Introduction
Fetal (FA) and perinatal asphyxia (PA) are major causes of neurological disability and account for ∼23% of all neonatal deaths worldwide.Reference Lawn, Cousens, Darmstadt, Paul and Martines 1 Owing to impaired gas exchange between the mother and fetus, fetal metabolic demands and cellular energy supplies are disturbed.Reference Alonso-Spilsbury, Mota-Rojas and Villanueva-Garcia 2 Consequently, severe asphyxia can result in hypoxic-ischemic encephalopathy (HIE) and multi-organ dysfunction such us hepatic, heart and renal failure.Reference Hankins, Koen and Gei 3 , Reference Shah, Riphagen, Beyene and Perlman 4 Only few studies have investigated hepatic injury after asphyxia, although the liver is usually involved.Reference Karlsson, Blennow, Nemeth and Winbladh 5 Human data revealed increased hepatic enzymes in the serum of asphyxiated newborns. In addition, the enzyme concentrations positively correlated with the severity of HIE and higher mortality rates.Reference Karlsson, Blennow, Nemeth and Winbladh 5 , Reference Islam, Islam and Mollah 6 Furthermore, the concentrations of several cytokines have been shown to be upregulated in the sera of asphyxiated neonates compared with healthy newborns.Reference Okazaki, Nishida and Kato 7 In a rat model of global PA, Ashdown et al.Reference Ashdown, Joita, Luheshi and Boksa 8 reported increased IL-6 and IL-1β in the liver. Inflammatory cytokines have been demonstrated to be involved in necrotic and apoptotic death of hepatocytes after hepatic ischemia/reperfusion injury.Reference Mari and Fernandez-Checa 9 , Reference Ding and Yin 10 In addition, ceramides have been shown to increase cell death after FA.Reference Vlassaks, Mencarelli and Nikiforou 11 This is a direct effect as the silencing of ceramide generation attenuated hepatocellular damageReference Zhai, Liu and Xue 12 , Reference Llacuna, Mari, Garcia-Ruiz, Fernandez-Checa and Morales 13 and hepatic inflammation.Reference Ding and Yin 10
Although these studies illustrate an important role of cytokines and ceramide in hepatic damage, no studies have been conducted to elucidate their role in neonatal asphyctic liver injury. Moreover, to the best of our knowledge, no studies addressed the consequences of asphyctic hepatic damage in adult life with respect to lipid metabolism. Accordingly, we investigated hepatic inflammation, ceramide signaling and hepatocellular damage after FA and PA during neonatal and adult life in a well-established rat model for global asphyxia.
Method
Animals and experimental procedures
All experiments were approved and conducted according to the Animal Ethics Board of Maastricht University on animal welfare, according to Dutch governmental regulations. Timed-pregnant Sprague–Dawley rats (Charles River, France) were housed individually under standard laboratory conditions and food and water were given ad libitum. Pregnant rats were randomly assigned to an experimental group. Unsexed fetuses, male neonates and male adults were used within this study.
FA was induced at embryonic day 17 (E17). Briefly, pregnant rats were anesthetized with isoflurane (1.5–2.0%) and uterine horns were exposed by performing a midline laparotomy. Both uterine and ovarian arteries were clamped for 30 min with removable clamps. Thereafter, reperfusion was permitted by detaching the clamps, the uterine horns were placed back intra-abdominally and the abdominal cavity was closed.Reference Vlassaks, Strackx and Vles 14
At E21/22, PA was induced. Pregnant rats were killed by decapitation to avoid the potential effect of the anesthetic. After hysterectomy, the uterine horns containing the pups were placed in saline (0.9% NaCl, 37°C) for exactly 19 min. Afterwards, pups exposed to the PA insult were delivered and stimulated manually to breathe in a closed incubator (37°C and 75% air humidity). The umbilical cords were ligated and cut to separate the pups from their placentas.Reference Vlassaks, Strackx and Vles 14 The effect of preconditioning (PC) was studied by inducing FA at E17, followed by PA at E21/22. Controls were born at the same time point by Caesarian section from untreated mothers. Pups were randomly cross-fostered with surrogate dams (maximally 12 pups each dam). Decreased fetal arterial blood pH and brain lactate accumulation post FAReference Cai, Sigrest, Hersey and Rhodes 15 and morphological and behavioral correlates after both FA and PA have been previously characterized.Reference Strackx, Van den Hove and Prickaerts 16 , Reference Strackx, Zoer and Van den Hove 17 For the collection of tissue samples, pups and adult rats were killed by decapitation at several time points after FA and at different short- and long-term time points after birth (sample size: n=5–8 for all groups at all time points) (Fig. 1). Pups belonging to the prenatal experimental groups were littermates from the same mother. The postnatal pups were born from at least two mothers. Liver and plasma samples were collected from all offspring, snap-frozen in liquid nitrogen and preserved at −80°C for further analysis.

Fig. 1 Experimental design. Fetal asphyxia (FA) was induced at E17 by clamping the uterine vasculature during 30 min. At term birth, global perinatal asphyxia (PA) was induced by placing the uterine horns containing the pups in a saline bath for 19 min. All animals were delivered by Caesarean section. Pups were euthanized at 6 h, 24 h and 96 h after FA (n=5/6 per group per time point) and 2 h, 6 h, 7 days and 8 months after birth (n=5–8 per group per time point). E=embryonic day.
RNA extraction and RT-PCR
Total RNA was prepared from the liver tissue using Trizol Reagent (Invitrogen, Breda, the Netherlands), according to the manufacturer’s guidelines. Reverse transcription was carried out using 1 µg of template RNA and the RevertAid First Strand cDNA Synthesis Kit (Fermentas, St. Leon Rot, Germany), according to the manufacturer’s instructions.
Five microliters of diluted cDNA (dilution 1:20) was amplified with LightCycler 480 SYBR Green I Master (Roche Applied Science, Almere, the Netherlands) in a final volume of 20 µl. Real-time PCR was performed using the Light Cycler 480 (Roche). Each PCR was carried out in duplicate and samples negative for RevertAid Reverse Transcriptase were used as negative control. Values are reported relative to the geomean mRNA expression of three housekeeping genes (HPRT, β-actin and GAPHD). Primers were designed using Primer3plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi, Boston, MA, USA) for interleukin 1β (IL-1β), tumor necrosis factor (TNF)-α, IL-10, IL-6, ceramide transport protein (CERT), goodpasture antigen-binding protein (GPBP), LAG1 homolog ceramide synthase 1 (Lass1), neutral sphingomyelinase (nSMase), acid sphingomyelinase (aSMase) and sphingomyelin synthase 1 (SMS1); BAX, BAD, Bcl-2 and Bcl-XL. Quantification cycle values were extracted with the Lightcycler 480 software (Conversion LC and Linge PCR) and calculated based on the cycle threshold (C t) values.
Western blot
Ten percent SDS-PAGE gels were loaded with 40 µg of total protein and transferred to a nitrocellulose membrane (Millipore, Amsterdam, the Netherlands). The membrane was blocked for 1 h with 5% BSA in TBS-T and incubated with mouse monoclonal anti-TNF-α (diluted 1:500; sc-80383; Santa Cruz Biotechnology, Santa Cruz, USA) or rabbit polyclonal anti-CERT (diluted 1:2500; epitope 300–350 of human GPBP; Bethyl Laboratories, Montgomery, TX, USA) overnight at 4°C. Monoclonal mouse anti-rabbit GAPDH (diluted 1:20,00,000; Fitzgerald industries, Concord, MA, USA) was used as loading control. After PBS washes, the membrane was incubated with donkey anti-mouse (diluted 1:10,000; Rockland Immunochemicals, Gilbertsville, PA, USA) and goat anti-rabbit (diluted 1:10,000, Rockland Immunochemicals) for 1 h at room temperature. Finally, the membrane was washed with PBS, dried and scanned using the LICOR Odyssey infrared imaging system.
Histological analysis
Snap-frozen livers were cut (7 µm at −13°C) and mounted on gelatin-coated slices (VWR International). General Hematoxylin and Eosin staining was performed to visualize general liver structure and inflammation. Scoring was performed in a blinded manner by a specialized animal pathologist. The sections from each animal were scored as 0 if they had no inflammatory cells present in the tissue, 1 for a few inflammatory cells (1–20 cells), 2 for moderate cell infiltration (21–40 cells), 3 for a large number of inflammatory cells (41–60 cells) and 4 if inflammation was spread all over in the tissue (>61 cells).Reference Kramer, Moss and Willet 18 To investigate fat deposition in the adult liver, frozen 7-μm-thick sections were stained with oil red-OReference Bieghs, Vlassaks and Custers 19 and qualified by a specialized animal pathologist.
Plasma and liver parameters
To assess hepatic function, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were determined in plasma on a Beckman Coulter Synchron LX20 PRO Clinical Chemistry analyzer (Beckman Coulter, Fullerton, CA, USA). The concentrations of AST, ALT and ALP were then calculated by an enzymatic rate method. Glucose was determined by the Hexokinase method and triglyceride was enzymatically measured with glycerol-3-phosphate oxidase (GPO-Trinder).
Total thiol groups (GSH) and 8-OH-dG (8-hydroxy-2-deoxy guanosine) determination
The tissue levels of the total thiol groups (GSH) were measured in 200 μl of tissue homogenate, using a spectrophotometric assay based on the reaction of thiol groups with 2,2-dithio-bis-nitrobenzoic acid at λ=412 nm (εM=13,600/M/cm, where εM is a wavelength-dependent molar absorbtivity coefficient). The limit of detection for this assay is ∼15 nM. The intra-assay coefficient of variation is 4%, whereas the inter-assay coefficient of variation is 5.6%. 8-OH-dG, a surrogate marker of DNA damage, was measured by a commercially available kit (Cayman Chemicals, Ann Harbor, MI, USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software (version 4.0; GraphPad Software Inc.) and Statistical Package for the Social Sciences (SPSS version 17.0 for Windows). Data are represented as mean+standard error of the means (s.e.m). For each parameter, normality was tested using a Kolmogorov–Smirnov test. Prenatal data were analyzed using Student’s t-test. All postnatal data were analyzed using one-way analysis of variance (ANOVA) test, followed by post-hoc comparisons using LSD correction. Statistical significance was established at P⩽0.05 and indicated by *.
Results
Prenatal effects after FA
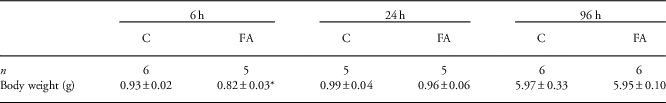
To have a longitudinal overview of the effects of FA on the fetal liver, we analyzed prenatal livers at 6, 24 and 96 h after 30 min of global FA (Fig. 1). At 6 h post FA, body weights of FA pups were significantly lower than controls (P=0.02). At later time points, when blood flow has completely recovered, the body weights between control and FA animals were similar (Table 1).
Table 1 Prenatal body weights (g) of control (C) and fetal asphyxia (FA) pups
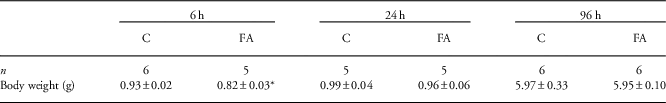
n Indicates the number of pups in each group.
*Indicates statistical significance.
FA induces an acute inflammatory response and changes in ceramide genes
We measured mRNA levels of IL-1β, TNF-α, IL-6 and IL-10; four cytokines that are known to be involved in the inflammatory response after asphyxia.Reference Ashdown, Joita, Luheshi and Boksa 8 , Reference Vlassaks, Strackx and Vles 14 An acute inflammatory response was observed, with increased IL-1β mRNA levels 6 h after FA (P=0.042; Fig. 2a) and elevated IL-6 mRNA levels 24 h after FA compared with control levels (P=0.05; Fig. 2b). No significant changes between control and FA animals were observed in TNF-α and IL-10 mRNA levels (data not shown).

Fig. 2 FA induced prenatal changes in mRNA levels of inflammatory cytokines and ceramide genes. Prenatal mRNA levels of IL-1β (a), IL-6 (b), Lass1 (c), CERT (d), GPBP (e), aSMase (f) and SMS1 (g) in control and FA animals. mRNA levels are relative to the geomean of β-actin, HPRT and GAPDH. *P<0.05 and **P<0.01 significantly different from respective control group. Data shown as mean+s.e.m. Lass1, LAG1 homolog ceramide synthase 1; CERT, ceramide transport protein; GPBP, goodpasture antigen-binding protein; aSMase, acid sphingomyelinase; SMS1, sphingomyelin synthase 1; FA, fetal asphyxia.
In addition, we studied the acute effect of FA in the ceramide signaling pathway as cytokines are suggested to affect the lipid metabolism.Reference Adibhatla, Dempsy and Hatcher 20 , Reference Alessenko, Galperin and Dudnik 21 By RT-PCR, we examined the level of several enzymes that are important in ceramide metabolism. We observed that increased IL-6 mRNA levels at 24 h after FA were associated with increased mRNA levels of ceramide synthase Lass1, the enzyme that converts sphinganine to ceramideReference Mencarelli and Martinez-Martinez 22 (P=0.094; Fig. 2c). Also at this time point, both ceramide transporters CERT (P=0.027; Fig. 2d) and GPBP (P=0.014; Fig. 2e) were higher expressed compared with controls. These transporters are responsible for transporting ceramide from the endoplasmatic reticulum to the Golgi to be converted to more complex sphingolipids.Reference Mencarelli, Bode and Losen 23 At 96 h after FA, a trend toward increased aSMase (P=0.064; Fig. 2f) and higher SMS1 mRNA levels (P=0.003; Fig. 2g) compared with controls was observed, pointing to the conversion of ceramide to sphingomyelin.Reference Mencarelli, Losen and Hammels 24
Decreased oxidative DNA damage 96 h after FA
To investigate whether the changes in inflammatory response and ceramide metabolism were associated with hepatocellular damage, we assessed mRNA levels of apoptotic genes belonging to the BAX family. In addition, glutathione (GSH) and 8-OH-dG were determined as markers for lipid peroxidationReference Al-Aubaidy and Jelinek 25 , Reference Marrazzo, Bosco and La Delia 26 and c-oxidative DNA damage,Reference Maddaiah 27 , Reference Li Volti, Musumeci and Pignatello 28 respectively. These have been shown to be important mediators in the development of post-ischemic liver damage.Reference Alessenko, Galperin and Dudnik 21 Most pronounced changes were observed at 96 h after FA with decreased mRNA levels of BAD compared with control (P=0.034; Fig. 3b). This decrease in BAD mRNA was accompanied by decreased concentration of 8-OH-dG (P=0.036; Fig. 3c), indicating less oxidative DNA damage 96 h post FA. No changes in GSH oxidation were observed for all investigated time points (Fig. 3d).

Fig. 3 Hepatic apoptosis and oxidative DNA damage seem to decrease 96 h after FA. Prenatal mRNA levels of Bcl-XL (a) and BAD (b) in control and FA animals. mRNA levels are relative to the geomean of β-actin, HPRT and GAPDH. Oxidative DNA damage measured by 8-OH-dG concentration (c) and total thiol groups (GSH) (d) corrected for their protein content. *P<0.05 significantly different from respective control group. Data are shown as mean+s.e.m. 8-OH-dG, 8-hydroxy-2-deoxy guanosine; FA, fetal asphyxia.
Liver structure and metabolism 96 h post FA
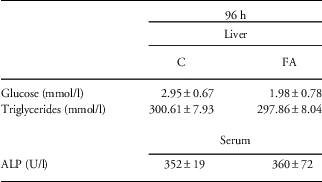
Given the decreased apoptosis and 8-OH-dG concentration at 96 h after the FA insult, we addressed whether this would lead to changes in liver structure or function. Therefore, we assessed general liver morphology by H/E-staining. However, no differences in inflammatory cell infiltration or general liver structure were observed between control and FA pups (data not shown). Although glucose concentration in liver homogenates decreased compared with control, this observation was not significant (Table 2). In addition, the concentration of triglycerides and ALP did not show any differences between controls and FA animals, indicating normal liver function (Table 2).
Table 2 Glucose, triglyceride and ALP concentrations of control (C), and fetal asphyxia (FA) pups at 96 h after FA
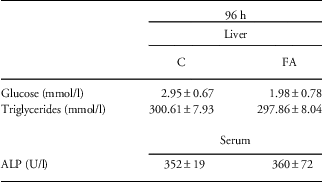
ALP, alkaline phosphatase.
Acute postnatal effects after PA and PC
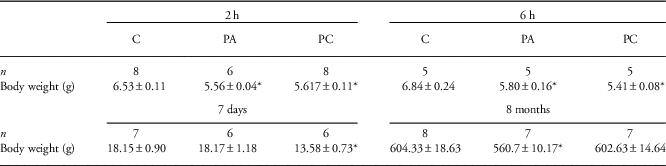
At 2 h, 6 h and 7 days after birth, we assessed hepatic inflammation, ceramide signaling and hepatocellular damage after PA and PC compared with control animals (Fig. 1). At 2 h and 6 h after birth, pups that experienced PA (with or without the combination of FA) weighted significantly less than control pups (P<0.01). At postnatal day 7, though, only preconditioned animals weighted significantly less than control animals (P<0.01; Table 3).
Table 3 Postnatal body weights (g) of control (C), perinatal asphyxia (PA) and preconditioned (PC) animals
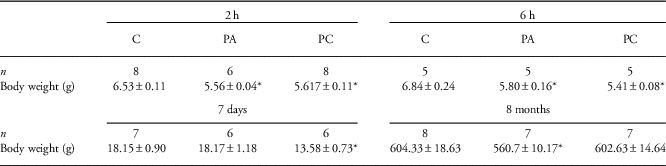
n Indicates the number of pups in each group.
*Indicates statistical significance.
Increased IL-6 and IL-10 mRNA levels 2 h after PA that are attenuated in PC animals
A duration of 2 h after birth, low levels of pro-inflammatory cytokines IL-1β and TNF-α were observed in animals exposed to both FA and PA (PC group) compared with controls (P=0.059 and 0.05, respectively; Fig. 4a and 4b). IL-6 mRNA levels were significantly increased 2 h after PA relative to controls (P<0.001; Fig. 4c). This increased IL-6 mRNA expression was blocked when animals were previously exposed to FA (PC group; P<0.001 for PA v. PC; Fig. 4c). Comparable results were found for IL-10, with increased mRNA levels 2 h after PA that were diminished in PC animals (P=0.036 for C v. PA and P=0.048 for PA v. PC; Fig. 4d). Importantly, a remarkable increase in IL-10 mRNA levels was observed at 6 h after birth in PC animals compared with control and PA animals (P<0.001; Fig. 4d), suggesting a strong anti-inflammatory environment because of PC.

Fig. 4 Increased IL-6 and IL-10 mRNA levels in PA animals, but baseline IL-6 and IL-10 mRNA levels in preconditioned animals. Acute postnatal mRNA levels of IL-1β (a), TNF-α (b), IL-6 (c) and IL-10 (d) in control, PA and PC animals. mRNA levels are relative to the geomean of β-actin, HPRT and GAPDH. *P<0.05; **P<0.01 and ***P<0.001 indicate significant differences between groups. Data are shown as mean+s.e.m. PA, perinatal asphyxia; PC, preconditioning.
Decreased lipid peroxidation 6 h after PA
At the 6 h time point, changes were observed in ceramide metabolism genes, apoptosis and lipid peroxidation. aSMase, the enzyme that is localized in lysosomes and regulates cellular membrane turnover,Reference Mencarelli and Martinez-Martinez 22 tended to decrease in PC animals compared with control levels (P=0.053; Fig. 5a, right panel). For SMS1, decreased mRNA levels were seen 6 h after PA (P=0.055; Fig. 5b, right panel), whereas PC animals showed baseline levels.

Fig. 5 Decreased expression of anti-apoptotic genes and lipid peroxidation 6 h after PA. Postnatal mRNA levels of nSMase and aSMase (a), Lass1 and SMS1 (b), BAX, BAD, Bcl-2 and Bcl-XL (c) are presented relative to the geomean of β-actin, HPRT and GAPDH. Lipid peroxidation (GSH) (d) and oxidative DNA damage measured by 8-OH-dG concentration (e) corrected for their protein content at 6 h after birth. *P<0.05 and **P<0.01 indicate significant differences between groups. Data are shown as mean+s.e.m. nSMase, neutral sphingomyelinase; aSMase, acid sphingomyelinase; 8-OH-dG, 8-hydroxy-2-deoxy guanosine; PA, perinatal asphyxia; PC, preconditioning.
A comparable pattern was observed for the apoptotic genes BAD and Bcl-2, which decreased 6 h after PA (P=0.056 and 0.05, respectively; Fig. 5c), whereas PC animals showed baseline levels. mRNA levels of the anti-apoptotic gene Bcl-XL were higher in the PC group compared with both control (P=0.015) and PA animals (P=0.004; Fig. 5c). These changes in apoptotic mRNA levels were associated with increased levels of GSH (P=0.01) and 8-OH-dG (not significant) in the PA animals. PC animals showed comparable levels to control levels (Fig. 5d and 5e).
Increased ALP concentration 2 h post PA
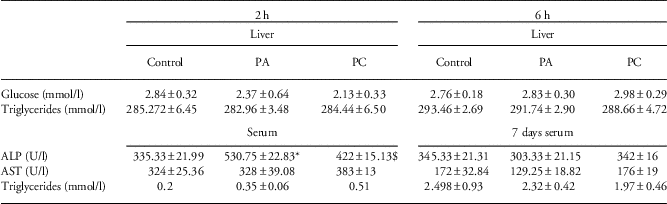
Finally, we assessed whether we could find changes in liver morphology or function because of asphyxia acutely after birth. On the basis of H/E-staining, we could not observe changes in hepatocellular damage between the groups for all time points (data not shown). To assess hepatic metabolism, we evaluated glucose and triglyceride concentrations in liver homogenates. Both 2 h and 6 h post birth, hepatic glucose and triglyceride levels were unchanged after either PA or PC (Table 4). Then, we also assessed liver enzymes in the serum of the pups as markers for liver function. Interestingly, 2 h after PA, when IL-6 and IL-10 mRNA levels were increased (Fig. 4c and 4d), ALP concentration was significantly higher compared with controls (P=0,02; Table 4). This increase was attenuated when the animals experienced FA before the PA insult (PC group) (P=0.057; Table 4). Although this effect seemed to be short-lasting, we did observe significantly elevated levels of AST in the serum of PC animals compared with controls at 6 h post birth (P=0,04; Table 4).
Table 4 Glucose, triglycerides, ALP and AST concentrations of control (C), perinatal asphyxia (PA) and preconditioned (PC) animals acutely after birth and in adult life (8 months after birth)
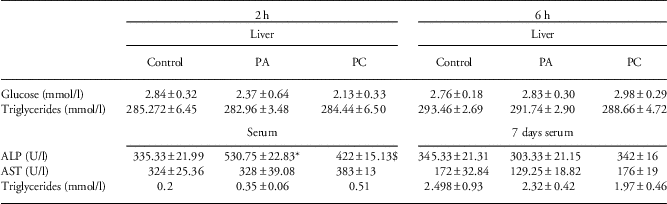
ALP, alkaline phosphatase; AST, aspartate aminotransferase.
*Indicates statistical significance.
$Statistical different from the PA group.
Chronic effects
To assess whether FA and PA also have hepatic consequences in adulthood, we analyzed the livers of 8-month-old animals. At this age, the PA animals weighted significantly less than control and PC animals (Table 2).
No changes in inflammatory response and ceramide metabolism at 8 months of age
No significant differences between groups were observed in hepatic mRNA levels for all cytokines and ceramide enzymes (data not shown). In the PC group, the expression of CERT was significantly decreased compared with the PA animals (P=0.049) and showed a declining trend compared with controls (P=0.07; Fig. 6a). GPBP was higher expressed in both PA (P=0.036) and PC animals (P=0.082) compared with controls (Fig. 6b). Western blot analysis of CERT and GPBP did not reveal any significant changes (data not shown).

Fig. 6 Long-term changes in CERT and GPBP mRNA levels. mRNA levels of CERT (a) and GPBP (b) at 8 months of age in control, PA and PC animals. Levels are presented relative to the geomean of β-actin, HPRT and GAPDH. *P<0.05 indicates significant differences between groups. Data are shown as mean+s.e.m. CERT, ceramide transport protein; GPBP, goodpasture antigen-binding protein; PA, perinatal asphyxia; PC, preconditioning.
Increased plasma concentration of ALP in PC animals at 8 months of age
At 8 months, measurements of genes of the BAX family and GSH concentration revealed no significant changes between groups (data not shown). Yet, 8-OH-dG levels seemed to be higher in animals that experienced PA during birth (both PA and PC animals), although levels did not reach significance (Fig. 7a). Significance was reached for plasma levels of ALP, showing higher concentration in PC animals (P=0.03) compared with controls (Fig. 7b). Nevertheless, no significant changes were observed in plasma concentration of AST and ALT (Fig. 7c and 7d).

Fig. 7 ALP concentrations are increased in the PC animals at 8 months of age. 8-OH-dG concentration corrected for protein content (a), plasma ALP (b), AST (c), ALT (d) concentrations in U/L and glucose (e) and triglyceride (f) concentration in mmol/l of control, PA and PC animals at 8 months of age. *P<0.05 and **P<0.01 indicate significant differences between groups. Data are shown as mean+s.e.m. 8-OH-dG, 8-hydroxy-2-deoxy guanosine; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PA, perinatal asphyxia; PC, preconditioning.
The general liver structure based on H/E staining was not different between groups. Overall, we observed a normal liver structure with no enlargement of the hepatocytes or compression of the sinusoids, and no hepatic inflammatory cell infiltration in all experimental groups. Also no changes in intracellular lipid accumulation were observed, based on Oil Red O staining (data not shown). This observation was confirmed by measuring hepatic glucose and triglyceride levels at 8 months, not revealing any significant changes between the groups at this age (Fig. 7e and 7f).
Discussion
This is the first study demonstrating that global FA and PA induce an acute hepatic inflammatory response. Interestingly, acutely after birth it seems that FA PC may induce hepatic protection by modulating the inflammatory, apoptotic and anti-oxidative response. This systemic protective response of FA might contribute to the dampened inflammatory response that is observed in the brain,Reference Vlassaks, Strackx and Vles 14 , Reference Anthony, Couch, Losey and Evans 29 and supports the notion that asphyctic liver damage correlates with the severity of HIE.Reference Karlsson, Blennow, Nemeth and Winbladh 5 , Reference Islam, Islam and Mollah 6
PA is associated with a systemic inflammatory response as is indicated by increased serum concentrations of IL-6, IL-8 and IL-10 in asphyxiated neonatesReference Okazaki, Nishida and Kato 7 and increased IL-6 and IL-1β in the asphyctic rat liver.Reference Ashdown, Joita, Luheshi and Boksa 8 Ceramides are lipid intermediates that have been determined to be important in hepatocellular damageReference Zhai, Liu and Xue 12 and are upregulated by inflammatory mediators such as cytokines.Reference Adibhatla, Dempsy and Hatcher 20 , Reference Alessenko, Galperin and Dudnik 21 Hence, in this study, we aimed to investigate in more detail the hepatic inflammatory response after both FA and PA. We examined whether this inflammatory response was associated with changes in ceramide metabolism and hepatocellular damage. We also considered whether FA and PA have any long-term hepatic impact. Finally, we assessed whether fetal asphyctic PC would have any beneficial effect on the liver.
An acute inflammatory response was observed after FA, initiated by increased mRNA levels of IL-1β and followed by increased mRNA expression of IL-6. Similar results were reported by Ashdown and coworkers, who found in a perinatal setting increased IL-6 and IL-1β in the rat liver 2 h after global PA.Reference Ashdown, Joita, Luheshi and Boksa 8 IL-1β is known to induce IL-6 productionReference Martin-Ancel, Garcia-Alix and Pascual-Salcedo 30 , Reference Silveira and Procianoy 31 and is associated with ceramide metabolism.Reference Adibhatla, Dempsy and Hatcher 20 At 24 h after FA, we found increased levels of Lass1 at the same time point as the increased IL-6 mRNA. Lass1 is an important enzyme involved in de novo ceramide synthesis and has been shown to be increased after hypoxia and oxygen/glucose deprivation.Reference Novgorodov and Gudz 32 Therefore, it seems that early after FA the inflammatory response that is driven by IL-1β and IL-6 promotes the generation of ceramide.Reference Jin, Hou and Mullen 33 , Reference Zhu, Lin and Cheng 34 However, as the mRNA levels of the transporters CERT and GPBP were upregulated, we assume that the produced ceramide is not accumulating in the cell but is transported from the endoplasmatic reticulum to the Golgi.Reference Mencarelli, Losen and Hammels 24 Thereafter, ceramide can be metabolized to sphingomyelin by the enzyme SMSReference Dobrowsky and Kolesnick 35 that was upregulated 96 h after FA, thereby diminishing the cellular ceramide-induced stress and apoptosis.Reference Mencarelli and Martinez-Martinez 22 , Reference Novgorodov and Gudz 32 , Reference Dobrowsky and Kolesnick 35 We indeed observed lower levels of apoptotic mRNA expression (Bcl-XL and BAD) and downregulated levels of 8-OH-dG 96 h post FA. Hence, although FA initially provokes a hepatic inflammatory response, the liver can modulate this reaction resulting in normal liver physiology. This is in agreement with the fact that FA is primarily induced as PC stimulus and thus a non-lethal asphyctic insult. It is worthwhile to mention that 96 h after FA is the time point in which the fetuses are primed to be born (E21 time point). Therefore, we speculate that this downregulation in apoptosis and DNA damage might be a hepatic protective mechanism in the fetus during birth.
A protective effect was indeed seen at 2 h after birth in the preconditioned animals concerning the inflammatory response. Hepatic inflammation was induced 2 h after PA compared with controls with increased mRNA levels of IL-6 and IL-10. As it has been shown in the brain,Reference Vlassaks, Strackx and Vles 14 the increased inflammatory response was abolished in the preconditioned FA–PA animals, revealing comparable mRNA levels as control animals. These results indicate a crucial role of IL-6 and IL-10 in the immune response after birth asphyxia. IL-6 and IL-10 are known to be secreted togetherReference Martin-Ancel, Garcia-Alix and Pascual-Salcedo 30 , Reference Aly, Khashaba, El-Ayouty, El-Sayed and Hasanein 36 and greater concentrations of these cytokines occur in the cerebrospinal fluid and serum from newborn infants with asphyctic encephalopathy.Reference Okazaki, Nishida and Kato 7 , Reference Silveira and Procianoy 31 The precise role of these molecules in asphyctic injury is, however, not clear as they are considered to have both pro- and anti-inflammatory properties.Reference Aly, Khashaba, El-Ayouty, El-Sayed and Hasanein 36 – Reference Suzuki, Tanaka and Suzuki 40 Nevertheless, as IL-10 is mainly considered as an anti-inflammatory cytokine,Reference Sabat, Grutz and Warszawska 41 the high levels of IL-10 in preconditioned animals at 6 h after birth might protect the fetuses by providing an anti-inflammatory environment. The observed baseline levels of Bcl-2 in preconditioned animals, but downregulated levels of this anti-apoptotic gene in PA animals support this assumption. In addition, Bcl-XL levels were increased compared with PA in the PC group at the same time point (6 h post birth). In a study by Maulik et al.,Reference Maulik, Engelman and Rousou 42 it has been shown that Bcl-2 regulates the inhibition of apoptosis after ischemic PC. In addition, Bcl-XL has been attributed to play an important role in PC induced protection as overexpression of Bcl-XL prevented apoptosis in the cortical and hippocampal neurons in a neonatal and adult mouse model of hypoxia/ischemia.Reference Parsadanian, Cheng, Keller-Peck, Holtzman and Snider 43 , Reference Wiessner, Allegrini and Rupalla 44 This observation might be applicable in our model as well as preconditioned animals showed the upregulation of this gene. In addition, at 6 h after birth, the PA animals showed lower expression of SMS1, the enzyme that converts ceramide to sphingomyelin.Reference Mencarelli, Losen and Hammels 24 Consequently, ceramide levels can accumulate and induce lipid peroxidation.Reference Alessenko, Galperin and Dudnik 21 However, as increased GSH levels were observed, indicating less oxidative damage, we assume that the upregulation of anti-oxidant products is an endogeneous protective mechanism of the neonatal livers to counterbalance the asphyctic damage. In a recent study by Bonestroo et al.,Reference Bonestroo, Nijboer and van Velthoven 45 an early downregulation of the hepatic pro-inflammatory response to brain damage was observed. Owing to the bidirectional communication that exists between the central nervous system and the peripheral immune system, it is plausible that glucocorticoids, catecolamines and acetylcholamine released after brain injury inhibit the systemic inflammatory response.Reference Chamorro, Urra and Planas 46 These studies indicate a central role of the liver in immunomodulation after asphyxia.
At 8 months of age, the liver still seemed to be affected by FA and PA, but only minor changes were observed with no significant consequences for normal liver pathology. Although the hepatic response to FA and PA seems to be short-lasting, animals born with PA weighted less than control and preconditioned animals. It might be that the animals that have experienced PA either do not receive enough energy (e.g. via food intake) or they have a normal food intake, but the metabolism has been disturbed because of asphyxia, resulting in defects in processing the food. The latter seems possible as we observed increased ALP levels at 8 months of age, indicating disturbed anabolic function of the liver. Hence, it is likely that the processing of metabolites is not performed properly, leading to growth restriction. Another possible explanation could be the disturbed gut homeostasis leading to malabsorption of nutrients across the gastrointestinal tract. It is known that hypoxic conditions in the gut are associated with damaged epithelium, altered intestinal motility and feeding intolerance.Reference Grenz, Clambey and Eltzschig 47 Therefore, we can speculate that the lower body weight of the PA animals is due to intestinal complications, leading to growth restriction.
According to the Barker hypothesis, lower birth weights, often reflecting adverse intrauterine circumstances, are associated with a higher risk of adult-onset diseases.Reference Barker 48 Previously, we have shown in a sheep model for chorioamnionitis that antenatal inflammation-related liver disturbances have long-lasting postnatal effects on liver metabolism.Reference Vlassaks, Gavilanes and Bieghs 49 Although the initial inflammatory response could be cleared in the mature liver, hepatic lipid metabolism was disturbed in animals that were exposed to inflammatory mediators during intrauterine life.Reference Vlassaks, Gavilanes and Bieghs 49 As in this study we could not find major changes in liver morphology and/or functioning, we assume that the 8-month time point may be too early to already see significant differences. More studies are thus needed to investigate the long-term consequences of global FA and PA on the liver.
In summary, we showed that PA induces hepatic inflammation with higher cytokine levels of IL-6 and IL-10. Asphyctic PC may modulate the inflammatory, apoptotic and anti-oxidative response, thereby protecting the neonate from PA damage. Although most of our data are restricted to mRNA measurements, these can already give an idea of the final response that takes place after FA and PA. Clearly, additional studies are needed to explore the hepatic response in more detail. Studies have already outlined that the systemic response has a pivotal role in determining the final outcome of brain injury.Reference Anthony, Couch, Losey and Evans 29 Therefore, investigating the hepatic response after FA and PA can provide more insights into the brain immunological responses occurring after asphyxia. In addition, more insights into the mechanisms and consequences of hypoxic hepatic damage are needed as disturbed hepatic function might lead to metabolic diseases in later life.Reference Vlassaks, Gavilanes and Bieghs 49 , Reference Rueda-Clausen, Dolinsky and Morton 50
Acknowledgments
The authors thank Claudia Clorefice (Clinical Chemistry, Maastricht University Medical Center) for technical support and Marion Gijbels (Molecular Genetics, Maastricht University) for pathological reviewing of the liver stainings.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory rats and has been approved by the Animal Ethics Board of Maastricht University.