptive, or do they reflect a more complex balance of costs and benefits? The implications are far-reaching, not only for basic research but also for prevention and treatment. In this paper, I show how theoretical work from evolutionary biology is providing new answers to these questions, potentially reshaping the way we think about the role of stress in human development. Indeed, the field is undergoing a conceptual revolution, as traditional approaches founded on notions of ‘toxic stress’Reference Shonkoff, Garner and Siegel 12 are revised in light of the potential of early stress to shift the developing organism along alternative adaptive trajectories. Even more recently, researchers have broadened their view beyond the individual organism, and have started to explore the role of genetic conflict between mother and fetus in the regulation of prenatal stress.
The goal of this paper is to offer a brief introduction to the evolutionary literature on early stress and behavioral development, with emphasis on recent theoretical contributions and emerging concepts in the field. Instead of attempting a detailed analysis of the neurobiological and genetic mechanisms involved in stress physiology and behavioral development, I will aim for the ‘big picture’ and focus on the key conceptual issues in this area of research. The functional approach I emphasize here is meant to provide conceptual grounding for the mechanistic analysis of neurobiological and endocrine processes. While some of the biological principles I discuss apply to a broad range of species, this paper will specifically deal with human behavior and human-centered models of early stress.
From dysregulation to adaptive plasticity
Stress and allostasis
Stressors can be defined as unpredictable and/or uncontrollable events that challenge an organism’s ability to maintain self-regulation and achieve key biological goals.Reference Koolhaas, Bartolomucci and Buwalda 13 Coping with stressors requires organisms to alter their physiological and psychological parameters so as to adapt to the changing demands of the environment, a process called allostasis (stability through change) to differentiate its dynamic quality from the static regulatory target of homeostasis.Reference Sterling and Eyer 14 , Reference McEwen and Wingfield 15
Allostatic responding is orchestrated by the stress response system (henceforth SRS). The SRS is an integrated, hierarchically organized system that comprises the hypothalamic–pituitary–adrenal (HPA) axis, the sympathetic and parasympathetic autonomic branches, and various limbic structures including the amygdala.Reference Ellis, Jackson and Boyce 16 – Reference Habib, Gold and Chrousos 19 Through the SRS, the brain coordinates whole-body reactions to stressors and other challenges, with both short- and long-term effects on cardiovascular functioning, metabolism, immune regulation, attention, memory, and so forth.Reference Flinn 1 , Reference Ellis, Jackson and Boyce 16 , Reference Schwabe and Wolf 20 , Reference Miller, Chen and Parker 21 The long-term effects of SRS activation are thought to be largely mediated by epigenetic modifications induced by stress-related hormones, with adrenal glucocorticoids (cortisol in humans) playing a prominent role.Reference Champagne 22 , Reference Meaney 23 It is important to note that the SRS does not only respond to stressors as defined above; instead, it activates in response to all sorts of events that require organismal readiness, including potential opportunities such as the presence of an attractive sexual partner.Reference Ellis and Del Giudice 9 , Reference Boyce and Ellis 24 , Reference López, Hay and Conklin 25
There is no question in the literature that allostasis is a fundamentally adaptive process. The short-term changes in physiology and behavior mediated by the SRS are designed to increase the organism’s ability to survive and reproduce – despite the metabolic, immune and psychological costs of SRS activity. However, theoretical models diverge considerably in how they address the long-term effects of sustained, chronic SRS activation.
Dysregulation models of early stress
The prevailing view in psychology and medicine is that while allostatic responses are usually adaptive in the short term, protracted SRS activation is maladaptive and toxic in the long term. In addition, gestation is such a critical period for development that even a comparatively brief exposure to elevated maternal stress hormones – in the order of weeks or months – can have disruptive effects on brain development, resulting in maladaptive outcomes that may last into adulthood. In total, early stress tends to impair behavioral development, leading to dysregulation of multiple neurobiological systems and subsequent maladaptation.Reference McEwen 4 , Reference Beauchaine, Neuhaus, Zalewski, Crowell and Potapova 5 , Reference Pechtel and Pizzagalli 7 , Reference Carr, Martins and Stingel 11 , Reference Shonkoff, Garner and Siegel 12 , Reference Lupien, Ouellet-Morin and Hupbach 26 – Reference Weinstock 28 The logic of dysregulation models of early stress is outlined in Fig. 1a. The leading example of this approach is the allostatic load model (ALM).Reference McEwen 4 , Reference McEwen and Wingfield 15 , Reference Lupien, Ouellet-Morin and Hupbach 26 Allostatic load can be defined as the cost of allostasis – the ‘wear and tear’ of biological systems that results from repeated adaptation to stressors. According to the ALM, chronic stress and the resulting allostatic load may lead to both hyper- and hypo-responsive profiles of SRS functioning;Reference McEwen 29 protracted exposure to cortisol negatively affects the development of critical brain structures such as the hippocampus, amygdala and prefrontal cortex,Reference McEwen 4 with a range of maladaptive behavioral correlates.
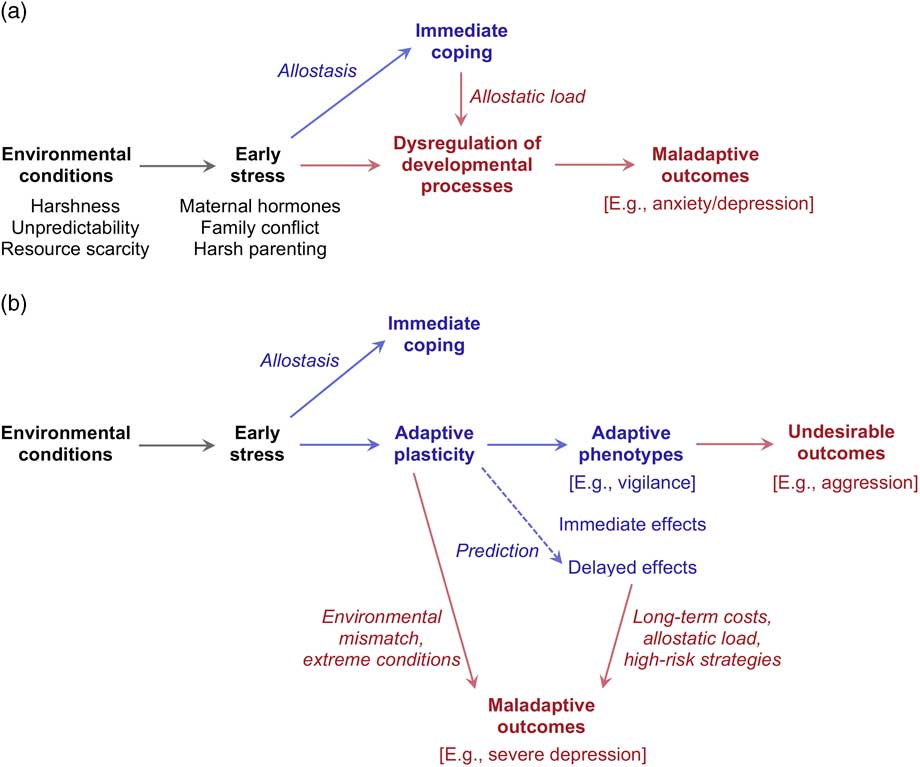
Fig. 1 Schematic representation of (a) dysregulation models and (b) adaptive models of early stress and behavioral development. Adaptive processes and outcomes are shown in blue; maladaptive and/or undesirable processes and outcomes are shown in red.
On the face of it, the evidence consistent with dysregulation models is impressive. Early exposure to chronic stress and/or elevated maternal stress hormones has been linked to reduced cognitive ability, impaired attention and memory, rigid learning strategies, and higher levels of anxiety, fearfulness, impulsivity, aggression and risk-taking. Early stress also increases the risk for conduct and personality disorders, attention deficit-hyperactivity disorder, depression, autism and schizophrenia.Reference Glover 2 , Reference Pechtel and Pizzagalli 7 , Reference Ellison 10 , Reference Carr, Martins and Stingel 11 , Reference Class, Abel and Khashan 30 – Reference Glasheen, Richardson and Kim 33 While chronic stress in childhood has been linked to both hyper- and hypo-responsive profiles of SRS activity,Reference McEwen 4 , Reference Beauchaine, Neuhaus, Zalewski, Crowell and Potapova 5 , Reference Del Giudice, Ellis and Shirtcliff 8 prenatal exposure to glucocorticoids appears to specifically predict increased SRS responsivity and behavioral vigilance, at least in infancy and early childhood.Reference Del Giudice 34 – Reference Werner, Zhao and Evans 37 While a recent study by O’Connor et al.Reference O’Connor, Bergman, Sarkar and Glover 38 found a blunted HPA response to separation in infants exposed to higher levels of prenatal cortisol, the data also showed higher pre-separation cortisol levels in the same infants, which may indicate a stronger anticipatory response rather than attenuated responsivity.Reference Gunnar and Adam 39
Adaptive models of early stress
Dysregulation models postulate the existence of a single optimal level of stress and a corresponding optimal trajectory of behavioral development. Depending on the model, the optimum may be found either at minimal levels of stress (the lower the better) or at some intermediate level – high enough to build up resilience but not so high as to become toxic.Reference Rutter 40 , Reference Seery, Leo, Lupien, Kondrak and Almonte 41 In contrast with this view, converging theoretical and empirical findings in evolutionary biology have brought about the realization that natural selection is unlikely to favor a single optimal strategy for survival and reproduction.Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van Ijzendoorn 42 , Reference Ellis and Boyce 43 What constitutes the optimal strategy in a given environment may prove detrimental to fitness in a different set of ecological circumstances. As a result, selection tends to favor adaptive phenotypic plasticity, the capacity of a single genotype to support a range of phenotypes in response to ecological conditions that recurrently influenced survival and reproduction during a species’ evolutionary history.Reference DeWitt and Scheiner 44 – Reference Schlichting and Pigliucci 46 The development of alternative phenotypes is often guided by environmental cues found in the organism’s early environment – for example cues to the presence of predators, the intensity of social competition, or the local mortality rate – and leads to durable or even irreversible changes in the individual’s morphology, physiology and behavior (developmental plasticity).
Building on the concept of developmental plasticity, researchers have been increasingly advancing alternative models in which early stress does not primarily impair or dysregulate children’s developmental trajectories, but rather shifts them toward behavioral strategies that have proven biologically adaptive in harsh or unpredictable conditions.Reference Flinn 1 , Reference Glover 2 , Reference Del Giudice, Ellis and Shirtcliff 8 , Reference Ellis and Del Giudice 9 , Reference Ellis, Jackson and Boyce 16 , Reference Cameron, Champagne and Parent 31 , Reference Belsky, Steinberg and Draper 47 – Reference Kaiser and Sachser 49 In evolutionary biology, adaptive traits are those that promote fitness and are thus favored by natural selection. An individual’s fitness is a function of its own reproductive success and that of genetically related individuals, with the latter discounted by a coefficient of relatedness (the concept of inclusive fitness).Reference Hamilton 50 – Reference Bourke 52 Adaptive traits may or may not improve happiness, well-being or health, and often carry substantial costs for the individual along with their reproductive benefits. The distinction between (biologically) maladaptive and (psychologically and/or socially) undesirable outcomes is a fundamental concept in the evolutionary study of human health and development.Reference Nesse 53 , Reference Nesse and Jackson 54
The logic of adaptive models of early stress is summarized in Fig. 1b. Exposure to stress works as a cue to local environmental conditions, and feeds into plasticity mechanisms that coordinate the development of alternative phenotypes. As shown in Fig. 1b, the effects of early stress can be either immediate or delayed – sometimes becoming manifest after years or even decades. When a plastic phenotype is induced by early cues but its benefits are only accrued at a later phase of the life cycle, the process can be described as a predictive adaptive response (PAR).Reference Gluckman, Hanson and Spencer 55 , Reference Gluckman, Hanson and Beedle 56 In PARs, early cues are employed to forecast the future state of the environment, and developmental trajectories are adjusted from the start to match the individual’s expected needs. In the literature, the term ‘programming’ is often used to describe the long-term effects of early stress;Reference Ellison 10 while the programming metaphor might suggest an inflexible and deterministic process, developmental trajectories often show considerable openness to later environmental inputs.Reference Gluckman, Hanson and Spencer 55 , Reference Gluckman, Hanson and Beedle 56
In this perspective, many putative maladaptive traits such as anxiety, aggression, and impulsivity can be reframed as costly but adaptive phenotypes that improve an individual’s survival and reproduction prospects in hostile, unpredictable contexts. For example, increased vigilance and anxiety can be regarded as defensive reactions to potential threats, whereas aggression and impulsivity can be effective competitive strategies in harsh, unstable social environments.Reference Glover 2 , Reference Del Giudice, Ellis and Shirtcliff 8 , Reference Cameron, Champagne and Parent 31 , Reference Belsky, Steinberg and Draper 47 , Reference Frankenhuis and de Weerth 48 , Reference Korte, Koolhaas, Wingfield and McEwen 57 Furthermore, there is evidence that high levels of physiological and emotional reactivity increase an individual’s sensitivity to context, making him/her more open to both negative and positive social influences.Reference Ellis, Jackson and Boyce 16 , Reference Boyce and Ellis 24 , Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van Ijzendoorn 42 , Reference Korte, Koolhaas, Wingfield and McEwen 57 Of course, some of these adaptive traits are going to have undesirable consequences for the individual and/or the social group, and may even be diagnosed as symptoms of psychopathology (e.g. conduct disorders). According to adaptive models, children exposed to early stress should exhibit patterns of impaired cognitive and emotional functioning when tested in safe, stress-free contexts and/or with tasks that mimic the demands of those contexts; but they should often perform better than their peers on tasks that reproduce key features of the dangerous, unpredictable environments they are adapted to. The initial evidence suggests that this may be the case.Reference Schwabe and Wolf 20 , Reference Frankenhuis and de Weerth 48
Adaptation or maladaptation?
While adaptive models emphasize the biological value of stress-related traits, they do not negate the possibility of genuinely maladaptive outcomes. For example, maladaptive outcomes may result from phenotype-environment mismatches – both at the individual level when the actual environmental state does not match the predicted one, and at the population level when the broader environment changes so that previously adaptive traits become maladaptive.Reference Frankenhuis and Del Giudice 59 , Reference DeWitt, Sih and Wilson 60 Other causes of maladaptation include exposure to extreme environments (e.g. conditions of severe sensory and affective deprivation) that exceed the evolved plasticity range of an organism; high-risk behavioral strategies that trade potential fitness benefits against the risk of severely maladaptive outcomes; and dysregulation of adaptive processes due to genetic and/or environmental causes, including deleterious mutations and the long-term effects of allostatic load.Reference Nesse 53 , Reference Frankenhuis and Del Giudice 59 , Reference Nesse 61 , Reference Crespi 62
In short, adaptive models incorporate the key insights of dysregulation models, as they acknowledge both the short-term benefits and the long-term costs of early stress (compare Fig. 1a and 1b).Reference McEwen 4 However, adaptive models give center stage to the long-term benefits of stress exposure as a determinant of developmental plasticity.Reference Ellis and Del Giudice 9 Within this general approach, specific models differ in the hypothesized balance between adaptation and maladaptation.Reference Ellison 10 Some theorists have speculated that the outcomes of early stress may be almost always beneficial, either for the individual or for the broader social group;Reference Glover 2 others have explicitly discussed various pathways to genuine maladaptation.Reference Ellis and Del Giudice 9 , Reference Del Giudice 34 Teasing apart adaptation and maladaptation in human development will require a great deal of empirical work, and a thorough understanding of the evolved function of psychological and physiological mechanisms.
Life history theory: an integrative framework
The logic of adaptive plasticity outlined in the previous section can be applied separately to various psychological traits such as anxiety, aggression and self-regulation. However, these behavioral traits cluster together in a way that suggests a high degree of functional coordination; moreover, they show reliable associations with individual differences in other domains including physical and sexual maturation, metabolism, and immune function.Reference McEwen 4 , Reference Del Giudice, Ellis and Shirtcliff 8 , Reference Ellis and Del Giudice 9 , Reference Gluckman, Hanson and Spencer 55
In evolutionary biology, a major framework for explaining coordinated patterns of developmental plasticity is life history theory.Reference Stearns 63 – Reference Kaplan and Gangestad 66 Life history theory deals with the way organisms allocate time and energy to the various activities – including growth, bodily maintenance, mating and parenting – that comprise their life cycle. Since all these activities contribute to the organism’s fitness, devoting time and energy to one will typically involve both benefits and costs, engendering trade-offs between different fitness components. For example, there is a trade-off between growth and reproduction because both require substantial energetic investment, and thus producing offspring reduces somatic growth. Natural selection favors organisms that schedule developmental tasks and activities so as to optimize resource allocation; this chain of resource-allocation decisions – expressed in the development of a coherent, integrated suite of physiological and behavioral traits – constitutes the individual’s life history strategy.
At the broadest level of analysis, life history-related traits covary along a dimension of slow v. fast life history. Variation along the slow-fast continuum is observed both between related species and between individuals of the same species.Reference Ellis, Figueredo, Brumbach and Schlomer 64 , Reference Réale, Garant and Humphries 67 , Reference Sæther 68 In humans, some individuals adopt slower strategies characterized by later reproductive development and behavior, a preference toward stable pair bonds and high investment in parenting, an orientation toward future outcomes, low impulsivity and allocation of resources toward enhancing long-term survival; others display faster strategies characterized by the opposite pattern.Reference Pigliucci 45 , Reference Ellis, Figueredo, Brumbach and Schlomer 64 , Reference Figueredo, Vásquez and Brumbach 69 , Reference Del Giudice 70 Fast life history strategies are comparatively high risk, focusing on mating opportunities (including more risky and aggressive behavior), reproducing at younger ages and producing a greater number of offspring with more variable outcomes. Trade-offs incurred by faster strategies include reduced health, vitality and longevity (of self and offspring). In most organisms, individual life histories are determined by a combination of genetic and environmental factors, and often exhibit a remarkable degree of developmental plasticity. In general, dangerous and unpredictable environments tend to entrain fast life history strategies, whereas safe and predictable environments favor slower strategies.Reference Ellis, Figueredo, Brumbach and Schlomer 64 , Reference Placek and Quinlan 71 – Reference Kuzawa and Bragg 73
Over the years, a number of authors have employed the concepts of life history theory to explain the long-term effects of early stress on development.Reference Del Giudice, Ellis and Shirtcliff 8 , Reference Ellis, Jackson and Boyce 16 , Reference Cameron, Champagne and Parent 31 , Reference Belsky, Steinberg and Draper 47 The central idea of these models is that early stress exposure – especially during the first 5–7 years of life – conveys predictive information about life history-relevant parameters of the environment (in particular danger and unpredictability), thus promoting the development of alternative life history strategies (Fig. 2). Higher levels of stress are predicted to entrain faster strategies, characterized by earlier sexual maturation (especially in females), impulsivity, and higher levels of both externalizing (aggression, attention-seeking) and internalizing symptoms (anxiety, depression). This perspective offers an elegant way to explain the coordination among stress-related behavioral traits and their associations with patterns of growth, maturation, metabolism and so forth.Reference Ellis and Del Giudice 9 , Reference Gluckman, Hanson and Beedle 56 , Reference Del Giudice and Belsky 74
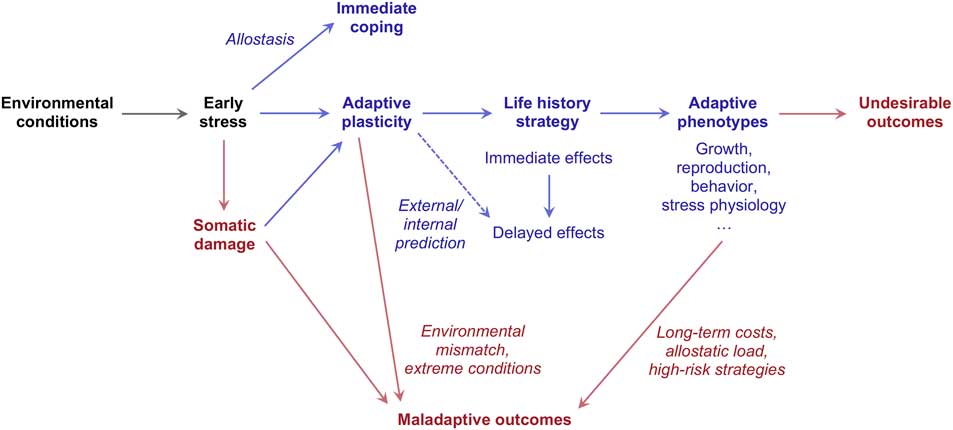
Fig. 2 An integrative life history perspective on early stress. Adaptive processes and outcomes are shown in blue; maladaptive and/or undesirable processes and outcomes are shown in red.
Internal v. external prediction
In life history models, the delayed effects of early stress – for example on the timing of sexual maturation – are usually interpreted as predictive-adaptive responses based on the anticipated state of the external environment (‘external’ PARs). In order to be adaptive, external PARs require a sufficient level of environmental stability between childhood and adulthood. In a recent paper, Nettle et al.Reference Nettle, Frankenhuis and Rickard 75 argued that early stress may influence life history development by a different route. If early stress causes permanent damage to the organism and thus reliably reduces life expectancy, it may be adaptive for individuals exposed to stress early in life to engage in faster strategies even if the environment improves later on. Such ‘internal’ PARs do not require environmental stability and seem likely to evolve under a wider range of conditions.Reference Del Giudice 76 In a nutshell, external PARs forecast the future state of the environment, whereas internal PARs forecast the future state of the organism.
Internal and external PARs are not mutually exclusive and may coexist in human development. Further elaborations of Nettle et al.’s model indicate that the degree of environmental stability required for the evolution of external PARs is likely to be lower than initially suggested (i.e. annual autocorrelations in the order of 0.80–0.85 instead of 0.95), thus broadening the scope for adaptive external prediction.Reference Del Giudice 76 The concept of internal PARs is theoretically intriguing because it suggests that the adaptive and maladaptive effects of early stress may be inextricably linked (see Fig. 2), as somatic damage (a maladaptive effect) contributes to inform and direct life history development (an adaptive effect). For this reason, internal prediction also raises new challenges for empirical research, by questioning the standard distinction between adaptive and maladaptive hypotheses on the developmental role of stress.
The ACM
My colleagues and I recently advanced an integrative evolutionary-developmental model of stress responsivity based on life history theory, the ACM.Reference Del Giudice, Ellis and Shirtcliff 8 , Reference Ellis and Del Giudice 9 , Reference Del Giudice, Hinnant, Ellis and El-Sheikh 77 The ACM synthesizes and extends previous models of early stress, and makes a host of detailed predictions about adaptive patterns of SRS functioning in different environments, their behavioral correlates, and their relations with individual variation in other neurobiological systems – including dopaminergic and serotonergic pathways and the hypothalamic–pituitary–gonadal axis. A simplified diagram of predicted responsivity patterns in the ACM is shown in Fig. 3. Sensitive patterns are hypothesized to develop in safe, predictable conditions and warm family environments. High stress responsivity in sensitive individuals increases their openness to the social and physical environment. Sensitive individuals are reflective, self- and other-conscious, and high in inhibitory control; collectively, these traits promote sustained learning and long-term cooperation in the context of slow life history strategies. Buffered patterns are predicted to develop preferentially in conditions of moderate environmental stress, where they strike a balance between the costs and benefits of responsivity. Buffered responsivity is predicted to arise primarily through moderate, repeated activation of the stress response system during the first years of life. Buffered individuals are predicted to be comparatively low in anxiety and aggression and moderately sensitive to social feedback, making intermediate exposure to stress look like a ‘protective factor’ in the development of psychopathology.Reference Rutter 40 , Reference Seery, Leo, Lupien, Kondrak and Almonte 41
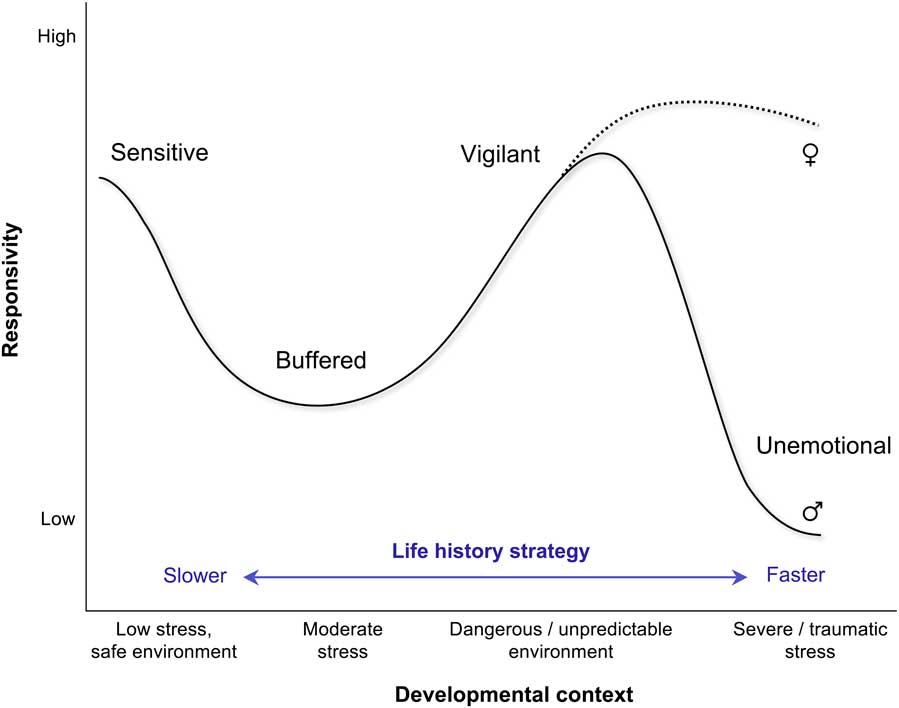
Fig. 3 Predicted relation between environmental conditions and physiological responsivity in the adaptive calibration model.Reference Del Giudice, Ellis and Shirtcliff 8
Moving toward faster life history strategies, vigilant patterns are predicted to develop in stressful contexts, where they enable people to cope with dangers and threats in the environment. High physiological responsivity mediates heightened attention to threats and high trait anxiety. In the ACM, vigilance is not associated with a single behavioral pattern, but rather with a distribution of patterns involving different mixtures of aggressive/externalizing and withdrawn/internalizing behaviors, also depending on an individual’s sex. Finally, unemotional patterns are marked by a profile of low stress responsivity. Generalized unresponsivity inhibits social learning and sensitivity to social feedback; it can also increase risk-taking by blocking information about dangers and threats in the environments. The predicted correlates of this pattern are low empathy and cooperation, impulsivity, competitive risk-taking and antisocial behavior – particularly in males. Because of sex differences in optimal strategies, the distribution of responsivity patterns and their behavioral correlates is expected to become more sex-biased at increasing levels of environmental stress; accordingly, unemotional profiles should be more common in males, especially after puberty (Fig. 3).
The ACM has significant implications for empirical research on early stress. Most notably, the predicted relation between environmental quality and physiological responsivity is strongly nonlinear (Fig. 3). If this prediction is correct, researchers should not expect simple linear relations between SRS responsivity and behavioral traits such as aggression and impulsivity. In addition, a nonlinear relation between environmental conditions and responsivity patterns may explain why, in studies of prenatal stress, associations between maternal self-reported distress/anxiety and cortisol levels (typically tested with linear correlation/regression models) are often found to be weak and inconsistent.Reference Del Giudice 34 , Reference Baibazarova, van de Beek and Cohen-Kettenis 35 , Reference Werner, Zhao and Evans 37
Another important feature of the ACM is that responsivity patterns are predicted to develop over time through a sequence of ‘switch points’ marked by hormonal transitions.Reference Del Giudice, Ellis and Shirtcliff 8 Especially in long-lived species like humans, life history development is likely to involve multiple stages, with opportunities for revision and recalibration after initial ‘decisions’.Reference Del Giudice and Belsky 74 , Reference Fischer, van Doorn, Dieckmann and Taborsky 78 Pre- and early postnatal development, the transition from early to middle childhood, and puberty are all potential switch points for the calibration of stress responsivity.Reference Del Giudice, Ellis and Shirtcliff 8 In the ACM, individual and sex differences in the functioning of the stress response system emerge according to the evolutionary function of each developmental stage; for example, some children (especially males) are predicted to switch from vigilant to unemotional responding as they move from early childhood to middle childhood and adolescence.
The broader implication is that superficially similar features of behavior and physiology (e.g. elevated SRS responsivity) may actually serve different life history strategies; conversely, the same overall strategy may be reflected in different types of behavior at different life stages. A life history framework promises to offers a more coherent picture of the relation between the immediate and delayed effects of early experience; for example, late-appearing traits (e.g. unemotional impulsivity and sexual promiscuity) and their developmental precursors (e.g. irritability and hyper-responsivity) may share deep functional connections even if they appear very different on the surface.Reference Ellis, Del Giudice and Shirtcliff 79 The same logic may explain why prenatal exposure to stress hormones seems to consistently increase SRS responsivity in infancy and early childhood (when survival and growth are the child’s main biological tasks), while chronic stress in childhood may lead to both hyper- and hypo-responsive profiles of SRS activity in adolescence and adulthood.
Cooperation and conflict in prenatal development
Adaptive models of early stress tend to view prenatal stress exposure as a cooperative transfer of information from mother to fetus. Hormones such as cortisol and catecholamines are released in the maternal bloodstream when stressful events challenge the mother’s coping ability; sustained exposure to stress-related hormones – perhaps especially to recurrent hormonal peaksReference Del Giudice 34 – provides the fetus with useful information about the predictability of the environment, the presence of threats, and the availability of social support. Since the fetus does not have direct access to the external environment, it benefits by letting maternal hormones shape its developmental trajectory. At the same time, the mother benefits by transmitting accurate information, thus maximizing phenotype- environment matching in her offspring and – indirectly – her own inclusive fitness.Reference Kaiser and Sachser 49 , Reference Pluess and Belsky 58 , Reference Sandman, Poggi Davis and Glynn 80 – Reference Kapoor, Dunn, Kostaki, Andrews and Matthews 82
The unstated assumption in this account is that the interests of the mother and fetus are 100% aligned, so that fully cooperative interactions can evolve. The biological reality, however, is both more complex and more interesting. As first shown by Trivers,Reference Trivers 83 the genetic interests of parents and offspring are only partially overlapping. Whenever a given trait or behavior results in a cost to the parent and a benefit to the offspring (or vice versa), parent and offspring can be expected to ‘disagree’ about the optimal level of expression of that trait. Stated otherwise, the level of a trait that maximizes the parent’s fitness will differ from the level that maximizes the offspring’s fitness, resulting in a biological conflict of interest about the trait in question. The logic of parent–offspring conflict is easiest to illustrate in the case of parental investment (e.g. food provision). The mother has the same genetic relatedness with each of her offspring, and – all else being equal – will maximize her own fitness by allocating her investment in equal proportions. However, any individual offspring is more closely related to itself than to its siblings (both present and future); thus, natural selection favors those offspring who increase their share of maternal resources above the mother’s optimum.Reference Trivers 83 Both the intensity of conflict and its likely resolution (e.g. whether a compromise is reached or one of the actors gets to control the outcome) are affected by ecological factors such as resource abundance and by the details of a species’ reproductive system.Reference Schlomer, Del Giudice and Ellis 84
Parent–offspring conflict in prenatal stress
Because of the inevitable divergence between maternal and fetal interests, prenatal development is characterized by an intricate mixture of cooperation and conflict.Reference Schlomer, Del Giudice and Ellis 84 – Reference Haig 86 The fetus – or, more precisely, the fetoplacental unit – is an active player rather than a passive target of maternal decisions; as a result, its hormonal interactions with the mother involve both honest signaling and reciprocal manipulation. Manipulative tactics and the countermeasures they evoke may evolve into full-fledged ‘arms races’, in which both actors pay significant physiological costs and expose themselves to the risk of occasional maladaptive outcomes when – for various reasons – conflict happens to escalate out of control.
The theory of parent–offspring conflict has been applied to various aspects of prenatal development,Reference Schlomer, Del Giudice and Ellis 84 most notably fetal nutrition (including blood glucose concentration and blood pressure)Reference Haig 85 – Reference Wells 88 and spontaneous abortion.Reference Flinn, Nepomnaschy, Muehlenbein and Ponzi 89 In both cases, stress-related hormones are involved in the physiology of conflict: placental corticotropin-releasing hormone is instrumental in raising maternal cortisol and blood glucose, while the abortogenic effects of cortisol at the beginning of pregnancy mediate the relation between maternal stress around conception and early pregnancy termination.Reference Flinn, Nepomnaschy, Muehlenbein and Ponzi 89 , Reference Nepomnaschy, Welch and McConnell 90 In both cases, prenatal conflict about the regulation of the mother’s SRS parameters is going to indirectly affect the child’s behavioral development, even if only as a side effect of passive hormonal exposure. However, there are reasons to believe that the behavioral effects of prenatal stress may become a matter of conflict in their own right.
In a recent paper,Reference Del Giudice 34 I argued that parent-offspring conflict may arise because of the effects of prenatal stress on postnatal plasticity. As noted in a previous section, there is accumulating evidence that high SRS responsivity and emotional reactivity result in increased sensitivity to the effects of the postnatal environment, so that highly reactive infants and children are also more behaviorally plastic.Reference Del Giudice, Ellis and Shirtcliff 8 , Reference Boyce and Ellis 24 , Reference Pluess and Belsky 58 , Reference Belsky and Pluess 91 In species with prolonged maternal care and extended family interactions such as humans, the mother has ample opportunity to shape her offspring’s behavior in (more or less subtly) self-interested ways. As a result, conflicts of interest arise in many areas of development, from feeding in infants to mate choice in young adults.Reference Schlomer, Del Giudice and Ellis 84 By definition, high postnatal plasticity means that the child will be more susceptible to the effects of maternal behavior, with beneficial long-term consequences for the mother. For this reason, selection should favor mothers who are able to increase their children’s plasticity beyond the children’s optimum; an obvious way to achieve this end is to increase fetal exposure to stress-related hormones by some (limited) amount. At the same time, fetuses should put up some resistance against maternal manipulation; however, they cannot simply ignore maternal signals, as the latter also provide useful information about the external environment.Reference Uller and Pen 92 Because of this strategic tension, the regulation of prenatal stress may evolve into a complex web of tactics and countermeasures revolving around the amount of stress-related hormones that reach the fetal brain.
While this hypothesis is still speculative, it has the potential to explain a number of puzzling features of prenatal stress physiology. For example, the placentally expressed enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) inactivates cortisol to cortisone, and is commonly understood to provide an adaptive ‘filter’ against maternal cortisol.Reference Brunton and Russell 93 However, the filtering action of 11β-HSD2 is opposed by that of 11β-HSD1, an enzyme that converts cortisone to cortisol and is expressed by maternal tissues in close contact with the placenta. Such paradoxical findings make little sense under the standard assumptions, but can be easily explained in a conflict perspective.Reference Del Giudice 34 In addition to making sense of this and other features of prenatal physiology, a conflict perspective can be employed to make new empirical predictions; for example, the idea that placental progesterone actively downregulates the responsivity of the mother’s HPA axis suggests the hypothesis that the maternal brain may express biochemical countermeasure against the effects of progesterone metabolites on SRS activity.Reference Del Giudice 34
As shown in Fig. 4, the logic of parent–offspring conflict adds a layer of complexity to adaptive models of early stress, and provides researchers with new insights as well as new challenges. To begin, the partial but pervasive conflict between the biological interest of parents and children raises the question of who is the real beneficiary of a given trait or outcome. Indeed, traits that are adaptive from the parent’s perspective may not be adaptive when viewed from the child’s perspective, and vice versa. Evidence that a given outcome is maladaptive for one of the actors (e.g. the mother) is no longer sufficient to infer dysregulation or mismatch, as the same outcome may be increasing the fitness of the other actor (e.g., the child).
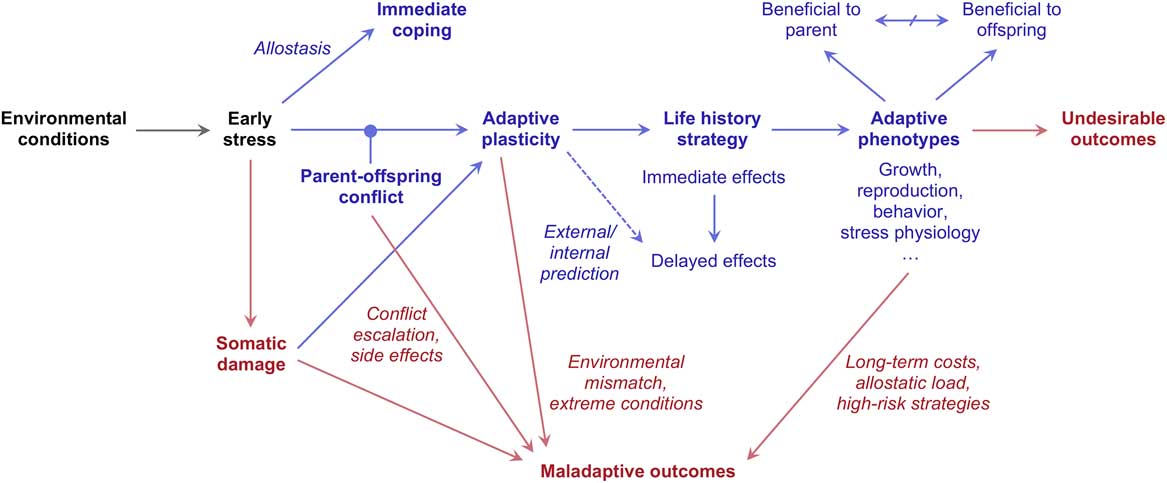
Fig. 4 Implications of parent-offspring conflict for adaptive models of early stress. Adaptive processes and outcomes are shown in blue; maladaptive and/or undesirable processes and outcomes are shown in red.
Besides complicating the study of adaptation, parent–offspring conflict also increases the scope for genuine maladaptation (Fig. 4). Because of the need to overshoot their target, physiological mechanisms involved in prenatal conflict are more likely to enter vicious cycles of escalation, with potentially catastrophic consequences and a range of maladaptive side effects. This logic has been invoked to explain the etiology of gestational hypertensionReference Haig 85 and may contribute to explain the most severe pathological outcomes of early stress, such as autism and schizophrenia.Reference Del Giudice 34 Finally, evolutionary conflict may arise not only between parent and child, but also between maternal and paternal chromosomes within the child’s genome. Imprinted genes are genes whose expression level changes according to the parent of origin; since the genetic interests of mothers and fathers are typically divergent, imprinted genes of maternal and paternal origin often evolve so as to have opposite effects on developmentReference Haig 94 , Reference Wilkins 95 While there is no room here for a detailed treatment of this topic, recent theoretical work suggests that imprinted genes may be involved in the regulation of early stress, the development of life history strategies and the etiology of mental disorders.Reference Del Giudice 34 , Reference Schlomer, Del Giudice and Ellis 84 , Reference Heijmans, Tobi and Stein 96 – Reference Úbeda and Gardner 99
Future directions
In many ways, the models reviewed in this paper should be regarded as initial, tentative steps toward a comprehensive theory of early stress. The insights gained so far need to be refined with the aid of mathematical modeling, further integrated across domains and levels of analysis, and subjected to stringent empirical tests. Mathematical modeling will play a crucial role in assessing the validity of evolutionary hypotheses about the timing and function of life history transitions, the adaptiveness (or lack thereof) of early plasticity, the costs and benefits of different responsivity profiles, and so forth. While theoretical biology offers a wealth of general results, the details of a species’ ecology and life history often matter a lot when it comes to finer-grained predictions. As more investigators incorporate the distinctive features of human ecology into mathematical models of development,Reference Del Giudice and Belsky 74 , Reference Nettle, Frankenhuis and Rickard 75 , Reference Úbeda and Gardner 99 – Reference Jones 101 theories of early stress will become increasingly powerful, detailed, and capable of generating robust quantitative predictions.
Another important avenue for future research concerns the interplay between the SRS and other neurobiological and endocrine systems involved in behavioral control. For example, the role of sex hormones in prenatal stressReference Kaiser and Sachser 49 is a crucial but under-investigated topic, especially in the human literature. Also, future extensions of life history models should give full consideration to stress-immune interactions in the development of life history strategies, behavior and psychopathology. There is extensive cross-talk between the SRS and the immune system, with inflammation emerging as a key functional link between psychosocial stress, immune functioning and disease.Reference Howerton and Bale 102 – Reference Murphy, Slavich, Rohleder and Miller 105 The immune response is an essential component of allostasis; like the SRS, the immune system collects life history-relevant information about mortality risk, and different life history strategies are likely to predict different patterns of immune functioning.Reference McDade 106 Indeed, the effects of early infections overlap considerably with those of psychosocial stress and prenatal exposure to stress-related hormones, including their association with later psychopathology.Reference Howerton and Bale 102 , Reference Raison and Miller 107 – Reference Patterson 109 Integrating immune functioning in life history models of early stress will be a major step toward a unified biological theory of human development.Reference Ellis and Del Giudice 9
Conclusion
Understanding the role of early stress in human development is a major scientific challenge with myriad implications for prevention, treatment, and basic research in the medical and behavioral sciences. Here I showed how evolutionary thinking has contributed to enrich and transform the study of early stress, giving rise to new models that incorporate concepts from developmental plasticity, life history theory and parent–offspring conflict. As models grow in scope and complexity, they become increasingly able to explain known phenomena and – even more importantly – generate novel, counterintuitive predictions. At the same time, they face researchers with new and sometimes formidable challenges in the design and interpretation of empirical studies. I hope the conceptual map sketched in this paper will serve as a useful starting point for explorations of this important, fascinating and rapidly evolving field.
Acknowledgment
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.






