Introduction
Manning et al. Reference Manning, Scutt, Wilson and Lewis-Jones1 suggested that the ratio of length between the second and fourth fingers (digit ratio or 2D:4D) is a negative correlate of prenatal testosterone (T) exposure and a positive correlate of prenatal estrogen exposure. As there are considerable practical and ethical constraints to measuring prenatal hormones more directly, there has been much interest in utilising 2D:4D as a tool for retrospective examination of the developmental origins of sexually differentiated outcomes; however, its validity has frequently been questioned Reference Leslie2–Reference Richards, Gomes and Ventura4 .
Experimental manipulation of fetal sex hormones is not permitted in human studies for obvious ethical reasons, and so researchers have developed a range of creative approaches to address this problem. These include investigation of patient groups exposed to atypical sex hormone concentrations (or sensitivity), such as congenital adrenal hyperplasia Reference Richards, Browne and Aydin5 and androgen insensitivity syndrome Reference Berenbaum, Bryk, Nowak, Quigley and Moffat6,Reference van Hemmen, Cohen-Kettenis, Steensma, Veltman and Bakker7 , and comparing same-sex and opposite-sex twins Reference Medland, Loehlin and Martin8–Reference Voracek and Dressler10 . Although theory-consistent effects have been reported in a number of studies and across different methodologies, these are typically present alongside null findings and replication failures.
A more direct approach has been to measure sex hormone concentrations in amniotic fluid. Amniocentesis is an invasive medical procedure by which amniotic fluid is extracted for genetic and chromosomal analysis in at-risk pregnancies. Although such samples may not be representative of the general population, amniocentesis has routinely been performed in typically developing pregnancies of advanced maternal age. Manning Reference Manning11 (see Fig. 2.4, p. 32) initially reported that maternal 2D:4D was negatively correlated with the level of T present in the amniotic fluid. Although this association could arise because aspects of maternal endocrine status affect pregnancy outcomes, it is difficult to interpret because (assuming that prenatal T levels really do influence offspring digit ratios) it could also be a spurious artefact resulting from digit ratios being correlated between mother and child Reference Richards, Bellin and Davies12,Reference Voracek and Dressler13 . Furthermore, it has been questioned whether the observed association could have been inflated by the presence of outliers Reference Lippa14 .
Lutchmaya et al. Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning15 reported that the ratio of testosterone to estradiol (T:E) present in amniotic fluid was significantly negatively correlated with right hand digit ratio (R2D:4D) in 29 2-year-old children. This finding indicates that a high level of T relative to estradiol (E) is associated with the development of a low, more ‘male-typical’, 2D:4D ratio in the right hand. However, the sample size was small, males and females were not analysed separately, no significant effect was observed for left hand digit ratio (L2D:4D), and neither T nor E on its own was a significant predictor. Although this study is frequently cited in support of the validity of 2D:4D, in the 16 years since its inception, no direct replication attempt has been published. The closest has been a study reporting a significant negative correlation between amniotic T and L2D:4D in female neonates Reference Ventura, Gomes, Pita, Neto and Taylor16 ; however, no significant effect was observed for R2D:4D in females, or for R2D:4D or L2D:4D in males. A reanalysis of these data Reference Richards, Gomes and Ventura4 showed a significant negative correlation with the average of R2D:4D and L2D:4D (M2D:4D) in females (but not in males); notably, there was no correlation with the right–left difference in 2D:4D (D[R-L]), an additional variable for which low values have been hypothesised to reflect high levels of fetal androgen exposure Reference Manning11 . Importantly, amniotic E was not measured in this study, meaning that no attempt at replicating the significant effect reported by Lutchmaya et al. Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning15 could be made.
The current paper reports the findings of a study in which we examined whether amniotic T, E, and T:E ratio were predictive of digit ratio variables measured in the children and mothers of these pregnancies at 4½-year follow-up.
Method
Participants
The sample for this study was obtained from women undergoing amniocentesis at the Queen Charlotte’s and Chelsea Hospital, London. Although the reason for amniocentesis was usually increased risk of Down syndrome, only women carrying healthy fetuses were retained. There were in total 66 mothers (with ages at birth ranging from 28.17 to 44.08 years; M = 37.68, SD = 4.01) from whom amniotic fluid samples were collected usually between weeks 15 and 22 of gestation. The women gave birth to 66 children (32 females and 34 males) whose digit ratios were measured around the age of 4½ years (range = 3.83–5.92, M = 4.501, SD = 0.629). Most of the women (78.8%, n = 52) were Caucasian, 9.1% (n = 6) were Asian, 6.1% (n = 4) were African, 4.5% (n = 3) were Middle-Eastern, and n = 1 (1.5%) was of mixed ethnicity. Regarding education, 19.7% (n = 13) had a postgraduate degree, 43.9% (n = 29) had an undergraduate degree, 16.7% (n = 11) had vocational training, 9.1% (n = 6) had A-levels, and 10.6% (n = 7) had GCSEs or equivalent. Study procedures were approved by national and institutional research ethics committees and conducted in accordance with the Declaration of Helsinki, and written consent was obtained from all the mothers who took part in this research.
Amniotic hormones
Amniotic fluid samples were obtained between 2002 and 2004 when women were recruited to the study as part of an ongoing larger scale project examining associations between hormones and behavior (see Bergman et al. Reference Bergman, Glover, Sarkar, Abbott and O’Connor17 ) Total T concentrations were measured in amniotic fluid samples by radioimmunoassay (RIA), Coat-a-Count (DPC Los Angeles, CA, USA), with intra- and inter-assay coefficients of variation of 7.5% and 8.9%. A random subset (18 males; 12 females) of the samples was also analysed for T and E by Liquid Chromatography/Mass Spectroscopy (LCMS). The correlation between T values using RIA and LCMS was strong, r(40) = 0.82, p < 0.001 Reference Bergman, Glover, Sarkar, Abbott and O’Connor17 .
Digit ratio (2D:4D)
2D:4D data were collected between 2005 and 2009 at approximately 4½ years follow-up. Measurements were taken directly (from the hands) and/or indirectly (from photocopies) using callipers measuring to 0.01 mm. The intra-class correlation (single measures, absolute agreement) for a subsample (n = 15) of participants’ photocopies for R2D:4D determined that the repeatability of measurement was high, ICC = 0.940, p < 0.001. To maximise the sample size that could be included in the analysis (and thereby increasing statistical power), we used the following calculation Reference Constantinescu18 to correct the measurements taken directly for those participants whose digit ratios had not also been measured from photocopies:
As photocopies were available for the majority of the 63 mothers (photocopies n = 59; callipers n = 35), 34 male children (photocopies n = 30; callipers, n = 27), and 32 female children (photocopies n = 30; callipers n = 24) for whom digit ratios could be calculated, only 4, 4 and 2 values, respectively, were corrected from the calliper measurements.
Statistical analysis
We used bootstrapped independent samples t-tests to examine sex differences and bootstrapped Pearson’s correlations to test for associations between hormonal variables and gestational age at the time of amniocentesis. We then used further bootstrapped Pearson’s correlations to examine associations between the hormonal and digit ratio variables, and calculated the bias-corrected accelerated 95% confidence intervals (BCa 95% CI) based on 2000 resamples. We took this approach because some variables were not normally distributed, and outliers were present for some of the hormonal variables. Using bootstrapping therefore allowed us to retain all biologically relevant data and produce more reliable estimates than would be obtained from standard parametric analyses.
Results
Amniotic T (both RIA and LCMS measurements) and T:E ratio were significantly higher when the fetus was male, though there was no sex difference for E. There were no sex differences for R2D:4D, L2D:4D, and M2D:4D. D[R-L] was marginally lower in males (p = 0.049), though the BCa 95% CIs overlapped zero (bootstrapped p = 0.057) (see Table 1).
Table 1. Sex differences for amniotic hormone and digit ratio variables

E, estradiol; LCMS, Liquid Chromatography/Mass Spectroscopy; RIA, radioimmunoassay; T, testosterone.
BCa 95% CI = bias-corrected accelerated 95% confidence intervals (calculated for mean difference via the bootstrapping procedure).
§ Equal variances are not assumed.
We used bootstrapped Pearson’s correlations to check for associations between amniotic hormone levels and gestational age at the time of amniocentesis (see Fig. 1 for scatterplots). No significant associations were observed for RIA T (males: r [31] = −0.159, p = 0.376, BCa 95% CI = −0.389, 0.166; females: r [29] = 0.003, p = 0.986, BCa 95% CI = −0.394, 0.313), LCMS T (males: r [15] = −0.334, p = 0.191, BCa 95% CI = −0.676, 0.126; females: r [10] = −0.243, p = 0.446, BCa 95% CI = −0.786, 0.916), or the T:E ratio (males: r [15] = 0.172, p = 0.508, BCa 95% CI = −0.312, 0.840; females: r [10] = −0.111, p = 0.732, BCa 95% CI = −0.810, 0.966). Although there was some evidence for a negative correlation between gestational age and E concentration in males (r [15] = −0.437, p = 0.079, BCa 95% CI = −0.657, = 0.239), the effect in females was in the opposite direction and not significant (r [10] = 0.450, p = 0.142, BCa 95% CI = −0.523, 0.789). Due to the inconsistent nature of these results, the relatively small variability in gestational age at amniocentesis, and in order to remain consistent with the approach taken by Lutchmaya et al. Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning15 , we elected to examine associations between amniotic hormones and digit ratios using zero-order correlations rather than including gestational age as a covariate.

Fig. 1. Scatterplots showing the association between gestational age at the time of amniocentesis and (a) RIA testosterone (T), (b) LCMS T, (c) LCMS estradiol (E), and (d) testosterone-to-estradiol ratio. Red circles = male; blue circles = female.
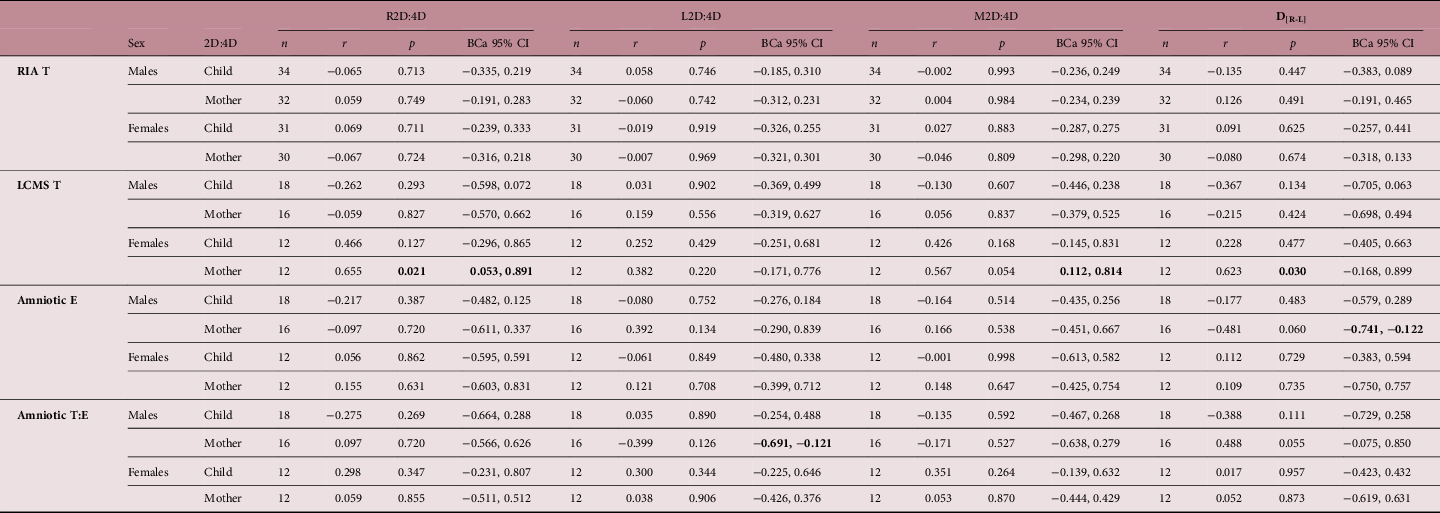
Pearson’s tests with BCa (i.e. bootstrapped) 95% confidence intervals for associations between amniotic fluid sex hormone concentrations and both maternal and child digit ratio variables at 4½-year follow-up are shown in Table 2. LCMS T levels in females correlated positively with R2D:4D, and there was some limited evidence of there being similar effects for M2D:4D (BCa 95% CIs did not cross zero but the parametric analysis was only marginally significant, p = 0.054) and D[R-L] (parametric analysis was significant, p = 0.030, but the BCa 95% CIs crossed zero). There was also a negative correlation between amniotic E and D[R-L] in males (BCa 95% CIs did not cross zero but the parametric statistic was not significant, p = 0.060). Each of these effects was in the opposite direction to that which would be predicted by theory. The only other finding of note was that the T:E ratio in males correlated negatively with maternal L2D:4D; although this effect was in the theory-consistent direction, and the BCa 95% CI did not cross zero, the conventional parametric statistical test was not significant (p = 0.126). There were no statistically significant correlations between amniotic T, E, or T:E ratio and any of the digit ratio variables measured in the children at follow-up.
Table 2. Associations between amniotic sex hormone concentrations and children’s digit ratio variables

E, estradiol; LCMS, Liquid Chromatography/Mass Spectroscopy; RIA, radioimmunoassay; T, testosterone.
BCa 95% CI = bias-corrected accelerated 95% confidence intervals (calculated via the bootstrapping procedure).
All statistical tests were Pearson’s correlations (two-tailed).
Discussion
We report the findings of a study examining associations between individual differences in amniotic sex hormone concentrations and digit ratio (2D:4D). Although previous reports have suggested that high levels of amniotic T are associated with low digit ratios in both mothers Reference Manning11 and neonates Reference Ventura, Gomes, Pita, Neto and Taylor16 , and that high ratios of amniotic T:E are associated with low digit ratios in 2-year-old infants Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning15 , we did not find evidence for such effects here. The findings, therefore, cast doubt on the idea that mid-trimester sex hormone concentrations are instrumental in the development of 2D:4D ratios in humans.
Most correlations between amniotic hormone levels and maternal digit ratio variables were not statistically significant, and the direction of these correlations was generally erratic. Of the five correlations for which some degree of statistical significance was indicated (i.e. a parametric p < 0.05 and/or BCa 95% CIs that did not cross zero), only one was in the theory-consistent direction. This was a negative correlation between T:E ratio in female pregnancies and the maternal L2D:4D. However, this effect should be interpreted with considerable caution considering (i) the high number of statistical tests that were run (and that we did not adjust for alpha inflation), (ii) the small sample size (n = 12) for this analysis, (iii) that although the BCa 95% CIs did not cross zero the conventional parametric statistical test was not significant (p = 0.126), and (iv) that similar effects were not observed for the other digit ratio variables (i.e. R2D:4D, M2D:4D, and D[R-L]) in females, and no such effects were observed in males.
The only other study that reports on an association between amniotic sex hormone levels and mothers’ 2D:4D Reference Manning11 found a negative correlation with T. The findings from our study generally contradict this observation, as the only significant correlations observed between amniotic T (specifically for LCMS measurements when the fetus was female) and maternal 2D:4D were in the opposite (i.e. positive) direction. These effects should of course be interpreted with caution: not only are they in the opposite direction to that predicted by theory, but the corresponding correlations observed for the larger sample for which RIA T measurements were available are in the negative direction and not statistically significant. It should also be noted that relatively little consideration has yet been given to the possibility of associations between maternal 2D:4D and amniotic hormone concentrations.
The most notable finding from the current study is that neither T nor E, nor the T:E ratio present in amniotic fluid was a significant predictor of children’s 2D:4D ratios measured at 4½-year follow-up. This observation may be interpreted in several ways. First, it could be that prenatal sex hormone exposure does indeed influence the development of 2D:4D, but that such processes occur earlier in pregnancy (i.e. towards the end of the first trimester) Reference Manning and Fink19 . Support for this idea comes from the finding that 2D:4D already shows sexual dimorphism by the 9th–12th weeks of gestation Reference Malas, Dogan, Evcil and Desdicioglu20 . However, as there appears to be a certain amount of lability in 2D:4D during infancy Reference Knickmeyer, Woolson, Hamer, Konneker and Gilmore21 and childhood Reference Trivers, Manning and Jacobson22 , postnatal exposure to sex hormones may also play a role. The second possible explanation for the current null findings is that second-trimester sex hormones do influence the development of 2D:4D, but that the concentrations measured in amniotic fluid simply do not accurately index those present in the fetal circulation Reference Rodeck, Gill, Rosenberg and Collins23 . For instance, as amniotic fluid is typically only sampled once during any individual pregnancy, it may not be representative of the fetal period as a whole, particularly as hormone levels can vary with gestational age; furthermore, the hormone levels present in amniotic fluid may represent what is excreted by the fetus rather than that which it actually experiences. The third possibility, of course, is that prenatal T and E do not determine variation in 2D:4D ratios (or that any association between these variables is smaller than initially thought).
It is noteworthy that we found no association between amniotic sex hormone concentrations and the children’s right–left difference in 2D:4D (D[R-L]). Although initially suggested by Manning Reference Manning11 as a further indicator of prenatal androgen action nearly two decades ago, there has been relatively little research into the validity of this proposed marker. We did find some evidence for D[R-L] being lower in males than females, but our findings are consistent with previous studies showing that this measure does not correlate with T measured from amniotic fluid Reference Richards, Gomes and Ventura4 , maternal circulation Reference Richards, Gomes and Ventura4,Reference Hickey, Doherty and Hart24 or umbilical cord blood Reference Hickey, Doherty and Hart24,Reference Hollier, Keelan and Jamnadass25 , and that it does not differ between patients with congenital adrenal hyperplasia and unaffected controls Reference Richards, Browne and Aydin5 . As D[R-L] is calculated as a ratio from two other noisy markers, its reliability can also be problematic Reference Voracek, Manning and Dressler26 . Taken together, these observations raise serious questions regarding the utility of D[R-L] as an indicator of prenatal androgen exposure.
The current findings should be considered in light of some important limitations. First, the method used for measuring finger lengths in this study was unusual in that some participants were measured directly, others were measured indirectly (i.e. from photocopies), and a subsample was measured using both techniques. It is, therefore, important to note that digit ratios measured from photocopies are typically lower (i.e. more male-typical) than those measured directly Reference Ribeiro, Neave, Morais and Manning27 . However, we corrected for this problem mathematically, and so all participants could be examined together. It should also be noted that T analyses specific to the direct and indirect measurements of finger lengths from this cohort have been reported in an unpublished MPhil thesis Reference Constantinescu18 , and showed the same general pattern of (null) results reported here. Another limitation was that we could only examine E and T:E concentrations in a subsample, meaning that the statistical power for the associated analyses was lower than that for the RIA T analyses. However, it should be recalled that the only other study to report associations between amniotic E (and T:E) Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning15 had a very similar sample size (n = 29; current sample for E/T:E: n = 30). It is also noteworthy that we did not observe the typical pattern of sex differences (i.e. M < F) for digit ratio variables in this sample. Although a non-significant effect of lower R2D:4D in males than females could simply reflect the study lacking statistical power, it remains unclear why L2D:4D and M2D:4D were slightly higher (though not significantly so) in males than females.
There are several potentially fruitful directions through which research might clarify the nature of the relationship (assuming there is one) between 2D:4D and prenatal sex hormones. First, as there are preliminary indications that second-trimester maternal T levels correlate with offspring digit ratio Reference Ventura, Gomes, Pita, Neto and Taylor16,Reference Barona, Kothari and Skuse28 (though see also Hickey et al. Reference Hickey, Doherty and Hart24 ), future studies could examine maternal T and E measured towards the end of the first trimester (i.e. the time at which digit ratios are thought to be most influenced by sex hormone exposure Reference Manning and Fink19 ). Additionally, considering that dihydrotestosterone (DHT) has a stronger affinity to the androgen receptor than does T, early DHT concentrations could be another target worth exploring.
Summary
The current study attempted to replicate the finding of Lutchmaya et al. Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning15 that T:E ratio in mid-trimester amniotic fluid was a significant negative correlate of children’s 2D:4D ratios. However, we found no evidence that individual differences in amniotic T, E, or T:E ratio could predict children’s digit ratios measured at 4½ years of age. We did observe some correlations between amniotic T (and T:E ratio) and maternal digit ratios, though the direction of these effects was more often than not in the opposite direction to that which would be predicted by theory. Furthermore, we observed no correlation between amniotic sex hormones and the children’s right–left difference in 2D:4D (D[R-L]). Taken together, the findings suggest that mid-trimester amniotic T and E do not significantly influence development of the 2D:4D ratio. However, the possibility remains that these hormones do influence the development of digit ratio at an earlier stage of gestation.
Acknowledgements
The authors wish to extend their gratitude to Prof Melissa Hines and Prof Vivette Glover for providing us with access to these data; we would also like to thank all of the parents and children who took part in this research.
Financial support
This research was supported by the National Institute of Health Research (NIHR) Cambridge Biomedical Research Centre, United States Public Health Service National Institutes of Health (grant numbers: HD24542, MH073019 and MH073842), and the March of Dimes. The funding sources had no involvement in designing the study, collecting, analysing and interpreting the data, writing the manuscript, or in the decision to submit the article for publication.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of relevant national guidelines on human experimentation, and with the Declaration of Helsinki.