Introduction
Uteroplacental insufficiency causes much of the intrauterine growth restriction observed in Western society and increases the predisposition to adult metabolic diseases.Reference Barker, Osmond, Golding, Kuh and Wadsworth1–Reference McMillen and Robinson3 Furthermore, fetal growth restriction in other species can also impair whole body insulin sensitivity, insulin secretion and glucose tolerance in offspring.Reference Simmons, Templeton and Gertz4–Reference Owens, Gatford and De Blasio6 Type 2 diabetes is characterized by compromised insulin inhibition of hepatic glucose production and stimulation of skeletal muscle glucose uptake, as well as impaired insulin secretion from the β-cell.Reference Kahn7 In fact, Type 2 diabetes is increasingly regarded as a disease of insulin insufficiency with failure to compensate for insulin resistance, due to inherent pancreatic β-cell dysfunction and reduced β-cell mass.Reference Masiello8, Reference Elayat, el Naggar and Tahir9 Local and systemic inflammation may be another important mechanism in the development of Type 2 diabetes in many individuals.Reference de Lemos, Reis and Baptista10 Increased circulating C-reactive protein (CRP) as a marker of systemic low-grade inflammation is associated with β-cell dysfunction and is an independent predictor of diabetes.Reference D’Alessandris, Lauro, Presta and Sesti11, Reference Goldberg12
Bilateral uterine vessel ligation in late gestation of rats to induce uteroplacental insufficiency restricts fetal growth and induces insulin secretory defects leading to impaired glucose tolerance in adult male offspring who are cross-fostered onto Control dams.Reference Simmons, Templeton and Gertz4, Reference Vuguin, Raab, Liu, Barzilai and Simmons5 When we cross-foster Restricted male offspring onto a different Restricted mother, this also adversely impacts on insulin secretion and glucose control compared to Control-on-Control male offspring.Reference Siebel, Mibus and De Blasio13 In contrast, we have demonstrated that glucose tolerance, peripheral insulin sensitivity and relative adiposity are unchanged in growth-restricted offspring induced by bilateral uterine vessel ligation that remain with their biological mothers.Reference Wadley, Siebel and Cooney14 We have shown that following uteroplacental insufficiency in late gestation, dams produce less milk per pup with altered composition.Reference Wlodek, Ceranic, O’Dowd, Westcott and Siebel15 Improving postnatal nutritional quality and quantity, however, by cross-fostering growth-restricted offspring onto a Control mother partially corrected the impaired glucose tolerance and compromised insulin secretion in Restricted-on-Restricted male offspring. Hence, adult glucose control in these rats may be modifiable by the early postnatal environment.
In rodents, regulation of β-cell mass is particularly susceptible to nutritional and hormonal disturbances during late gestation when the majority of β-cell formation occurs.Reference Schwitzgebel, Somm and Klee16, Reference Stoffers, Desai, DeLeon and Simmons17 Growth and remodeling of pancreatic β-cells continue into the immediate postnatal period in the rat.Reference Scaglia, Cahill, Finegood and Bonner-Weir18 It is during this time that the perinatal β-cell population, which is somewhat unresponsive to glucose, is replaced with mature β-cells, which are highly glucose responsive.Reference Masiello8 Growth-restricted offspring are commonly born with a reduced β-cell mass that is less capable of proliferating at a normal rate, due to altered β-cell gene transcription and metabolism.Reference Simmons, Templeton and Gertz4, Reference Kahn19, Reference Messer and I’Anson20 We have recently shown that the percentage of pancreatic islets per whole pancreas and β-cell mass was reduced by 45–65% in male rats exposed to uteroplacental insufficiency that remained with their biological mothers, when compared to Controls at 9 and 24 weeks of age.Reference Gallo, Wlodek, McConell, Laker and Siebel21 As greater metabolic stress is exerted later in life, β-cell exhaustion of the limited population may occur, leading to the impairment of glucose tolerance.Reference Masiello8 The question that remains, however, is whether β-cell mass and key underlying molecular determinants can be restored or normalized by improving postnatal nutrition via cross-fostering. The latter include insulin-like growth factors (IGFs), vascular endothelial growth factor (Vegf) and the transcription factor, pancreatic duodenal homeobox-1 (Pdx1).Reference Gallo, Wlodek, McConell, Laker and Siebel21–Reference Chamson-Reig, Thyssen, Arany and Hill29 β-cell mass expansion is reliant on normal glucose uptake in the β-cell via the glucose transporter (Glut2) and the preservation of insulin signaling, including the downstream protein kinase, v-akt murine thymoma viral oncogene homolog 2 (Akt2). Upon insulin binding to its receptor in the liver, activation of Akt2 inhibits transcription of phosphoenolpyruvate carboxykinase (Pepck) and glucose-6-phosphatase, thus inhibiting hepatic glucose production.Reference Vuguin, Raab, Liu, Barzilai and Simmons5, Reference Kiraly, Bates and Kaniuk30–Reference Tanaka, Gleason, Tran, Harmon and Robertson33 These mechanisms are also required to maintain adaptive hyperinsulinemia during worsening insulin sensitivity.
In this study, we explored the effects of uteroplacental insufficiency on endocrine pancreas morphology, intrinsic β-cell and metabolic function and its molecular determinants in the pancreas of cross-fostered male offspring at 6 months of age. In addition, we examined molecular determinants of metabolic function in the pancreas one week after cross-fostering to determine the earlier effects of growth restriction. We also examined molecular determinants of hepatic contributions to metabolic control at these ages. We hypothesized that cross-fostering Restricted offspring onto Control mothers would rescue the pancreatic phenotype via differential regulation of its molecular determinants. Our previous studies have shown a clear metabolic phenotype in adult male cross-fostered offspring.Reference Siebel, Mibus and De Blasio13 Of particular relevance to this study was the deficit in first-phase insulin secretion with concomitant impaired glucose tolerance in Restricted-on-Restricted males, which was absent in female offspring. This was then ameliorated by improving postnatal nutrition, by cross-fostering Restricted male pups onto a Control mother.Reference Siebel, Mibus and De Blasio13 Therefore, this study analyzed samples from male offspring only. This is the first time that this characterization has been performed in a well-established rat model of growth restriction, incorporating cross-fostering protocols to delineate both prenatal and postnatal influences.
Method
Animals and growth measurements
All experiments were approved by The University of Melbourne Animal Ethics Committee prior to commencement. Wistar Kyoto rats (11 weeks of age) were obtained from the Australian Resource Centre (Murdoch, WA, Australia). They were provided with 12 h light/dark cycle and had access to food and water ad libitum. On day 18 of gestation, pregnant rats were randomly allocated to Restricted or Control (sham surgery) groups. The Restricted group underwent bilateral uterine artery and vein ligation to induce uteroplacental insufficiency as described previously.Reference Wlodek, Mibus and Tan34–Reference Wlodek, Westcott and O’Dowd36 Although the uteroplacental insufficiency surgery induced a reduction in litter size, our previous studies have shown no clear effect on metabolic outcomes of including a matched litter size control in addition to a sham-surgery control.Reference Siebel, Mibus and De Blasio13 Pups were cross-fostered one day after birth onto a different sham-operated or Restricted mother as previously described.Reference Siebel, Mibus and De Blasio13, Reference Wlodek, Mibus and Tan34, Reference Di Nicolantonio, Koutsis, Westcott and Wlodek37, Reference Di Nicolantonio, Koutsis, Westcott and Wlodek38 This generated four experimental groups: (Pup-on-Mother) Control-on-Control, Control-on-Restricted, Restricted-on-Control and Restricted-on-Restricted, with a similar number of male and female pups in each litter. Offspring studied at 6 months of age were weaned at postnatal day 35, as in previous studies with only one randomly selected male offspring from each litter studied from eight independent litters (n = 8 total offspring studied).Reference Siebel, Mibus and De Blasio13, Reference Wlodek, Mibus and Tan34, Reference Wlodek, Westcott and Serruto39 A separate cohort was generated on postnatal day 7 in which tissues collected from male offspring were pooled within the same litter (n = 8 total litters studied), with weights and dimensions also recorded.
Post-mortem tissue and blood collection and analyses
Non-fasted male rats analyzed at 6 months of age were anesthetized with an intraperitoneal injection of Ketamine (30 mg/kg body weight) and Ilium Xylazil-20 (225 mg/kg body weight). Blood was collected via cardiac puncture at post-mortem (6 months) and plasma stored at −20°C. The whole pancreas was rapidly excised, weighed and a representative portion (∼1 cm3) from the hepatic end was fixed in 10% NBF for immunohistochemical analyses at 6 months. The liver and remaining pancreatic tissue at 6 months, and the liver and whole pancreata at 7 days pooled from males of the same litter, were snap-frozen in liquid nitrogen and stored at −80°C. Circulating CRP, total cholesterol, non-esterified fatty acids (NEFA), pancreatic-amylase and triglycerides were measured in duplicate by colorimetric enzymatic analysis on an automated centrifugal analyser (COBAS Mira, Roche Diagnostics, Castle Hill, NSW, Australia) in non-fasted plasma samples collected at post mortem at 6 months. Although all assays were designed for measuring specified metabolites in human plasma, validation confirmed 100% cross-reactivity with rat plasma in our analyses. The lower detection limits (sensitivity) and ranges for each of the assays were as follows: CRP 0.4–239 ng/ml; total cholesterol 3.43–5.45 mmol/l; NEFA 0.81–1.24 mmol/l; pancreatic-amylase 3–2386 mmol/l; triglycerides 1.23–2.52 mmol/l.
Immunohistochemistry and morphometric analysis
At 6 months, one pancreatic section (10 μm; n = 5–8 per group) was immunostained for insulin using polyclonal guinea-pig anti-rat insulin as previously described.Reference Gatford, Mohammad and Harland25 Samples were code-blinded to remove potential bias while performing analyses. Stained sections were visualized using a Light Zeiss microscope, camera and software (AxioCam MRc5, Carl Zeiss Pty. Ltd, North Ryde, NSW, Australia) at 20 × magnification. For each section, pancreatic islet number and area were expressed relative to total sectional area (per mm2) with islet area arbitrarily divided into small (<5000 μm2), medium (5000–10,000 μm2) and large (>10,000 μm2), similar to Chamson-Reig et al.Reference Chamson-Reig, Thyssen, Arany and Hill29 Random-systematic sampling was then used to select 50 fields per section and relative islet and β-cell volume density (Vd) were quantified by point-counting morphometry using a 700-point grid (700 points/field, Vd equals the number of intercepts on an islet or insulin-positive cells as a proportion of intercepts on a pancreas).Reference Gatford, Mohammad and Harland25 Under the assumption that 1 cm3 tissue weighs approximately 1 g, Vd and pancreatic weight can be multiplied to determine the absolute islet and β-cell mass, expressed in milligrams.Reference Bonner-Weir40 Percent of islet occupied by β-cells was also determined.
Real-time polymerase chain reaction (PCR) analysis
Total RNA was extracted from the pancreata and liver at postnatal day 7 and 6 months using the Micro-to-Midi Total RNA Purification System kit (Invitrogen Life Technologies, Carlsbad, CA, USA). Reverse transcription and real-time PCR were performed as previously described.Reference Wlodek, Westcott and O’Dowd36, Reference Wlodek, Westcott, Siebel, Owens and Moritz41 Primers and TaqMan® probes (Biosearch Technologies, Novato, CA, USA) were designed from GenBank gene sequences for 18S, Akt2, Glut2, Hprt1, Igf1, Igf1r, Insr, Pdx1, Pepck and Vegf (see Table 1). Optimal concentration for forward and reverse primers was 300 nM and for TaqMan® probes it was 100 nM. Results were analyzed using the sequence detector software (Rotor-Gene v6, Corbett Research, Mortlake, NSW, Australia). Relative quantification of gene expression was performed by the comparative threshold cycle (ΔΔCT) method with 18S rRNA and hypoxanthine guanine phosphoribosyl transferase-1 (Hprt1) optimized and validated as suitable endogenous controls for the liver and pancreas, respectively.
Table 1 GenBank accession numbers, primer and probe sequences for genes quantified using real-time PCR relative to 18S or Hprt1

Statistical analyses
All measures were analyzed using one-way ANOVA, with Duncan’s post hoc analysis where appropriate. Relationships between size at birth, gene expression, pancreatic morphology and other outcomes were analyzed using Pearson’s correlations (n = 20–32). All data were normally distributed and presented as mean ± s.e., with the level of significance set at P < 0.05.
Results
Body, organ weights and dimensions
The effects of uteroplacental insufficiency on litter size and birth weight have been previously reported.Reference Siebel, Mibus and De Blasio13, Reference Wlodek, Mibus and Tan34, Reference Wlodek, Westcott and O’Dowd36, Reference Wlodek, Westcott and Serruto39, Reference Wlodek, Westcott, Siebel, Owens and Moritz41 Briefly, uteroplacental insufficiency reduced (P < 0.05) total litter size (mean ± s.e.; 5.6 ± 0.5 v. 8.2 ± 0.7) and male offspring birth weight (3.6 ± 0.1 g v. 4.3 ± 0.1 g) compared to sham-operated Controls. Restricted-on-Control and Restricted-on-Restricted remained lighter (P < 0.05) than Control-on-Control males at post mortem on postnatal day 7 and 6 months (Table 2). Restricted-on-Restricted males had shorter crown rump length (−13.5%, P < 0.05), head length (−12%, P < 0.05) and hind limb length (−14%, P < 0.05) compared to Controls on postnatal day 7, whereas Restricted-on-Control head length and hind limb length were intermediate between Restricted-on-Restricted and Control-on-Control demonstrating a positive effect of improved lactation on growth (Table 2). This cross-foster effect on growth was absent by 10 weeks as reported previously.Reference Siebel, Mibus and De Blasio13 Absolute, but not relative, pancreas and liver weights were lower (P < 0.05) in Restricted-on-Control and Restricted-on-Restricted than Control-on-Controls on postnatal day 7 (Table 2). Absolute and relative pancreas and liver weights were not different across groups at 6 months of age (Table 2).
Table 2 Effect of prenatal and postnatal growth restriction and cross-fostering on body weight, dimensions and organ weights on postnatal day 7 and at 6 months
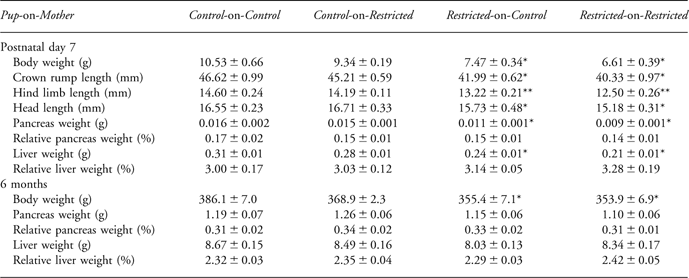
Body weight, crown rump length, hind limb length, head length, absolute pancreas and liver weight, relative pancreas and liver weight measured for male offspring. All data are expressed as mean ± s.e. (n = 8).
*Indicates significantly different (P < 0.05) to Control-on-Control.
**Indicates significantly different (P < 0.05) to all other groups.
Plasma analyses
At 6 months, non-fasted plasma CRP in Restricted-on-Restricted and Control-on-Restricted offspring was 45–47% higher (P < 0.05) than in Control-on-Control (Fig. 1a). There were no differences in circulating concentrations of total cholesterol, NEFA, pancreatic-amylase or triglycerides (Fig. 1b–e).
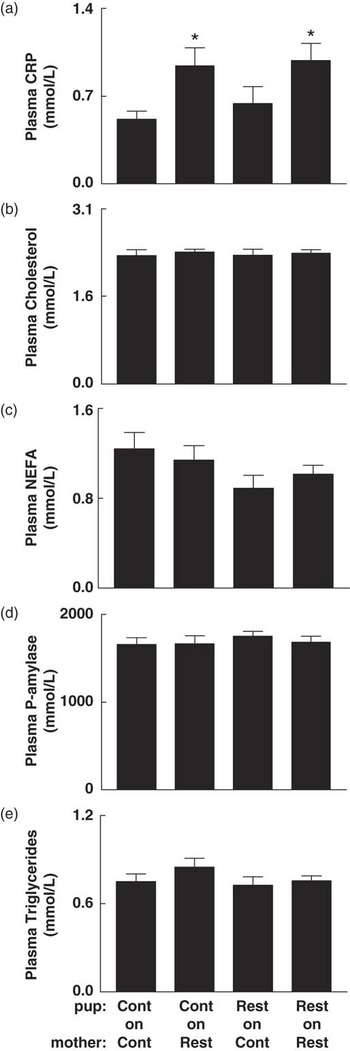
Fig. 1 The effect of prenatal and postnatal growth restriction and cross-fostering on plasma metabolite concentrations in non-fasted male offspring at 6 months. (a) C-reactive protein (CRP), (b) total cholesterol, (c) non-esterified fatty acids (NEFA), (d) pancreatic-amylase and (e) triglycerides measured at 6 months of age for male offspring. Cont = Control; Rest = Restricted, Pup-on-Mother. All data are expressed as mean ± s.e. (n = 8/group). *Indicates significantly different (P < 0.05) to Control-on-Control.
Endocrine pancreatic morphology
Pancreatic β-cells comprised 73–77% of total islet mass at 6 months of age with no differences across groups, which is comparable to that previously reported.Reference Ackermann and Gannon42, Reference Edlund43 Mean pancreatic islet number was 51% less (P < 0.05) in adult Restricted-on-Restricted male offspring than Controls (Fig. 2a). This substantial decrease in islet number in Restricted-on-Restricted males was in parallel with decreased (P < 0.05) absolute islet (−62%) and β-cell (−65%) mass compared to Control-on-Control males at 6 months (Fig. 2b and c). Exposing Restricted offspring to improved lactation attenuated these decreases, with increased (+60%; P < 0.05) islet and β-cell mass in Restricted-on-Control males when compared to Restricted-on-Restricted, equating to 95–98% of Control-on-Control pancreatic mass. Importantly, we observed comparable outcomes when islet and β-cell mass were expressed as absolute or relative to body weight (data not shown). There were no differences in the relative number of small, medium or large islets across groups at 6 months (Fig. 2d). Birth weight correlated positively with the average number of total islets (r = 0.566, P = 0.005, n = 23), average number of small islets (r = 0.522, P = 0.011, n = 23) and proportion of β-cells per islet (r = 0.538, P = 0.010, n = 22, data not shown). Both absolute (r = 0.581, P = 0.005, n = 21) and relative (r = 0.543, P = 0.009, n = 21) β-cell mass correlated positively with post-mortem body weight, despite having no direct relationship to birth weight (data not shown).
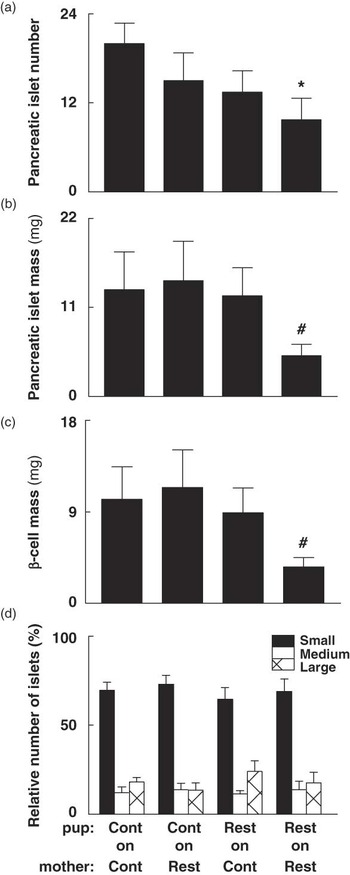
Fig. 2 The effect of prenatal and postnatal growth restriction and cross-fostering on (a) islet number per section area, (b) absolute islet mass, (c) absolute β-cell mass and (d) percentage of small, medium and large islets in male offspring at 6 months. Small (<5000 μm2), medium (5000–10,000 μm2) and large (>10,000 μm2) pancreatic islets relative to the total number of islets. Cont = Control; Rest = Restricted, Pup-on-Mother. All data are expressed as mean ± s.e. (n = 6–8 for all groups). *Indicates significantly different (P < 0.05) to Control-on-Control. Indicates significantly different (P < 0.05) to all other groups.
Pancreatic and hepatic gene expression
Pancreatic gene expression of Glut2, Igf1r, Insr, Pdx1 and Vegf was elevated (P < 0.05) in Restricted-on-Control offspring on postnatal day 7 compared to Control-on-Controls (Fig. 3). Pancreatic Insr and Igf1r mRNA expression were also upregulated (P < 0.05) in Restricted-on-Restricted offspring at this age compared to Control-on-Controls (Fig. 3). In contrast, there were no differences in pancreatic gene expression observed at 6 months, whether expressed in absolute terms (Fig. 4) or relative to β-cell mass (data not shown). Furthermore, absolute and relative β-cell mass did not correlate with pancreatic gene expression at 6 months (data not shown). Restricted-on-Restricted offspring had decreased (P < 0.05) hepatic Glut2 mRNA compared to Control-on-Controls on postnatal day 7 with no other differences identified on postnatal day 7 or at 6 months (Table 3).
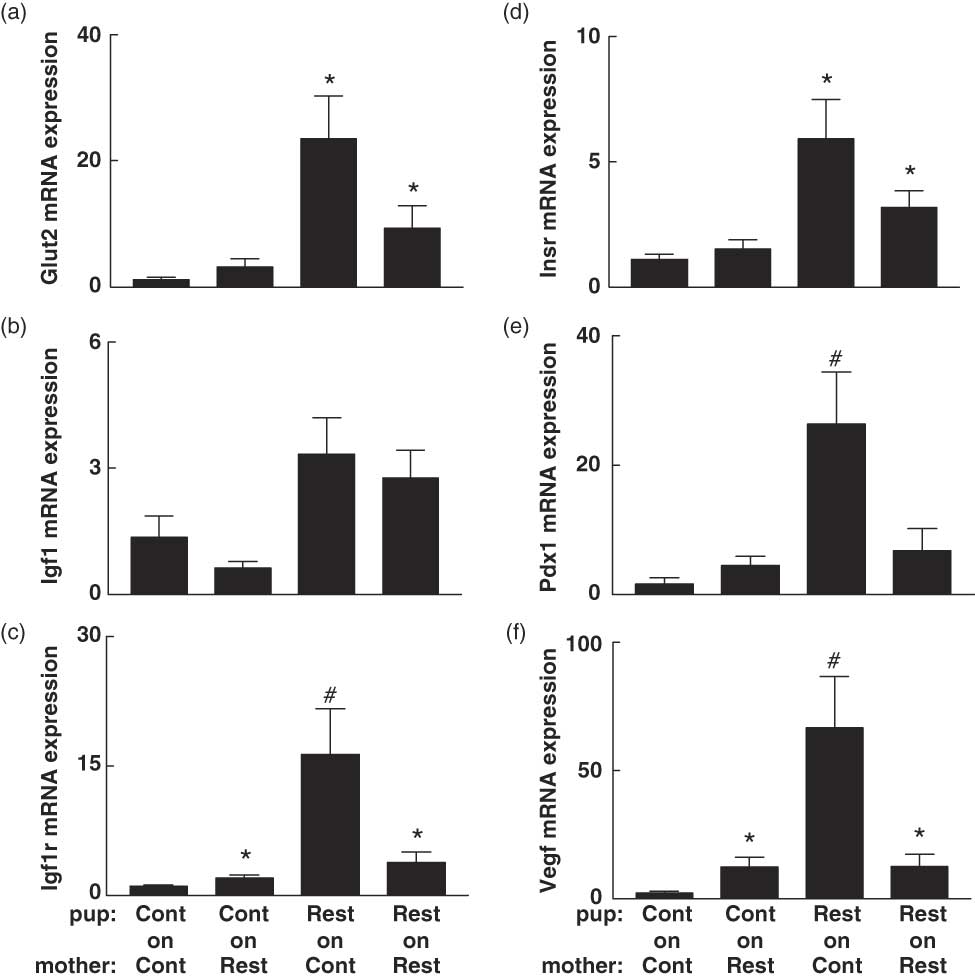
Fig. 3 The effect of prenatal and postnatal growth restriction and cross-fostering on pancreatic (a) Glut2; (b) Igf1; (c) Igf1r, insulin-like growth factor-1 receptor; (d) Insr, insulin receptor; (e) Pdx1, pancreatic duodenal homeobox-1 and (f) Vegf, vascular endothelial growth factor mRNA expression relative to the endogenous reference gene (Hprt1) in postnatal day 7 male offspring. Cont = Control; Rest = Restricted, Pup-on-Mother. All data are expressed as mean ± s.e. (n = 6–8 for all groups). *Indicates significantly different (P < 0.05) to Control-on-Control. Indicates significantly different (P < 0.05) to all other groups.
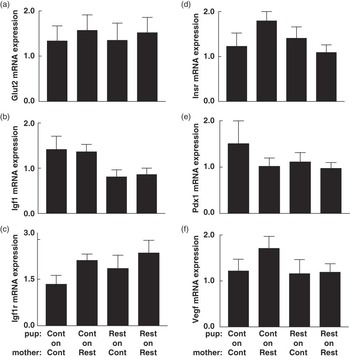
Fig. 4 The effect of prenatal and postnatal growth restriction and cross-fostering on pancreatic (a) Glut2; (b) Igf1; (c) Igf1r, insulin-like growth factor-1 receptor; (d) Insr, insulin receptor; (e) Pdx1, pancreatic duodenal homeobox-1 and (f) Vegf, vascular endothelial growth factor mRNA expression relative to the endogenous reference gene (Hprt1) in male offspring at 6 months. Cont = Control; Rest = Restricted, Pup-on-Mother. All data are expressed as mean ± s.e. (n = 6–8 for all groups). There were no differences in pancreatic gene expression across groups at 6 months.
Table 3 Effect of prenatal and postnatal growth restriction and cross-fostering on hepatic mRNA expression on postnatal day 7 and at 6 months
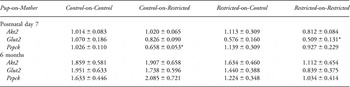
Akt2, v-akt murine thymoma viral oncogene homolog-2; Glut2, glucose transporter-2; Pepck, phosphoenolpyruvate carboxykinase. Values are expressed as mean ± s.e. (n = 8 per group), relative to a calibrator (the control group).
*Indicates significantly different (P < 0.05) to Control-on-Control.
Discussion
Despite previously reporting hypoinsulinemia and impaired glucose tolerance in Restricted-on-Restricted rats,Reference Siebel, Mibus and De Blasio13 there is no adverse effect on intrinsic β-cell function, estimated by insulin secretion relative to β-cell mass in this study. This suggests that the lower β-cell mass in overall fewer islets in these offspring impairs the ability of the pancreas to meet normal metabolic demands following uteroplacental insufficiency. Importantly, we have shown that improving lactation for Restricted-on-Control male rat offspring can attenuate deficits in adult pancreatic islet number, islet mass and β-cell mass induced by uteroplacental insufficiency. Together, these data support previous studies in the rat, in which growth-restricted offspring had reduced pancreatic islet number and β-cell mass in adulthood,Reference Simmons, Templeton and Gertz4, Reference Kahn19, Reference Messer and I’Anson20 as well as our previous findings of improved glucose tolerance in Restricted-on-Control rats.Reference Siebel, Mibus and De Blasio13 Furthermore, the average number of total islets, small islets and proportion of β-cells per islet correlated positively with birth weight in our adult male offspring. However, these deficits in endocrine pancreatic morphology were in the absence of any changes in regulatory gene expression in the whole pancreas or relative adiposity.
Male offspring born small and provided with improved milk quality and quantity (Restricted-on-Control)Reference Wlodek, Ceranic, O’Dowd, Westcott and Siebel15 showed a marked increase in relative pancreatic gene expression of the β-cell specific transcription factor (Pdx1) and growth factors (Igf1r and Vegf) on postnatal day 7 compared to all other groups. This upregulation above that of the Control-on-Control male pups may be a compensatory response following exposure to a poor intrauterine environment, in the face of improved postnatal nutrition. Following this lactational improvement and upregulation of pancreatic genes early in life, pancreatic islet and β-cell mass in Restricted-on-Control offspring were increased when compared to Restricted-on-Restricted offspring at 6 months of age. This correlates well with the improved metabolic function in these Restricted-on-Control male adults from the same rats.Reference Siebel, Mibus and De Blasio13 Therefore, this demonstrates that both the intrauterine and lactational environments are important determinants of adult endocrine pancreatic morphology and metabolic function.
It has been well-documented that increased weight gain between the ages of 2 and 7 years in low birth weight children independently predicts an increased risk of cardiovascular disease, obesity and diabetes in adulthood.Reference Eriksson, Forsen, Tuomilehto, Osmond and Barker44–Reference Parsons, Power and Manor47 Furthermore, placental restriction in the sheep is associated with rapid catch-up growth during the 1st month of life resulting in visceral obesity and insulin resistance.Reference Owens, Gatford and De Blasio6, Reference De Blasio, Gatford, McMillen, Robinson and Owens48 In previous studies using the Sprague-Dawley rat, uteroplacental insufficiency induced by bilateral uterine artery ligation resulted in growth-restricted offspring catching up in weight to Controls by 7 weeks of age with concomitant mild glucose intolerance, when fostered onto an unoperated mother after birth.Reference Simmons, Templeton and Gertz4, Reference Lane, Chandorkar, Flozak and Simmons49 By 10 weeks of age, these rats weighed more than Controls, developed insulin resistance and were obese with virtually absent acute first-phase insulin secretion by 6 months.Reference Simmons, Templeton and Gertz4 In contrast, we observed no accelerated growth or obesity in our cross-fostered Restricted offspring, and no evidence of insulin resistance following an insulin tolerance test,Reference Siebel, Mibus and De Blasio13 supported by no alterations in key hepatic genes involved in the insulin-signaling cascade at 6 months of age. A reduction in hepatic Pepck mRNA expression in the Control-on-Restricted offspring on postnatal day 7 may be suggestive of impaired hepatic glucose production. However, no further glucose or insulin tolerance testing has been performed in this cohort at this age. These differences in growth profiles and subsequent metabolic control are potentially due to a number of factors including strain differences (Wistar-Kyoto v. Sprague-Dawley rats) in response to the bilateral uterine vessel ligation surgery, cross-fostering, varying litter sizes, altering lactation and increased adiposity.
Altered growth profiles in late gestation, early postnatal life and the environments that induce these can severely impact on adult metabolic health, as previously reviewed.Reference McMillen and Robinson3, Reference Holemans, Aerts and Van Assche50 The critical period around birth is important for the development and later function of pancreatic β-cells, the primary determinants of glucose tolerance, in a number of different species and paradigms.Reference Masiello8 Nutritional perturbations, such as uteroplacental insufficiency and maternal undernutrition during gestation and lactation in rodents, can adversely affect endocrine pancreas development, resulting in reduced β-cell mass,Reference Simmons, Templeton and Gertz4, Reference De Prins and Van Assche51–Reference Garofano, Czernichow and Breant53 impaired glucose tolerance and first-phase insulin secretion.Reference Simmons, Templeton and Gertz4, Reference Garofano, Czernichow and Breant53 It appears that the insulin secretion deficiency and resulting impaired glucose tolerance in our adult male rats with prenatal and postnatal restraint may be due to a pancreatic β-cell mass deficit programmed around birth, since there was no impairment in intrinsic β-cell function. The importance of the immediate postnatal environment for later metabolic function is highlighted in a study which exposed growth-restricted rat offspring to the long-acting glucagon-like peptide-1 analog, Exendin-4, in early postnatal life.Reference Stoffers, Desai, DeLeon and Simmons17 They showed that the rate of β-cell proliferation was normalized, reversing the adverse consequences of perinatal insults, thereby preventing the development of diabetes in adulthood.Reference Stoffers, Desai, DeLeon and Simmons17 This strongly supports our results, which clearly show amelioration of pancreatic deficits following cross-fostering of Restricted offspring onto a Control mother.
It seems likely that the intrauterine nutritional environment can not only program β-cell mass in offspring via altered organogenesis of the endocrine pancreas but also change to key lineage determinants, such as Pdx1.Reference Chamson-Reig, Thyssen, Arany and Hill29 There is evidence that intrauterine growth restriction induced by uteroplacental insufficiency can both increase and decrease Pdx1 mRNA expression within the pancreas depending upon the stage of development and life examined.Reference Lesage, Blondeau, Grino, Breant and Dupouy54 Stoffers et al. observed that Pdx1 mRNA in the pancreatic islet is reduced by more than 50% before birth, remains downregulated postnatally and is almost absent by adulthood in growth-restricted rats.Reference Stoffers, Desai, DeLeon and Simmons17 This initial downregulation and sustained repression of Pdx1 gene transcription in the pancreatic islet appears to be acting via epigenetic modifications, particularly histone deacetylation and methylation that generally suppress gene transcription.Reference Park, Stoffers, Nicholls and Simmons55 Furthermore, this process is reversible at the neonatal stage, defining a critical developmental window for potential intervention. Presently, however, we found no evidence of uteroplacental insufficiency suppressing Pdx1 gene transcription or of any other growth factor or functionally relevant gene studied in the whole early postnatal or adult pancreas. This may be complicated by the fact that pancreatic islets were not isolated and that the pancreas predominantly comprises exocrine tissue. Although pancreatic and hepatic gene expression does not appear to be adversely affected by uteroplacental insufficiency or cross-fostering at 6 months of age, whole pancreatic Pdx1, Igf1r and Vegf mRNA expression was increased in Restricted-on-Control pups when compared to Restricted-on-Restricted at postnatal day 7. This upregulation of key genes did not persist into adulthood, suggesting that it was a transitory response to improved milk quality and quantity, but one that nevertheless appears to prevent later structural and functional deficits observed in adult Restricted offspring exposed to a poor lactational environment.
Adult growth-restricted offspring born to mothers with 50% global food reduction throughout pregnancy have increased plasma CRP levels, despite being nursed by Control dams.Reference Magee, Han and Cherian56 Although it is not known how CRP specifically affects insulin signaling and β-cell function, the increased circulating CRP levels in both the Control-on-Restricted and Restricted-on-Restricted male offspring at 6 months of age may be predictive of low-grade systemic inflammation and later impaired glucose tolerance.Reference de Lemos, Reis and Baptista10, Reference D’Alessandris, Lauro, Presta and Sesti11 When lactation was improved in Restricted male offspring cross-fostered onto a Control mother, circulating CRP concentrations returned to Control-on-Control levels, concomitant with restored insulin secretion and glucose tolerance as reported earlier.Reference Siebel, Mibus and De Blasio13 Considering that we previously found that Restricted-on-Restricted male offspring were not insulin resistant or obese,Reference Siebel, Mibus and De Blasio13 it was not surprising that there were no other differences in circulating total cholesterol, NEFA or triglycerides.
In summary, we have shown that adult male offspring exposed to uteroplacental insufficiency followed by a poor lactational environment (Restricted-on-Restricted) have endocrine pancreatic mass deficits that are ameliorated by improved lactation (Restricted-on-Control). We have characterized an underlying deficit in the mass of the endocrine pancreas that may be responsible for the insulin secretion deficiency and impaired glucose tolerance observed in these growth-restricted adult male offspring. Further determination of the mechanisms involved in this amelioration by improved early postnatal nutrition may provide additional insight into other early life interventions that can modulate these long-term consequences for low birth weight offspring.
Acknowledgments
The authors wish to thank Mr Chris Chiu and Dr Miles De Blasio for technical assistance with real-time PCR and COBAS analysis, respectively. The authors also thank Kerryn Westcott for professional assistance with animal surgery and handling. ALS present contact address: Baker IDI Heart & Diabetes Institute, Victoria, Australia. This research was supported by a grant from the National Health and Medical Research Council of Australia to MEW (NHMRC; no. 400004), The University of Melbourne ECR grant to ALS and ANZ trusts grant to ALS. ALS was supported by an NHMRC Peter Doherty Biomedical Research Fellowship.
Statement of Interest
None.









