Introduction
The emergence of the ‘fetal origins of adult disease’ hypothesis [later termed the ‘developmental origins of health and disease’ (DOHaD) hypothesis] has placed considerable and new responsibilities on the obstetrician. The quality of antenatal care is no longer simply to be seen as a determinant of perinatal outcomes, but also of long-term health for mother and baby. Ground-breaking work by David Barker and colleagues demonstrated that intrauterine growth, influenced by maternal lifestyle factors not only impacts on perinatal morbidity and mortality but also on the offspring’s body composition and risk of numerous non-communicable diseases in later life. These include cardiovascular disease,Reference Barker, Gluckman and Godfrey 1 obesity,Reference Ravelli, van Der Meulen, Osmond, Barker and Bleker 2 type 2 diabetes,Reference Barker, Hales and Fall 3 osteoporosis,Reference Gluckman, Hanson, Cooper and Thornburg 4 chronic obstructive pulmonary diseaseReference Stocks, Hislop and Sonnappa 5 and depression.Reference Suri, Lin, Cohen and Altshuler 6 As these associations were first reported, a new scientific field of endeavor has sought to explain the underlying mechanisms. Key findings on how long-term health is shaped by periconceptional and prenatal life are now further supported by recent human data. Further, advances in the field of epigenetic processes such as DNA methylation,Reference Godfrey, Sheppard and Gluckman 7 histone modificationReference Sun, Denisenko and Sheth 8 and miRNA regulationReference Lillycrop and Burdge 9 now provide a plausible mechanism by which the environment may alter gene expression, although its translation to the development of human diseases is still being investigated.
Research in rodent models has allowed for further detailed examination of the DOHaD hypothesis in a controlled setting. Data in mice show that even before implantation the developing embryo is exquisitely sensitive to its nutritional environment.Reference Watkins, Ursell and Panton 10 In rats, a maternal low protein diet in the pre-implantation period led to a reduced number of cells in the trophectoderm and inner cell mass of the blastocyst and hence an abnormal trajectory of growth including over compensatory adolescent growth and alterations of relative organ size in the offspring.Reference Kwong, Wild, Roberts, Willis and Fleming 11 Concerns about the impact of pre-implantation embryo exposure to synthetic, in vitro culture conditions has appeared to be borne out by recent studies showing that the culture medium in which a pre-implantation human embryo develops has significant implications for birth weight and placental size,Reference Dumoulin, Land and Van Montfoort 12 , Reference Eskild, Monkerud and Tanbo 13 although this remains a controversial area.Reference Carrasco, Boada and Rodriguez 14 , Reference Lin, Li, Lian, Chen and Liu 15
More recently, transgenerational effects have been demonstrated with the observation that the intrauterine environment impacts on fertility of the offspring by modulating fetal gametogenesis in utero. The first evidence orginated from observations that women with a particularly low (<2500 g) or high (>4500 g) birth weight have decreased fecundity.Reference Nohr, Vaeth, Rasmussen, Ramlau-Hansen and Olsen 16 Similarly, men who were born small for gestational age demonstrated decreased plasma testosterone concentrations,Reference Vanbillemont, Lapauw and Bogaert 17 although others showed no difference in semen quality according to birth weight.Reference Ramlau-Hansen, Hansen and Jensen 18 In animals, poor maternal nutrition has been associated with delayed sexual maturation in male offspring and reduced sperm count.Reference Zambrano, Rodriguez-Gonzalez and Guzman 19 Smoking at the time of conception may also have a profound effect on germ cell numbers in male offspring and somatic cells within the developing female gonads.Reference Mamsen, Lutterodt and Andersen 20
This article focuses on developmental origins of ovarian function and the role of the placenta and periconceptional nutrition as determinants of long-term health. We argue that emerging evidence in these areas supported the need for a proactive clinical approach to optimizing periconceptional as well as antenatal care in order to optimize health and well-being through the life course.
DoHAD and the ovary
One of the key determinants of fertility is the rate of ovarian ageing. As women continue to delay childbirth, poor ovarian reserve is becoming an increasingly challenging clinical problem. There is growing evidence that early developmental conditions impact on ovarian reserve and hence long-term fertility and reproductive health. Understanding the mechanisms involved may provide additional insights into the regulation of ovarian reserve and suggest potential interventions, which could modulate the rate of ovarian ageing.
Extensive germ cell death occurs during early development as oocytes become assembled into the primordial follicle pool.Reference Baker 21 These mechanisms result in a peak number of 6–7 million primordial follicles at around 18 weeks’ gestation, which reduces to about 1–2 million at birth through apoptosis.Reference Fulton, Martins da Silva, Bayne and Anderson 22 This establishes and fixes the ovarian reserve available in individual women before birth.Reference Skinner 23 Subsequent decline in oocyte numbers continues through follicular atresia and apoptosis.Reference Vaskivuo, Anttonen and Herva 24 By puberty, ∼300,000 oocytes remain and then further loss throughout reproductive life leads to a virtual exhaustion of follicle numbers by the menopause where <1000 will remain.Reference Faddy, Gosden, Gougeon, Richardson and Nelson 25 If ovarian reserve is reduced at birth, the reproductive life ‘window’ is likely to be shorter.
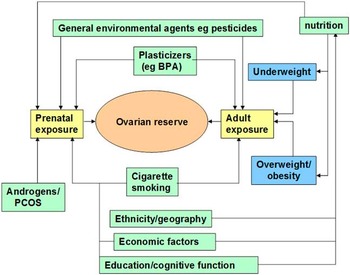
It is well established that organ development during prenatal life is influenced by the prevailing intrauterine environment, and that adverse conditions during fetal life can lead to an increased risk of adult-onset diseases, such as type-2 diabetes and hypertension.Reference Barker 26 The formulation of the DOHaD hypothesis raises the question as to whether poor maternal nutrition, the prenatal endocrine environment and toxin exposure could have an adverse effect on ovarian reserve. Casting the net wider, it is possible that circulating hormones during pregnancy can influence ovarian reserve. Also, the potential effects of maternal exposure to environmental contaminants need to be considered.Reference Mark-Kappeler, Hoyer and Devine 27 The range of possible factors affecting ovarian reserve is summarized in Fig. 1 as recently reviewed.Reference Richardson, Guo, Fauser and Macklon 28

Fig. 1 Environmental and developmental determinants of ovarian reserve. Multiple factors have been shown to influence both the size of the primordial follicle pool laid down during fetal organogenesis via prenatal exposure, and the adult exposures, which modulate the rate at which follicles are depleted throughout reproductive life. Reproduced with permission from Barker.Reference Barker 26
Intrauterine nutrition
A number of studies have examined the effect of maternal diet on the development of follicles in the fetal ovary. Maternal feed restriction during pregnancy in sheepReference Rae, Palassio and Kyle 29 leads to the development of smaller ovaries in the fetal lambs, with fewer advanced follicles but without changing germ cell numbers. A longer-term impact of such restriction is manifest as a lower ovulation rate in the adult.Reference Rae, Kyle and Miller 30 In cows, maternal nutrient restriction during the first third of gestation leads to diminished ovarian reserve as measured by anti-Mullerian hormone (AMH), follicle-stimulating hormone (FSH) and antral follicle count.Reference Mossa, Carter and Walsh 31
Young women born small for gestational age have been shown to have reduced ovarian volume, increased FSH and reduced ovulation ratesReference Ibanez, Potau, Enriquez and de Zegher 32 suggesting poor ovarian reserve. However, anatomical analysis of human fetal ovaries does not suggest that slow fetal growth is associated with a smaller follicle pool size or accelerated depletion.Reference de Bruin, Nikkels and Bruinse 33 Moreover, later studies, which examined indicators of ovarian reserve in adolescent girls and young women found no significant reduction with earlier poor intrauterine growthReference Hart, Sloboda and Doherty 34 or low birth weight allowing for gestational age.Reference Lem, Boonstra and Renes 35 Also, a recent study showed no association between low birth weight and later development of ovulatory dysfunction in women.Reference Shayeb, Harrild and Bhattacharya 36
The importance of adequate lactation to support establishment of ovarian reserve during early postnatal growth has been confirmed by a study in rats where maternal malnutrition during lactation was shown to adversely affect follicular numbers.Reference Ferreira, Gombar and da Silva Faria 37
Socioeconomic adversity in childhood has also been demonstrated to affect ovarian reserve by reducing the age at which women experience the menopause. The mechanism(s) by which this occurs is unknown but it is thought that it is likely to be secondary to poor nutrition and emotional stress.Reference Hardy and Kuh 38 The Study of Women’s Health Across the Nation also shows that higher educational level and being employed were significantly associated with later age at final menstrual period.Reference Gold, Crawford and Avis 39 Research has also demonstrated differences in ovarian reserve depending on the woman’s ethnicity, with African-Americans completing the menopause earlierReference Bromberger, Matthews and Kuller 40 and Japanese later.Reference Gold, Bromberger and Crawford 41
Prenatal endocrine environment
There is considerable evidence that fetal programming through androgens contributes to the development of polycystic ovary syndrome (PCOS) experienced in adult life (reviewed by Xita and Tsatsoulis.Reference Xita and Tsatsoulis 42 ) The impact of exposure of female fetuses to excess androgens has been studied in cases of ‘fetal androgen excess disorders’, where the subsequent development of a PCOS phenotype was observed even when androgen levels had been normalized after birth.Reference Hague, Adams and Rodda 43 In utero exposure to excess androgens may also influence ovarian reserve. In a study of Rhesus monkeys, early prenatal androgenization diminished ovarian reserve and reduced yield of oocytes for in vitro fertilization (IVF).Reference Dumesic, Patankar and Barnett 44 In a rat model, adult ovarian morphology has been shown to be dependent on the timing and level of androgen exposure in utero.Reference Wu, Li and Wu 45
However, long-term follow-up of women with PCOS does not provide evidence of earlier menopause. Indeed, assessment of ovarian reserve through measurement of serum AMH reveals higher levels of this factor in women with PCOS.Reference La Marca, Broekmans, Volpe, Fauser and Macklon 46 This may reflect a higher level of AMH production per granulosa cell in PCOS patients and the larger number of small follicles present.
Environmental exposures
It is well established that many chemicals present within the environment, including natural and artificial components of the diet, have the potential to interfere with the physiological role of hormones. These ‘endocrine disrupting chemicals’ may interfere with hormone biosynthesis, signaling or metabolism.Reference Diamanti-Kandarakis, Bourguignon and Giudice 47 Many of these agents act as steroid receptor agonists and antagonists, particularly with regard to estrogenicity and androgenicity.Reference Andersen, Vinggaard, Rasmussen, Gjermandsen and Bonefeld-Jorgensen 48 , Reference Shanle and Xu 49
Cigarette smoking has an adverse impact on the reproductive health of women.Reference Hruska, Furth, Seifer, Sharara and Flaws 50 Smoking effects on ovarian reserve may involve the action of polycyclic aromatic hydrocarbons (PAHs) contained in cigarette smoke. Murine fetal ovaries cultured in the presence of PAHs showed extensive germ cell loss, which was prevented by a selective AHR antagonist.Reference Matikainen, Moriyama and Morita 51 In human studies, smoking was not found to reduce the number of oogonia but there was a significant decrease in the number of somatic cells with prenatal exposure to maternal smoking.Reference Lutterodt, Sørensen and Larsen 52 Because oocytes cannot survive without enclosure in somatic cells, the authors concluded that the observed lack of somatic cells at this early stage could have long-term consequences on ovarian reserve and fertility later in life.
The mechanisms underlying smoking toxicity on reproductive and ovarian function are complex because of the considerable array of circulating metabolites associated with inhalation of tobacco smoke.Reference Hoffmann and Hoffmann 53 Some of these will interact directly with the gamete pool through receptors such as AHRReference Andersen, Vinggaard, Rasmussen, Gjermandsen and Bonefeld-Jorgensen 48 or by causing a disruption of ovarian developmental signaling.Reference Fowler, Childs and Courant 54 Others mechanisms have been postulated including an effect of smoking on ovarian vascularization.Reference Motejlek, Palluch, Neulen and Grummer 55
Another environmental compound of concern is bisphenol A (BPA). BPA has been shown to have detrimental effects on fetuses and young children including induction of immune, cognitive and behavioral changes and an increasing risk of premature secondary sexual characteristic development.Reference Erler and Novak 56 Work in rats has also demonstrated a significant decrease in total follicle number the ovaries of offspring of dams exposed to high BPA levels.Reference Manikkam, Guerrero-Bosagna, Tracey, Haque and Skinner 57 Detectable urinary BPA levels have been found in the majority of women undergoing IVF and are inversely associated with the number of oocytes retrieved.Reference Mok-Lin, Ehrlich and Williams 58 As a result of this research, the use of BPA has been banned within the European Union in any products used by babies such as feeding bottles. Other environmental compounds that demonstrate a similar transgenerational decrease in the primordial follicle pool size include pesticides (permethrin and insect repellant DEET), dioxin (TCDD) and a hydrocarbon mixture (found in jet fuel).Reference Manikkam, Guerrero-Bosagna, Tracey, Haque and Skinner 57
DoHAD and the placenta
The placenta is a vital organ in determining life in utero. Fetal size at birth depends heavily on placental function, because fetal growth relies on the capacity of the placenta to transfer nutrients from the mother to the fetus.Reference Lewis, Cleal and Hanson 59 , Reference Kaiser 60 Indeed, most of the variation in birth weight can be explained by differences in placental function, set against a background of genetic growth potential.Reference Constancia, Hemberger and Hughes 61 – Reference Horikoshi, Yaghootkar and Mook-Kanamori 63 Other than determining birth weight, the placenta can also directly affect the shape, size and composition of most fetal organ systems, including the heart and circulatory system,Reference Thornburg and Challis 64 , Reference Musa, Torrens and Clough 65 the metabolic system,Reference Lewis, Demmelmair and Gaillard 66 the inflammatory response,Reference Zhu, Du and Ford 67 boneReference Lewis, Cleal and Ntani 68 and the brain.Reference Keverne 69 In recent years, it has become clear that the placenta is not a ‘static’ barrier organ, but has evolved to actively adapt and respond to the environment, continuously challenged by alterations in maternal physiology and health and fetal demand during the course of pregnancy. Recent evidence suggests that gene expression and epigenetic changes in the placenta may translate directly to fetal developmental effects, and thus provoke adverse effects on offspring health. Studies linking placental biology to short-term and long-term offspring health have now become an intriguing area of research that may provide meaningful insight into the placental origins of adult-onset diseases.
Maternal health and placental function
Babies born to mothers with obesity and diabetes during pregnancy have an increased risk of being large for gestational age and show several metabolic, inflammatory and vascular developmental effects that may herald long-term health risks.Reference Sebire, Jolly and Harris 70 However, substantial variation exists between infants with respect to their size and body composition at birth.Reference Landon, Spong and Thom 71 This is likely attributable to placental function, in particular to placental transfer of nutrients and placental production of growth factors and inflammatory cytokines.Reference Lewis, Demmelmair and Gaillard 66 Recent studies have demonstrated that the placenta has a remarkable capacity to adapt to the maternal environment, as well as to fetal cues to adjust its function in support of optimal prenatal growth and development.Reference Lewis, Cleal and Hanson 59 Understanding the complex nature of the placental genetic and epigenetic response to maternal health is far from unraveled, but new research may provide crucial insight into the origins of several major obstetric complications (e.g. intrauterine growth restriction, pre-eclampsia, preterm birth and placental abruption), as well as explain pathways directly affecting fetal development with potential long-term health consequences.
The placenta can sense maternal health by using a system of placental membrane transporters that respond to nutritional and endocrine signals from the mother.Reference Lewis, Cleal and Hanson 59 Indeed, a reduction of placental transporter gene expression and activity is associated with impaired fetal growth.Reference Jansson, Pettersson and Haafiz 72 Furthermore, placentas from women with gestational diabetes and obesity can respond by altering insulin-dependent gene pathways and mTOR signaling.Reference Li, Hadden and Singh 73 , Reference Jansson, Rosario and Gaccioli 74
Fetal effects of placental function
From an evolutionary perspective, placental function is more likely to have adapted to undernutrition than overnutrition. A large number of transporters for amino acids, glucose, fatty acids and micronutrients are found in the placenta’s two plasma membranes; microvillus and basal plasma. Research has demonstrated that transporters of amino acids are significantly decreased in placentas of intrauterine growth-restricted offspring when compared with controls.Reference Jansson and Powell 75 Work in rats has demonstrated that the ability of the placenta to transport amino acids is decreased in late pregnancy as a result of protein restriction. This occurs before the occurrence of intrauterine growth restriction, thus implying that placental amino acid transport is a cause rather than a consequence of intrauterine growth restriction.Reference Jansson, Pettersson and Haafiz 72 Another theory is that increased exposure to maternal glucocorticoids caused by a reduced activity of 11 β-hydroxysteroid dehydrogenase (which converts cortisol to the less active cortisone) may result in intrauterine growth restriction and early programming of later disease.Reference Edwards, Benediktsson, Lindsay and Seckl 76
The placental response to protect the fetus against a high abundance of glucose, lipids and other endocrine and inflammatory factors, may not be well established in humans.Reference Lewis, Cleal and Hanson 59 How the placenta responds to excess nutrients is not entirely clear. For glucose metabolism, parallel changes in methylation and expression of glucose transporter genes have been implied.Reference Novakovic, Gordon, Robinson, Desoye and Saffery 77 Similarly, in patients with hypercholesterolemia and gestational diabetes, the placenta responds to the high lipid levels by increasing placental cholesterol metabolism.Reference Marseille-Tremblay, Ethier-Chiasson and Forest 78 In addition to enhanced maternal-to-fetal transfer, lipids cause inflammatory changes in the placenta most likely through recruitment and activation of macrophages.Reference Challier, Basu and Bintein 79 , Reference Zhu, Du, Nathanielsz and Ford 80 The consequences of this low-grade chronic placental inflammation are currently unknown, but shaping and priming of the fetal immune response may well be sensitive to this effect.
In addition to the metabolic effects of placental function, evidence has emerged to suggest placental function also affects the development of the heart, as recently reviewed by Thornburg and Challis.Reference Thornburg and Challis 64 Prenatal development of the heart appears susceptible to a combination of haemodynamic, metabolic and epigenetic stressors that can result in reduced cardiomyocyte numbers and altered heart remodeling at birth, with lifelong consequences for healthy cardiac ageing. Similarly, recent evidence supports an important epigenetic basis for placental origins of brain development, primarily based on imprinting effects of a conserved number of matrilineally imprinted genes shared between the placenta and the hypothalamic region, as well as with 5HT-dependent pathways, which may have important implications for neuromotor development and adult-onset psychiatric disorders.Reference Keverne 69
Placental weight, size and surface area correlates to long-term health
One of Barker’s later interests was the study of placental dimension at birth as predictors of long-term disease. His studies of the birth record data of the Helsinki Birth Cohort, which consisted of 20,431 men and women reported several associations between placental shape and size, and subsequent health during the offspring’s life course and into adulthood.Reference Barker and Thornburg 81 Placental weight can be considered a crude proxy of its function, and substantial variation exists between placental weights for a given birth weight.Reference Alwasel, Abotalib and Aljarallah 82 In the Helsinki Birth Cohort, both low placental weight and high placental weight in relation to birth weight were predictive of hypertension and coronary heart disease in adulthood.Reference Barker, Bull, Osmond and Simmonds 83 Other placental characteristics, such as placental thickness and surface area, have also been shown to be associated with elevated adult chronic diseases. In particular, heart disease and cancer risk can be related back to characteristic variations in shape and size of the placenta at birth (Table 1). Some of these effects are sex specific, mostly with stronger associations found in male offspring. There is no clear explanation for this, although an evolutionary basis has been suggested.Reference Eriksson, Kajantie, Osmond, Thornburg and Barker 84
Table 1 Placental size and shape in relation to adult-onset chronic disease risk

a This association was only found in men.
Perspectives and targets for intervention
Obstetric intervention studies have traditionally been designed to aim for improvement of maternal and/or neonatal outcomes. Less attention has been focused towards the simple fact that most periconceptional and prenatal intervention studies will also affect the placenta. Treatments to improve offspring health may therefore have greater benefit if interventions are aimed at improving placental function to create an optimal environment for normal fetal development. This may sometimes be a challenging endeavor, as some seemingly favorable interventions (e.g. metformin treatment of women with gestational diabetes to prevent fetal overgrowth), may have certain side-effects not immediately visible at childbirth, but which translate to long-term offspring health hazards (e.g. overproduction of placental inflammatory cytokines, alterations in fat mass only becoming apparent from 2–3 years of age). In view of these findings, it is desirable that any intervention trial involving women either becoming pregnant or with an on-going pregnancy include the careful follow-up of their children’s health. A helpful starting point would be to have a closer look at how these interventions have affected placental function and architecture, as well as to study the link between placental adaptation and offspring health.
DoHAD and nutrition before and during pregnancy
Periconceptional nutrition and outcomes
Despite the fact that for a number of decades health care professionals have seen pregnancy as a ‘window of opportunity’ to change a detrimental lifestyle,Reference Bille and Andersen 85 research has shown that women planning a pregnancy do not tend to alter their nutritional intake.Reference Crozier, Robinson, Godfrey, Cooper and Inskip 86 This is intriguing in light of recent evidence, which suggests that diet affects the nutritional environment of uterine fluid.Reference Kermack, Finn-Sell and Cheong 87 Moreover, there is a growing body of evidence that periconceptional nutrition is important not only to the couples’ chance of conceiving and maintaining a healthy pregnancy but also to the well-being of the offspring. A Dutch study recently demonstrated that couples who consumed a Mediterranean diet in the weeks before IVF were 65% more likely to achieve an ongoing pregnancy.Reference Twigt, Bolhuis and Steegers 88 Interestingly, the change of diet may not need to be for a prolonged period; studies in rodents have shown that dietary manipulation in the 3 days before implantation can impact on fetal growth trajectories and even behavioral development.Reference Fleming, Lucas, Watkins and Eckert 89
Data suggest that suboptimal maternal nutrition (either over- or undernutrition) may have negative effects on the offspring. Mothers who eat a diet containing a high saturated fat intake during pregnancy are more likely to produce offspring with abnormal β-cell function and hence a predisposition to type 2 diabetes in later life.Reference Cerf 90 Similar adult phenotypes can be seen in offspring born to mothers who have a diet with poor intakes of energy, protein and micronutrients during pregnancy.Reference Yang and Huffman 91 This outcome is thought to be secondary to changes in the insulin-like growth factor axis leading to insulin resistance and hypertension.Reference Setia and Sridhar 92
A group of micronutrients of significance in the preconception period are the B vitamins. In 1991, a double-blind randomized controlled trial, performed across seven countries, demonstrated the need for folic acid supplements to be used by women before conception and in the first 12 weeks of pregnancy in order to prevent neural tube defects. 93 Moreover, recent research has demonstrated the role of preconception folate supplementation on the homocysteine pathway in follicular fluidReference Boxmeer, Brouns and Lindemans 94 and the correlate with improved embryo quality.Reference Boxmeer, Macklon and Lindemans 95 Further building on the work of David Barker and colleagues, research in Southampton has focused on the importance of vitamin D supplementation during pregnancy. A systematic review of the studies undertaken to date demonstrated evidence that increased vitamin D intake in pregnancy improved offspring birth weight, bone mass and serum calcium concentrations, although more randomized controlled trials in this area were recommended.Reference Harvey, Holroyd and Ntani 96 In addition, extra vitamin D in pregnancy is known to enhance maternal absorption of dietary calcium and bone resorption, leading to increased calcium levels required for fetal skeletal growth and tooth enamel production.Reference Lewis, Lucas, Halliday and Ponsonby 97 Studies of the long-term effects of vitamin D deficiency in utero and in early infancy have demonstrated links with an increased risk of developing multiple sclerosis, schizophrenia, insulin-dependent diabetes mellitus and some types of cancer (prostate, breast and colorectal).Reference McGrath 98
The importance of nutrition in the periconceptual period is an area of increasing interest. More randomized controlled trials are required to ascertain which nutrients are important and therefore should be supplemented around the time of conception and during pregnancy.
The role of omega-3 polyunsaturated fatty acids in pregnancy
Omega-3 polyunsaturated fatty acids play important roles in the structure and function of all cell membranesReference Calder 99 and they interact with various transcription factors thus playing a role in the regulation of gene expression in many cells types.Reference Calder 99 , Reference Calder 100 Furthermore, omega-3 fatty acids modulate the production of bioactive lipid mediators like prostaglandins, leukotrienes, resolvins and protectins that are central to cell and tissue responses.Reference Calder 101 The main biologically active omega-3 fatty acids are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The richest source of EPA and DHA is seafood, especially fatty fish like salmon, sardines and mackerel and they are also found in omega-3 supplements like fish oils.Reference Calder 100 Other foods are poor sources of EPA and DHA, although the human body has the capacity to endogenously synthesize them from a precursor plant omega-3 fatty acid, alpha-linolenic acid (ALA). However, the rate of this endogenous biosynthesis is believed to be relatively poor.
The importance of lipid nutrition in pregnancy is highlighted by the observation that more than 50% of the dry weight of the human brain is lipid, particularly structural lipid (i.e. phospholipids). The human brain and retina contain an especially high proportion of DHA relative to other tissues. The human brain growth spurt occurs from approximately the beginning of the third trimester of pregnancy to 18 months after birth. The amount of DHA in the brain increases dramatically during the brain growth spurt. In humans, brain weight increases from about 100 g at 30 weeks of gestation to about 1100 g at 18 months of age; during this time there is three- to four-fold increase in DHA concentration in the brain and a 35-fold increase to total brain DHA. This DHA is provided by the mother across the placenta during pregnancy and in breast milk after birth. Maternal blood plasma and breast milk DHA comes from maternal diet, maternal stores (e.g. adipose tissue, although some have argued that maternal brain may also give up DHA during pregnancy), and maternal biosynthesis from dietary or stored ALA.
Placental fatty acid transporters act to concentrate DHA on the fetal side of the placenta.Reference Haggarty 102 There is evidence that maternal blood DHA declines during pregnancyReference Al, van Houwelingen and Kester 103 and that it becomes progressively lower with each successive pregnancy.Reference Al, van Houwelingen and Hornstra 104 There is much evidence that increasing maternal intake of DHA increases the DHA content of both maternal and fetal blood.
These observations reinforce the need for pregnant women to consume preformed DHA, consistent with current recommendations. For example, the UK Government recommendation equates to a minimum daily intake of 450 mg EPA plus DHA. 105 The European Food Safety Authority recommended that pregnant women consume an additional 100 to 200 mg per day of DHA on top of the adult recommended daily intake of 250 mg EPA+DHA. 106
An adequate supply of omega-3 fatty acids, especially DHA, seems essential for optimal visual, neural and behavioral development of the infant/child. The need for DHA early in life was demonstrated in studies with pre-term infants, where feeds that included DHA (and often also the omega-6 fatty acid arachidonic acid) were shown to improve visual development.Reference Lauritzen, Hansen, Jorgensen and Michaelsen 107 The literature on the effect of DHA on visual and cognitive outcomes in term infants is mixed with some studies reporting benefits and others not. One reason for this might be that an early beneficial effect of DHA is lost with time so that early assessments show benefit and later assessments do not; at least one study has shown this.Reference Agostoni, Riva, Trojan, Bellu and Giovannini 108 Nevertheless, there are reports of later neurocognitive benefits in children as a result of higher omega-3 exposure early in life.Reference Bakker, Hornstra, Blanco and Vles 109 , Reference Helland, Smith, Saarem, Saugstad and Drevon 110 Despite the inconsistencies in the literature, it still seems important that pregnant and breastfeeding women and infants consuming formula instead of breast milk have adequate intakes of omega-3 fatty acids, especially DHA. The balance of EPA to DHA is believed to be important and this is reflected in regulations for the composition of infant formula, although the optimal balance is not clear.
Early life effects of omega-3 fatty acids go beyond eye and brain development and function. EPA and DHA are important structural and functional components of other systems including the cardiac, vascular and immune systems. Hence, an appropriate supply of omega-3 fatty acids influences the development and early and later functioning of these systems. For example, there is some evidence that increased intake of EPA and DHA during human pregnancy has an effect on the developing immune system of the baby, modulating various T cell phenotypes, and that this may reduce allergic symptoms later in life.Reference Miles and Calder 111
Because of effects of omega-3 fatty acids on eicosanoids involved in uterine contraction and cervical ripening, a diet rich in these fatty acids can prolong pregnancy by several daysReference Olsen, Sorensen and Secher 112 and can result in larger babies with greater head circumference. Moreover, omega-3 fatty acids have been reported to protect against early delivery.Reference Olsen and Secher 113
Conclusions: the emerging field of periconceptional medicine
The impact of the DOHaD concept on clinical and academic obstetrics and gynecology continues to grow. As highlighted in this article, many of the factors which will determine long-term development, health and fertility are active during the periconceptional and antenatal periods, and this presents both challenges and opportunities to clinicians caring for women, and their concepti during this crucial phase. Despite the growing body of evidence about the importance of preconceptional care, both provision and uptake is still low.Reference Mazza, Chapman and Michie 114 A survey of general practitioners identified a number of barriers to preconception care; women not presenting before pregnancy; time constraints; and limited unbiased resources from credible organizations for both the patient and the GP.Reference Mazza, Chapman and Michie 114 It is recognized that a large number of women use the internet to look for preconception advice.Reference Larsson 115 Recently, in The Netherlands, Steegers-Theunissen and her team have developed an e-health intervention, which assesses risk factors and then provides coaching by means of a readily accessible smart phone app. Such strategies may hold promise for effective and timely implementation of preconceptional care.
At the interface of reproductive medicine and perinatology, two subspecialities within O and G, which have diverged in recent years, a new field, which resonates with Barker’s insights is emerging. As obstetricians recognize that perinatal complications have their routes in the peri-implantation period, and fertility specialists understand the long-term impact of their interventions, both are seeing the potential opportunities of focusing diagnosis and interventions in this early phase of pregnancy. Periconceptional Medicine represents a new area in which expertise in obstetrics, reproductive medicine, (epi)genetics, nutrition and reproductive epidemiology can come together to increase our understanding of the key mechanisms underlying periconceptional determinants of health, and provide the scientific rationale to design and test clinical and lifestyle interventions aimed at improving long-term health.Reference Calder 100
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Research conducted by AJK, PCC, ITC and NSM is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre.
Conflicts of Interest
None of the authors have any conflict of interest to declare.