The rate of conceiving twins has increased over the last 40 years in part due to older maternal age, and more recently from greater maternal body mass index and use of assisted reproductive technologies (ART).Reference Hoekstra, Zhao and Lambalk 1 Twinning is associated with greater rates of complications during pregnancy and delivery for the mother and twins, compared with singleton pregnancies. For example, compared with women carrying singletons, women carrying twins or other higher order multiples have up to five times the risk of developing preeclampsia, a common cause of maternal and fetal morbidity.Reference Ros, Cnattingius and Lipworth 2
In addition to risks in the perinatal period, twinning may be associated with subsequent cancer risk in the motherReference Kim, Leyva and Diano 3 and in the adult offspring.Reference Xue and Michels 4 Results of epidemiologic studies comparing breast cancer risk in the mothers of twins and the mothers of singletons have been inconsistent,Reference Nechuta, Paneth and Velie 5 although data from several large, Scandinavian cohort studies, mainly in younger women,Reference Albrektsen, Heuch and Kvale 6 – Reference Ji, Forsti, Sundquist and Hemminki 10 have suggested a protective effect of multiple births, with decreases in maternal breast cancer risk of 10–30%. Twins themselves have been hypothesized to be at increased risk of breast cancer owing to higher hormone concentrations deriving from two placentae.Reference Xue and Michels 4 , Reference Ekbom, Hsieh, Lipworth, Adami and Trichopoulos 11 , Reference Potischman and Troisi 12 Some,Reference Ekbom, Hsieh, Lipworth, Adami and Trichopoulos 11 , Reference Hsieh, Lan and Ekbom 13 – Reference Braun, Ahlbom, Floderus, Brinton and Hoover 16 but not allReference Sanderson, Daling, Doody and Malone 17 – Reference Verkasalo, Kaprio, Pukkala and Koskenvuo 19 studies show elevations in breast cancer risk in twins compared with singletons. Increases in placenta derived hormones in twin pregnancies have been hypothesized to mediate the differences in cancer risk,Reference Ekbom, Hsieh, Lipworth, Adami and Trichopoulos 11 but there are scant data addressing this mechanism, particularly in the fetal circulation.
The biological mechanisms underlying the association of twinning and altered breast cancer risk are unknown. To determine if twinning is associated with a distinct hormonal exposure in mothers and children, we compared the concentrations of several placenta-derived hormones that are also implicated in breast cancer etiology in the maternal circulation in the third trimester and at delivery, and in the umbilical cord at delivery, between twin and singleton pregnancies.
Methods
Study subjects
Pregnant women >18 years of age who intended to deliver at Dartmouth Hitchcock Medical Center were eligible for the study. Women carrying twin gestations and presenting for prenatal care or hospitalized for antenatal surveillance from 2003 to 2007 were recruited in the third trimester. We attempted blood draws at recruitment and at labor and delivery. The next woman with a singleton pregnancy that met the eligibility criteria and could be matched to the twin pregnancy on gestational age at third trimester blood draw (within 1–2 weeks), parity at the time of the pregnancy (nulliparous/parous) and maternal age (±5 years), in that order, was recruited for the study. Additional women with singleton pregnancies were recruited at admission for labor and delivery and matched to twin mothers according to the criteria above but with gestational age at labor and delivery instead of at third trimester blood draw. As the motivation for the study was to characterize hormones in uncomplicated pregnancies, five twin pregnancies and three singleton pregnancies that developed preeclampsia after enrollment were excluded. The current analysis included 41 twins and a total of 62 singleton controls (40 with blood samples in the third trimester and 52 with blood samples at delivery; some of the prenatal controls were also labor and delivery controls).
The study protocol was approved by institutional review boards at the Geisel School of Medicine (Lebanon, NH) and the U.S. National Cancer Institute, and all participating women gave written, informed consent.
Biospecimen collection and processing
A 10-ml red-top tube of whole blood was collected from the mother in the third trimester (31–39 weeks) and at the earliest possible time after admission for labor and before administration of any medication. Blood was collected from the umbilical cord (henceforth referred to as cord) after delivery. After allowing samples to clot at room temperature, they were centrifuged and the sera were stored at −70°C. Samples were shipped on dry ice to a biorepository in Rockville, MD, where they were stored at −80°C. Placenta were routinely examined by the pathology department to determine chorionicity.
Laboratory assays
Hormones were measured in maternal and cord serum at the Reproductive Endocrine Research Laboratory at the University of Southern California, Keck School of Medicine under the direct supervision of one of us (FZS). Androstenedione and estriol were not measured in the cord because of limited sample volume. Concentrations of androstenedione, testosterone and estradiol were measured by radioimmunoassay (RIA) following extraction with organic solvent and purification by Celite column partition chromatography as described previously.Reference Goebelsmann, Horton and Mestman 20 – Reference Scott, Stanczyk, Goebelsmann and Mishell 22 Estriol was measured by RIA with preceding dual organic solvent extraction steps.Reference Goebelsmann, Horton and Mestman 20 , Reference Goebelsmann, Arce, Thorneycroft and Mishell 23 Insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) were quantified by a solid phase enzyme-labeled chemiluminescent immunometric assay on the Immulite 2000 analyzer (Siemens Healthcare Corporation, Deerfield, IL, USA). The laboratory technicians were blind to quality control samples which constituted 10% of the study batch samples. The within- and between-assay coefficients of variations were 7.3 and 6.8% for androstenedione, 4.8 and 17.5% for testosterone, 5.6 and 14.9% for estradiol, 5.9 and 7.8% for estriol, 3.0 and 6.7% for prolactin, 1.6 and 7.0% for IGF-1 and 2.9 and 3.1% for IGFBP-3.
Clinical data
Information on mother’s age, race/ethnicity, parity, and whether conception was ART assisted, and baby’s sex and birth anthropometrics were abstracted from medical records and a form completed at delivery. Z-scores were developed for birth weight values to account for gestational age and fetal sex using an external standard.Reference Yudkin, Aboualfa, Eyre, Redman and Wilkinson 24 Gestational age was estimated based on current American College of Obstetricians and Gynecologists guidelines 25 and confirmed by ultrasound.
Statistical methods
Clinical characteristics were compared between twin and singleton pregnancies using Student’s t-test for continuous variables, and χ2 test for categorical variables. All concentrations of hormones were log-transformed to normalize their distributions, and are presented on the logarithmic scale. We fit multivariate linear regression models to estimate mean maternal hormone concentrations by twin/singleton status and offspring sex. For estimating sex-specific average maternal hormone concentrations, we restricted to same-sex twins and included an interaction term between twin/singleton status and offspring sex to allow sex effects to differ for twins and singletons. To determine mean differences in cord hormones between singletons and twins, we used a generalized estimating equation (GEE) model with each mother as an outcome cluster and an exchangeable correlation matrix. This approach allowed us to account for the fact that fetal hormone concentrations of related twins are correlated. For all models, maternal hormone concentrations from samples drawn during pregnancy were adjusted for mean-centered gestational week at blood draw, whereas, maternal and cord concentrations from samples drawn at delivery were adjusted for mean-centered gestational week at delivery. We calculated geometric means of the hormones by exponentiating the coefficients from the GEE models. Statistical analyses were performed using Stata 13. 26
Results
Clinical and demographic characteristics
Mothers of twins (33.4 years) were 2.4 years older on average than mothers of singletons (31.0 years), and approximately a third of both twin and singleton mothers were experiencing their first pregnancy (Table 1). Most mothers were white (95.1% of singleton and 97.6% of twin pregnancies, data not shown). Mean gestational week at delivery was earlier among the twin pregnancies (37.1 weeks) compared with the singleton pregnancies (38.5 weeks), as was gestational week at blood draw in the third trimester (32.2 v. 34.2 weeks, respectively). Prevalence of ART use was higher among mothers of twins (39%) than mothers of singletons (8%). Mean birth weight was lower in twins (z-score −0.72) than singletons (z-score 0.20), even when accounting for gestational age and fetal sex.
Table 1 Maternal, perinatal and neonatal characteristics of singleton and twin pregnancies

Summary statistics for characteristics are mean (s.e.) for continuous variables and number (%) for proportions.
a Birth weight is the average of the twins.
Maternal and cord hormones comparing singletons and twins
Maternal hormone concentrations were higher in twin than in singleton pregnancies (Table 2), though differences did not exceed 20% and not all were statistically significant. Differences in cord hormone concentrations between singletons and twins were 5% or less except for IGFBP-3 concentrations, which were 200% lower in twin than singleton pregnancies (Table 3). As a sensitivity analysis we adjusted for maternal prepregnancy body mass index (BMI) and the observed associations remained unchanged.
Table 2 Maternal serum hormone concentrations in the third trimester and at delivery in singleton and twin pregnancies

IGF-1, insulin-like growth factor-1; IGFBP-3, IGF-binding protein-3.
a Third trimester hormones are on the log scale and adjusted for pregnancy week at time of blood draw.
b Hormones at delivery are on the log scale and adjusted for gestational age at delivery.
c Per cent dif (difference) was calculated as (meantwins−meansingletons)/meantwins.
Table 3 Cord serum hormone concentrations in singleton and twin pregnancies
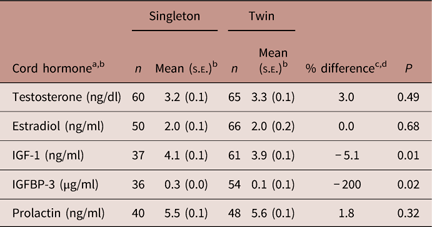
IGF-1, insulin-like growth factor-1; IGFBP-3, IGF-binding protein-3.
a Androstenedione and estriol were not measured in the cord samples because of limited volume.
b All hormones are log-transformed.
c Means were adjusted for gestational week at birth.
d Per cent difference was calculated as (meantwins–meansingletons)/meantwins.
Maternal and cord hormones by chorionicity of twins
Only five of the twin pregnancies were monochorionic and 36 were dichorionic, limiting statistical power to assess differences in hormones by chorionicity. Hormone concentrations were higher in mothers of dichorionic compared with singleton twins during the third trimester, but were more similar at delivery (online Supplementary Table 1). Cord IGF-1 and IGFBP-3 were higher in both dichorionic and monochorionic twins compared to singletons (online Supplementary Table 2).
Maternal hormone concentrations by offspring sex of singletons and twins
In singleton pregnancies, maternal hormone concentrations differed <10% between males and females (online Supplementary Table 3). Androstenedione, however, was 30.8% lower in mothers of females than males (female=0.9 ng/ml and male=1.3 ng/ml; P=0.04) in the third trimester but not different at delivery. In twin pregnancies, there was no difference in third trimester maternal hormones by sex of the twins (online Supplementary Table 4) except for higher estriol in mothers carrying opposite sex twins compared with two male twins (male/female=3.4 ng/ml and male/male=3.1ng/ml; P=0.05). At delivery, the estrogens and androstenedione were higher in mothers carrying twins with at least one female compared with all males (online Supplementary Table 5).
Cord hormone concentrations by offspring sex of singletons and twins
In singletons, only cord testosterone differed by sex with females having concentrations 28.3% lower than males (online Supplementary Table 6). Among twins, hormone concentrations in those with a female co-twin were similar to those with a male co-twin (online Supplementary Table 7). This was also true for males: hormone concentrations were similar in those with a male co-twin compared with those with a female co-twin (online Supplementary Table 8).
Discussion
Hormone concentrations in late gestation in the current study were modestly higher in mothers of twin than singleton pregnancies. There was little difference in cord hormone concentrations except for a large deficit of IGFBP-3 in twins compared with singletons. Maternal and cord androgens were lower in singleton females than males, but there was little evidence of any other differences in maternal or cord hormones by sex of the twins. Analysis of hormones by zygosity was limited by the small number of monozygotic pregnancies in the study.
Previous studies using older assay methodology provided less accurate measurements but, like ours, showed higher circulating estrogenReference Sanderson, Daling, Doody and Malone 27 – Reference Verkasalo, Kaprio, Pukkala and Koskenvuo 29 and testosterone concentrationsReference Thomas, Murphy and Key 29 in women carrying twins. While we found IGFs also to be higher in women carrying twins, previous studies of maternal IGF-1 and IGFBP-3 in twin and singleton pregnancies have suggested no differences.Reference Kazer, Cheng, Unterman and Glick 30 , Reference Langford, Nicolaides and Jones 31 Higher concentrations of these hormones in mothers of twins could be due to gestational weight gain, but we were unable to measure this maternal characteristics in this study.Reference Lof, Hilakivi-Clarke and Sandin 32 – Reference Wuu, Hellerstein and Lipworth 34
Hormone concentrations in the cord were generally similar in the present study, except for substantially lower IGFBP-3 concentrations in twin than singleton pregnancies. IGFBP-3 binds the insulin-like growth factors IGF-1 and IGF-2 with high affinity. Although IGF-1 concentrations in the cord did not differ significantly between twins and singletons in our data, IGF-2 is the major growth factor in fetal development. As IGFBP-3 blocks IGF-2 access to the IGF-1 receptor, lower IGFBP-3 concentrations in twins might allow for the abundant free IGF-2 necessary for growth and development in an environment of shared nutritional resources. Mothers of twins gain more weight during gestation, but the difference in gestational weight gain between mothers of singletons and twins most likely would not account for the observed differences in cord IGFBP-3 because gestational weight gain has been positively associated with cord levels of IGFBP-3.Reference Rifas-Shiman and Fleisch 35
The association of twinning and hormone exposure may depend on chorionicity or zygosity of twins since dizygotic twins, which are also dichorionic pregnancies, are more likely to have two placenta possibly leading to altered hormonal exposures. Differences in estrogen levels between monochorionic and dichorionic pregnancies are not well studied,Reference Kappel, Hansen, Moller and Faaborg-Andersen 36 possibly because monochorionic pregnancies are rare as was the case in our study, which also limited our ability to draw valid conclusions based on the data.
Sex of the co-twin has also been hypothesized to affect hormone exposure for monozygotic and dizygotic twins compared with singletons. For example, the female co-twin of a same sex dizygotic twin pregnancy might be exposed to higher amounts of estrogen than a singleton or female co-twin of a monozygotic twin pregnancy.Reference Xue and Michels 4 Our data suggested that differences in cord hormones by sex of the co-twin was minimal. However, the small sample size of our study is a limitation in that the null findings may reflect a lack of statistical power to detect small differences in hormone concentrations. We attempted to include information on the major twinning risk factors (e.g. age) in the analysis, but other possible confounders that we did not measure, such as genetic predisposition to twinning or gestational weight gain, could have affected our results. Study eligibility was strict and the study sample was ethnically homogeneous so our findings may not be generalizable to other populations.
In conclusion, the association between twinning and hormone concentrations was different in the maternal and cord circulation. Sex hormones were modestly higher in mothers of twin than singleton pregnancies, and there was little difference in the twins and singletons themselves, except for much lower concentrations of IGFBP-3 in the former. In general, these data do not support the hypothesis that twinning leads to altered sex steroid hormone exposure in mothers, although the findings for cord concentrations of IGFBP-3 warrant further investigation given its ability to modulate bioavailability of the major growth hormones, IGF-1 and IGF-2.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174418000697
Acknowledgments
None.
Financial Support
This research was funded by the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services.
Conflicts of Interest
R.T. and R.N.H. are employed by the Federal Government.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards described in the United States Federal Policy for the Protection of Human Subjects and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees at Geisel School of Medicine (Lebanon, NH) and the U.S. National Cancer Institute.





