The development of novel functional foods, containing probiotic lactic acid bacteria and prebiotics, is of utmost importance because of their widely known health benefits (i.e.: immunological stimulation, improvement of digestion and absorption, synthesis of vitamins, decrease of cholesterol levels, inhibition of growth of potential pathogens, reduction of gas distension and recovery of the normal flora after antibiotic therapy (Gibson & Roberfroid, Reference Gibson and Roberfroid1995; Manning & Gibson Reference Manning and Gibson2004; Wang, Reference Wang2009; Wallace et al. Reference Wallace, Guarner, Madsen, Cabana, Gibson, Hentges and Sanders2011).
Prebiotics have been defined as non-digestible food components that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improving host health. These compounds include oligosaccharides such as inulin, lactulose and fructo, gluco or galacto-oligosaccharides (Playne & Crittenden Reference Playne, Crittenden, Nasser and German2004, Reference Playne, Crittenden, Fox and McSweeney2009). Gibson & Roberfroid (Reference Gibson and Roberfroid1995) introduced the concept of ‘Synbiotic’ for the synergic effect produced on the gut microbiota by the ingestion of prebiotics and probiotics in functional foods.
Galacto-oligosaccharides (GOS) are present in human breast milk and their presence in infant formulas have been described as responsible for the stimulation of bifidobacteria and lactobacilli in a similar way to oligosaccharides in human breast milk (Boehm & Stahl Reference Boehm, Stahl and Mattila-Sandholm2003; Boehm et al. Reference Boehm, Fanaro, Jelinek, Stahl and Marini2003; Weaver, Reference Weaver2003). GOS are composed of a variable number of galactose units linked to a terminal glucose. The degree of polymerisation is variable (from 2 to 8 monosaccharide units), tri and tetra-saccharides being the main components and also those with the highest prebiotic effect (Panesar et al. Reference Panesar, Panesar, Singh, Kennedy and Kumar2006). In addition to their prebiotic properties, the ability of GOS to act as cryoprotectants has been recently demonstrated (Tymczyszyn et al. Reference Tymczyszyn, Gerbino, Illanes and Gómez-Zavaglia2011, Reference Tymczyszyn, Sosa, Gerbino, Hugo, Gómez-Zavaglia and Schebor2012).
The synthesis of GOS consists of a kinetically controlled enzymatic reaction of lactose transgalactosylation catalysed by β-galactosidases (E.C.3.2.1.23). In this reaction, the produced galactosyl-enzyme intermediate is either hydrolysed or attacked by another nucleophile acceptor (lactose in this case) to form new glycosidic bonds leading to GOS (Splechtna et al. Reference Splechtna, Nguyen, Steinböck, Kulbe, Lorenz and Haltrich2006; Neri et al. Reference Neri, Balcão, Costa, Rocha, Ferreira, Torres, Rodrigues, Carvalho and Teixeira2009; Vera et al. Reference Vera, Guerrero and Illanes2011a, Reference Vera, Guerrero, Illanes and Conejerosb).
Whey is a by-product of cheese making usually managed as a waste that, having a high biochemical oxygen demand (BOD), is costly to remove. Hence, the use of whey represents a very interesting option to give an added value to effluents (Marwaha & Kennedy Reference Marwaha and Kennedy1988). Whey proteins are generally separated from cheese whey by ultrafiltration, and employed as food additives or protein supplements. Therefore, the permeate remaining after whey protein recovery, mostly composed by lactose and salts, can be dried and used for pig feeding (Nessmith et al. Reference Nessmith, Nelssen, Tokach, Goodband and Bergström1997). Due to the high lactose concentration, cheese whey permeate (WP) can also be used to produce different added-value products, such as GOS (Vera et al. Reference Vera, Guerrero, Conejeros and Illanes2012), lactic acid, ethyl alcohol, or even as a culture medium for probiotic bacteria (Cui et al. Reference Cui, Wan, Li, Liu and Rajashekara2012).
Probiotic cultures for food applications are most frequently provided in frozen and dried forms. Drying techniques to obtain dehydrated probiotic microorganisms in a viable state have proven to be useful. Despite its high cost, freeze-drying (lyophilisation) has been the most widely used technique. Spray-drying is a lower cost technique and therefore, it is more convenient for producing large quantities of bacterial probiotic cultures (Desmond et al. Reference Desmond, Stanton, Fitzgerald, Collins and Ross2001; Silva et al. Reference Silva, Carvalho, Teixeira and Gibbs2002; Corcoran et al. Reference Corcoran, Ross, Fitzgerald and Stanton2004; Golowczyc et al. Reference Golowczyc, Silva, Abraham, De Antoni and Teixeira2010, Reference Golowczyc, Silva, Teixeira, De Antoni and Abraham2011).
With this background, the aim of this work was to optimise the enzymatic synthesis of GOS in WP using β-galatosidase from Aspergillus oryzae as biocatalyst. WP and enzymatically treated WP containing GOS (WP-GOS) were used as culture media for Lactobacillus plantarum 299v, a microorganism with recognised probiotic properties (Ducrotté et al. Reference Ducrotté, Sawant and Jayanth2012). The composition of WP-GOS before and after fermentation was determined by HPLC. The fermented WP and WP-GOS were dehydrated by freeze-drying or spray-drying and stored to obtain a low cost product with promising applications in functional foods.
Materials and methods
Synthesis of GOS from WP
Reactions were carried out at a constant temperature of 37 °C in 200 ml Erlenmeyer flasks using A. oryzae β-galactosidase as biocatalyst. The β-galactosidase preparation employed in this study is traded as Enzeco Fungal lactase® and was kindly donated by Enzyme Development Corporation (EDC, New York, NY). The enzyme was stored at 4 °C and remained fully active throughout this investigation. Enzyme has a transgalactosylation activity of 56 000 IUT/g of enzyme preparation. One international unit of transgalactosylation (IUT) was defined as the amount of β-galactosidase that catalyses the transgalactosylation of 1 μmol galactose per minute at 40 °C, pH 4·5 and 40% (w/w) initial lactose monohydrate concentration (Vera et al. Reference Vera, Guerrero and Illanes2011a, Reference Vera, Guerrero, Illanes and Conejerosb).
WP was donated by a local dairy industry (Ilolay S.A, Sante Fe, Argentina). It was obtained by drying desprotenised sweet whey and contains approximately 80% (w/w) lactose, 6% (w/w) of ashes and 3% (w/w) of proteins (Leon Pelaez Reference Leon Pelaez2013).
A proper amount of WP was mixed with 100 mm McIlvaine citrate-phosphate buffer at pH 4·5, in order to obtain a reaction medium containing 20 to 60 g solids/100 g of suspension. The previous suspension was heated over 95 °C to promote lactose dissolution and then the temperature was adjusted to 37 °C. Afterwards, 10 g of enzyme solution were added to start the reaction of synthesis, so that the enzyme dosage was 100 IUT per gram of lactose. The progress of synthesis was followed by taking samples at regular time intervals and the reaction was stopped by adding 0·5 ml 200 mm NaOH (Chemical Co., St. Louis, MO). The oligosaccharides content was analysed by HPLC (see section HPLC Analysis below). After analysis, the WP-GOS composition containing the maximum concentration of GOS was selected as growth medium for the microorganism. At these conditions, the reaction was stopped by boiling. All experiments were done in duplicate, differences among replicates never exceeding 5%. The following parameters were considered:
- Conversion (X): represents the percentage of initial lactose consumed during the synthesis.
where L 0 and L represents the initial and final concentration of lactose, respectively.
- Yield (Y): represents the mass of total GOS obtained per unit mass of initial lactose. For the purpose of this work, it was evaluated at the maximum GOS concentration.
where M GOS represents the mass of total GOS produced.
- Specific productivity (π): represents the mass of total GOS produced per unit of enzyme mass and unit of reaction time. For the purpose of this work, it was evaluated at the maximum GOS concentration.
where E represents the enzyme mass and t, the time of reaction at which maximum GOS is obtained.
HPLC analysis
The content of oligosaccharides and monosacharides was determined by HPLC in a Jasco RI 2031 HPLC equipment, provided with refractive index detector, isocratic pump (Jasco PU2080) and autosampler (Jasco AS 2055), using BP-100 Ag+ columns (300 mm×7·8 mm) for carbohydrate analysis (Benson Polymerics, Reno, NV, USA). Samples were eluted with mili-Q water at a flow-rate of 0·5 ml/min. Column and detector temperatures were 80 and 40 °C, respectively. Chromatograms were integrated using the software ChromPass. Composition of samples was determined by assuming that the area of each peak is proportional to the percentage of the corresponding sugar. The accuracy of such assumption was checked by making a material balance. Retention times were determined using standards of galactose, glucose, galactobiose, lactose and 3α–4β–3α galactotetraose supplied by Sigma (St. Louis, MO).
Bacterial strain
Lb. plantarum 299v, deposited at the Deutsche Sammlung von Mikroorganismen (DSM) under the code DSM 9843, was used in this work. The strain was maintained frozen at −80 °C in 120 g/l non-fat milk solids. Microbial cells were reactivated in MRS broth (Biokar Diagnostics, Beauvais, France) (De Man et al. Reference De Man, Rogosa and Sharpe1960) at 37 °C before conducting the experiments. This microorganism was selected because of its probiotic properties (Ducrotté et al. Reference Ducrotté, Sawant and Jayanth2012) and preliminary results demonstrated that it can grow in WP suspensions at high concentrations of solids.
Growth conditions
WP and WP-GOS, containing 40 and 20 g solids/100 g suspension, were neutralised with NaOH to pH 7, and autoclaved for 15 min at 121 °C. The content of GOS, determined by HPLC, was not affected by this process (P>0·05) (data not shown). WP and WP-GOS were inoculated with Lb. plantarum 299v and incubated at 37 °C. For spray-drying, WP and WP-GOS were centrifuged and the insoluble solids were removed before inoculating Lb. plantarum 299v.
Culture aliquots were taken at different times of growth (0, 8, 12, 16, 20, 24 and 36 h) to determine viable cells by plate count (see section Bacterial plate counts) and microorganism sugar consumption by HPLC.
Freeze-drying procedure
Lb. plantarum 299v was cultivated for 24 h in WP and WP-GOS at 37 °C, at the best concentration determined above (see Synthesis of GOS from WP and HPLC sub-sections). Aliquots of 1 ml of culture containing Lb. plantarum 299v and GOS, neutralised or not, where then transferred into 5 ml glass vials under aseptic conditions and frozen for 48 h at −80 °C. The freeze-drying process was carried out at −50 °C using a Heto FD4 freeze drier (Heto Lab Equipment, Denmark) and lasted 48 h.
Spray-drying procedure
Lb. plantarum 299v was cultivated for 24 h at 37 °C in WP and WP-GOS (prepared as described above). Microorganisms were dried directly from the growth medium (WP and WP-GOS). A laboratory-scale spray-dryer (model B290 Büchi mini spray-dryer) was used to process the samples at a constant air inlet temperature of 180 °C and an outlet temperature of 65–70 °C.
Bacterial plate counts
Viable bacterial plate counts were determined before and after freeze-drying and spray-drying and during storage at 4 °C. Freeze-dried samples were rehydrated in 1 ml salt solution (0·85 g NaCl/100 ml). One gram of spray dried powder was rehydrated in 9 ml of the salt solution, homogenised for 1 min in a vortex mixer and maintained at room temperature for 30 min. Bacterial suspensions were serially diluted and plated on MRS agar plates (Biokar Diagnostics, Beauvais, France). Bacterial counts were determined after 48 h incubation at 37 °C of samples stored for 120 d at 4 °C. The recovery of microorganisms after different times of storage was analysed by plate counts.
Water activity measurements
Water activity was determined after drying the samples using an Aqualab water activity instrument (Aqualab, Model Series 3TE, USA). The equipment was calibrated using standard salt solutions provided by the manufacturer.
Reproducibility of the results
All experiments were done on duplicate samples using three independent cultures of bacteria. The relative differences were reproducible independently of the cultures used. Analysis of variance (ANOVA) of the viable counts corresponding to the different treatments was carried out using the statistical program Statistix 8 Software (Analytical Software, Florida USA). Comparison of means by Tukey methods were tested, and if P<0·05 then the difference was considered statistically significant.
Results
Optimisation of synthesis of GOS from WP
In a first stage, the conditions of the enzymatic reaction leading to WP-GOS were optimised. Maximum Y was 27·3±0·28 g GOS/100 g lactose, and was obtained with 40 g solids/100 g suspensions (Fig. 1). A remarkable increase of 35% in Y was obtained when increasing WP concentration from 20 to 40 g solids/100 g suspension. Over 40 g/100 g, a decrease in Y was detected. From Fig. 1, it can also be noticed that an increase in the WP concentration from 20 to 60 g solids/100 g suspension produced a decrease in π of 67%. These results allowed us to define the best conditions for further experiments. Then, a concentration of 40 g solids/100 g of suspensions, where the percentage of total GOS with respect to total sugars is maximum, was selected.

Fig. 1. Effect of the concentration of WP on the synthesis of GOS at 37 °C and pH 4·5, using A. oryzae β-galactosidase as biocatalyst. (⧫) Yield (Y) and (○) specific productivity (π).
Figure 2 shows the kinetics of synthesis of GOS. The decrease in the concentration of lactose, concomitant with the increase in the concentration of total GOS, galactose and glucose is presented in Fig. 2a. Figure 2b shows that the highest concentration of GOS was produced when X is close to 57% conversion, reaching a total GOS concentration of 27±0·15% of the total sugar content. Therefore, the reaction was stopped at this point and the product obtained was used as culture medium for Lb. plantarum 299v in the following experiments.

Fig. 2. Kinetics of synthesis of GOS from of WP using A. oryzae β-galactosidase as biocatalyst. (a): Composition of the reaction product. (●) lactose, (■) galactose, (▲) glucose and (⧫) total GOS. (b) Production of total GOS as a function of the conversion rate in the enzymatic reaction. The enzymatic reaction was carried out with an initial concentration of 40 g WP/100 g of suspension, at 37 °C and pH 4·5.
Fermentation of WP and WP-GOS
The growth kinetics of Lb. plantarum 299v in WP and WP-GOS containing 40 and 20 g solids/100 g suspensions was analysed at pH 7. Taking into account that fermented products will be dehydrated by freeze-drying or spray-drying, low concentrations of solids are not suitable for these processes and thus, they were not tested. The highest bacterial counts were obtained when the strain was grown in WP and WP-GOS containing 20 g solids/100 g suspensions after 24 h of incubation. Concentrations of 40 g solids/100 g suspensions inhibited microbial growth.
No significant differences between the growth kinetics of Lb. plantarum 299v in WP and WP-GOS (P>0·05) were observed (Fig. 3). Lb. plantarum 299v reached stationary phase in approximately 16 h in both culture media, attaining bacterial counts higher than 108 CFU/ml.

Fig. 3. Growth kinetics of Lb. plantarum in WP (■) and WP-GOS (●) at 37 °C.
The composition of GOS in WP-GOS before and after fermentation was analysed by HPLC. Table 1 shows the relative composition of sugars in WP-GOS prior to fermentation. The GOS present in WP were composed of trisaccharides (GOS 3), tetrasaccharides (GOS 4) and oligosaccharides with degree of polymerisation ⩾5 (GOS 5). GOS 3 were those in higher proportion.
Table 1. Sugar composition of whey permeate enzymatically treated (WP-GOS) containing 20 g solids/100 g of suspensions
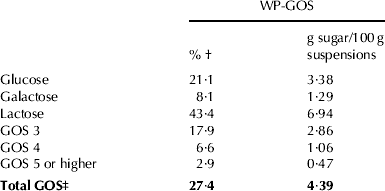
† g sugar/100 g of total sugar
‡ Total GOS denotes GOS 3, GOS 4, GOS 5 and GOS of higher degree of polymerisation
Figure 4 shows the evolution of the relative concentration of GOS 3, 4 and 5 during growth of Lb. plantarum 299v in WP-GOS. GOS 3 showed a clear increase in relative concentration after 20 h of fermentation. Also after 20 h fermentation, the concentration of GOS 4 slightly increased and that of GOS 5 slightly decreased at such conditions.

Fig. 4. Evolution of Galacto-oligosaccharide concentration during the growth of Lb. plantarum in WP-GOS: (■) GOS 3, (⧫) GOS 4 and (○) GOS 5.
Dehydration process
In the following step, we studied the survival of Lb. plantarum 299v in the freeze-drying and spray-drying processes and subsequent storage, as well as the effect of GOS as protectants.
Lb. plantarum 299v grown in WP and WP-GOS containing 20 g solids/100 g suspensions at 37 °C and pH 7, was harvested in the stationary phase (as described in Fig. 3) and freeze-dried. The recovery after freezing at −80 °C and after freeze-drying was analysed. The effect of neutralising or not the fermentation medium was also studied. Figure 5 depicts the decrease in the number of viable cells (N/N0) after freezing and freeze-drying. No significant differences (P>0·05) were observed when the fermented WP-GOS medium was neutralised before dehydration. For non-neutralised fermentation media, a decrease of one log cycle in the CFU/ml was observed. This indicates that it is the acidity of the growth medium and not the preservation process, which is the principal factor affecting the bacterial survival.

Fig. 5. Logarithm of N/N0 of Lb. plantarum 299v grown in WP (white bars) and WP-GOS (grey bars) after freezing and freeze-drying, with or without neutralisation. *Significant differences respect to control (non-dried microorganisms) (P<0·05).
In parallel experiments, WP and WP-GOS were fermented for 24 h, neutralised and then spray-dried. The final a w in both media was 0·262 and 0·250, respectively. No significant differences between the survival of Lb. plantarum 299v grown and dehydrated in WP and WP-GOS were observed (P>0·05). The high counts of viable microorganisms obtained after spray-drying (above 108 per gram) indicate that Lb. plantarum 299v is a thermo-resistant microorganism (Table 2).
Table 2. Survival and water activity (a w) of Lb. plantarum 299v grown in whey permeate (WP) and in whey permeate containing GOS (WP-GOS) after spray-drying. sd=±0·50×108 CFU
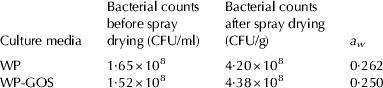
Storage stability
Bacterial survival was assessed at different times at 4 °C, the temperature usually employed in the industry for the storage of starters. Figure 6 shows the storage stability of Lb. plantarum v299 dehydrated by freeze-drying or spray-drying. For the freeze-drying process (Fig. 6a), no significant differences in the viability of Lb. plantarum 299v grown in WP-GOS were observed up to 90 d storage. However, in the same period of storage, the viability of freeze-dried Lb. plantarum 299v grown in WP decreased significantly. The viability of spray-dried Lb. plantarum v299 grown in both WP and WP-GOS was stable during storage up to 120 d (Fig. 6b).

Fig. 6. (a) Stability of freeze-dried Lb. plantarum 299v as a function of the time of storage at 4 °C. ■: grown and dehydrated in WP, ▲: grown and dehydrated in WP-GOS. (b) Stability of spray-dried Lb. plantarum v299 as a function of the time of storage at 4 °C. ■: grown and dehydrated in WP, ▲: grown and dehydrated in WP-GOS.
Discussion
Whey proteins are widely used for several industrial purposes and therefore, the remaining whey permeate, mainly composed of lactose, represents the final effluent of cheese production. For this reason, strategies addressed to generate added value products are constantly being developed. The use of whey permeate as a source of lactose for the synthesis of GOS and its subsequent use as a culture medium for probiotic bacteria, as carried out in this work, appears as an appropriate way to add value to this effluent.
The synthesis of GOS was firstly optimised to obtain an appropriate culture medium. The increase in GOS yield observed at WP concentrations up to 40 g solids/100 g suspensions can be explained considering the higher initial lactose concentration required to favour the reaction of synthesis over the reaction of hydrolysis (Neri et al. Reference Neri, Balcão, Costa, Rocha, Ferreira, Torres, Rodrigues, Carvalho and Teixeira2009). Despite this, a decrease in GOS yield was observed over 40 g of solids/100 g suspensions, which is in agreement with the results reported by Das et al. (Reference Das, Sen, Sarkar, Bhattacharyya and Bhattacharjee2011). This can be explained considering that at high WP concentrations, the concentration of salts is also high and the water activity is low. In this regard, van Griethuysen et al. (Reference van Griethuysen, Flaschel and Renken1985) highlighted the inhibitory effect of whey salts on A. oryzae β-galactosidase activity. In the same way, the decrease in π at high WP concentrations is probably the consequence of low water activities and intensification of the inhibitory effect of WP salts. Cruz-Guerrero et al. (Reference Cruz-Guerrero, Gómez-Ruiz, Viniegra-González, Bárzana and García-Garibay2006) have reported that the reaction rate of synthesis of GOS decreases significantly with a reduction in the water activity, thus explaining the decrease observed in π at higher WP concentrations.
The concentrations of total GOS synthesised from WP are comparable to those obtained using pure lactose as substrate (Neri et al. Reference Neri, Balcão, Costa, Rocha, Ferreira, Torres, Rodrigues, Carvalho and Teixeira2009; Vera et al. Reference Vera, Guerrero and Illanes2011a, Reference Vera, Guerrero, Illanes and Conejerosb). At optimum conditions for the synthesis of GOS, Y was only 4% lower than the value obtained using pure lactose (Vera et al. Reference Vera, Guerrero, Conejeros and Illanes2012). In addition, the relative composition of oligosaccharides with different degrees of polymerisation is similar to that obtained using A. oryzae β-galactosidase and pure lactose as reagent, GOS 3 being the prebiotic in higher concentration (Guerrero et al. Reference Guerrero, Vera, Plou and Illanes2011).
It has been reported that several species of lactic acid bacteria are capable of growth in WP 5 g/100 ml (Cui et al. Reference Cui, Wan, Li, Liu and Rajashekara2012). WP used in this work as culture medium has a much higher content of solids (20 g solids/100 g suspension), a clear adverse condition for bacterial growth. In spite of that, WP was used as culture medium because of two facts: (a) they were the best ones for GOS synthesis and (b) the content of solids is required for the subsequent dehydration processes. Even under these unfavourable conditions, bacterial counts of Lb. plantarum 299v in WP and WP-GOS were comparable to those obtained using MRS containing GOS or glucose as the only carbon source (Hernandez-Hernandez et al. Reference Hernandez-Hernandez, Muthaiyan, Moreno, Montilla, Sanz and Ricke2012). This represents a significant economic advantage in the growth of this microorganism at industrial level.
It must be pointed out that the relative increase in the concentration of GOS 3 and GOS 4 during growth in WP-GOS is due to the consumption of glucose as carbon source, since the absolute concentration of GOS remained unchanged. In this way, GOS remain available for their consumption by the gut microflora. Numerous health claims have been made on behalf of prebiotics from investigations undertaken in vivo and in vitro. Most of the research has been focused on the fermentability and bifidogenicity activity of the GOS. Many authors have reported that GOS are substrate for the growth of human faecal flora, including bifidobacteria and lactobacilli (Bouhnik et al. Reference Bouhnik, Flourié, D'Agay-Abensour, Pochart, Gramet, Durand and Rambaud1997; Gopal et al. Reference Gopal, Sullivan and Smart2001; Amaretti et al. Reference Amaretti, Bernardi, Tamburini, Zanoni, Lomma, Matteuzzi and Rossi2007; Cardelle-Cobas et al. Reference Cardelle-Cobas, Fernández, Salazar, Martínez-Villaluenga, Villamiel, Ruas-Madiedo and de los Reyes-Gavilán2009; Ignatova et al. Reference Ignatova, Iliev, Kirilov, Vassileva, Dalgalarrondo, Haertlé, Chobert and Ivanova2009; Hernandez-Hernandez et al. Reference Hernandez-Hernandez, Muthaiyan, Moreno, Montilla, Sanz and Ricke2012).
GOS are mainly known because of their prebiotic properties, but little attention has been paid to other interesting properties already demonstrated in vitro. For example, it has been reported that GOS can mimic surface receptors of eurkaryotic cells inhibiting the attachment of certain pathogens to intestinal cells (Shoaf et al. Reference Shoaf, Mulvey, Armstrong and Hutkins2006). It was also reported that the growth of some Lactobacillus strains in the presence of different prebiotics may increase the bacterial resistance to gastrointestinal conditions (Hernandez-Hernandez et al. Reference Hernandez-Hernandez, Muthaiyan, Moreno, Montilla, Sanz and Ricke2012). Considering this, the appropriate growth of Lb. plantarum 299v in WP-GOS reported in this work could enhance its probiotic properties. Since Lb. plantarum 299v was grown and directly dehydrated in WP-GOS, the high concentration of GOS in the culture supernatants contributes to obtain a dried product with high concentration of probiotics and prebiotics in a single step.
Preservation of probiotic bacteria is necessary for potential applications in functional foods. Spray drying and freeze drying have proved to be suitable methods to obtain viable dehydrated microorganisms, although their success is strain-dependent. Lb. plantarum 299v grown in both WP and WP-GOS proved to be highly resistant to both dehydration processes, and also to storage. The high stability (>108 CFU/g) of spray-dried can be ascribed to the fact that water activity was close to the optimal values reported by Laroche et al. (Reference Laroche, Fine and Gervais2005).
This indicates that spray-drying is a highly efficient process for dehydration and storage of Lb. plantarum v299. Considering that spray-drying is about 10-fold cheaper than freeze drying (Ananta et al. Reference Ananta, Birkeland, Corcoran, Fitzgerald, Hinz, Klijn, Mättö and Mercernier2004), the use of this process is highly recommended for obtaining a synbiotic product at a lower cost.
The higher stability during storage of freeze-dried Lb. plantarum 299v grown in WP-GOS than grown in WP (without GOS) could indicate a protective effect of GOS during storage, as already demonstrated in Lactobacillus delbrueckii subsp. bulgaricus (Tymczyszyn et al. Reference Tymczyszyn, Gerbino, Illanes and Gómez-Zavaglia2011, Reference Tymczyszyn, Sosa, Gerbino, Hugo, Gómez-Zavaglia and Schebor2012).
Finally, the strategy developed in this work may contribute to expand the applications of GOS derived from WP in the elaboration of products containing both prebiotics and probiotics, which is important in the design of new functional foods and feeds.
This work has been funded by the Argentinean Agency for the Promotion of Science and Technology (ANPCyT) [Projects PICT 2008-145; PICT 2010-0586, PICT 2011-226], Argentinean National Research Council (CONICET) [Project PIP 114-201101-00024], the Chilean Fondecyt (Grant 110050), the Pontificia Universidad Católica de Valparaíso (Grant 037-112/2008) and Spanish MCINN (Project BIO2007-67708-C04-01) the bilateral Argentinean/Chilean project MinCyT/Conicyt [CH913]; and the Ibero-American Program for the Development of Science and Technology (CYTED; Network 312RT0463). MG, AGZ, and EET are members of the Research Career, Conicet (National Research Council, Argentina). MS and PC are fellows from CONICET. CG is fellow from the Chilean government (MECESUP2 UCV 0608) and CV is fellow from Conicyt, Chile (ref. 21080173).










