Cheese whey is the green-yellowish liquid produced during the traditional cheese manufacture process resulting from the precipitation and removal of milk casein (Siso, Reference Siso1996). This wastewater possesses a relatively high organic load, monitored by BOD (biological oxygen demand) and COD (chemical oxygen demand), due mainly to the presence of milk carbohydrates (lactose) and proteins (Prazeres et al. Reference Prazeres, Carvalho and Rivas2012). Cheese whey is simultaneously an effluent with strong organic and saline content but also with a high nutritional value and with many possibilities for technological exploitation. For instance it can be further processed for ricotta production, where another wastewater, named ricotta whey, is produced. The first step in most procedures for cheese whey valorisation consists in the recovery of the protein fraction, typically achieved by ultrafiltration, to produce whey protein concentrates (WPC). High volumes of a lactose-rich stream, whey permeate, is obtained at the same time (Guimarães et al. Reference Guimarães, Teixeira and Domingues2010). The production of whey powder is the most elementary form of adding value to whey. While the value of whey powder seems to be real enough compared with the alternative of feeding the liquid whey to pigs and/or cattle, cheese manufacturers are faced with large investments if they want to transform liquid whey into saleable products (Peters, Reference Peters2005).
Probiotics were defined as live microorganisms that when administered can confer a health benefit on the host (FAO/WHO, 2002). To produce probiotics in adequate amounts for industrial applications, the growth media need to be optimised for the specific strain aiming for increased biomass yield and reduction of production costs. This is achieved in two types of fermentation media: synthetic and dairy based media. For industrial use, and before probiotics can be supplied to the market, the cultures need to be prepared for transport and storage. Live bacteria used in functional foods are generally stored and shipped in dried or deep-frozen forms. Dried cultures are preferred over frozen forms because of the ease of long-term storage and shipping without the use of specialised refrigerated containers. Freeze drying is the main form of drying probiotics for application in foods. Other drying methods include spray drying, vacuum drying, fluidised bed drying or a combination of drying techniques (Muller et al. Reference Muller, Ross, Fitzgerald, Stanton, Charalampopoulos and Rastall2009). Spray drying is a low-cost alternative to freeze drying because it is relatively inexpensive and allows the continuous production of large amounts of dried cells in a continuous process (Gardiner et al. Reference Gardiner, O'Sullivan, Kelly, Auty, Fitzgerald, Collins, Ross and Stanton2000). However, cell dehydration by spray drying implies harsher conditions than freeze drying. This might inevitably cause membrane damage and inactivation, depending on the technological conditions applied and the intrinsic resistance of the strain used. In our laboratory, the practical application of freeze drying to lactic acid bacteria (LAB) cultures, compared with spray drying, suggests that the former is appropriate for almost all LAB strains, whereas the feasibility of application of spray drying seems to be much more strain dependent (Gardiner et al. Reference Gardiner, O'Sullivan, Kelly, Auty, Fitzgerald, Collins, Ross and Stanton2000; O'Riordan et al. Reference O'Riordan, Andrews, Buckle and Conway2001; Desmond et al. Reference Desmond, Stanton, Fitzgerald, Collins and Ross2002; Lian et al. Reference Lian, Hsiao and Chou2002; Corcoran et al. Reference Corcoran, Ross, Fitzgerald and Stanton2004; Ananta et al. Reference Ananta, Volkert and Knorr2005). In particular in Argentina, many medium to large-size dairy industries possess spray drying infrastructure, which could be exploited for the production of spray dried probiotics in the future. In addition, freeze drying is still rare at the industrial scale for LAB in our country. Spray drying can offer a 6 times less expensive alternative for every kg water removed compared with freeze drying (Knorr, Reference Knorr1998). A more recent work estimates that, whereas fixed cost may be similar, the manufacturing costs of freeze drying are 5 times more expensive than spray drying (Dominguez, Reference Dominguez and Moo-Young2011). In a previous work, we observed the suitability of cheese whey for the biomass production of commercial probiotic bacteria (Burns et al. Reference Burns, Molinari, Vinderola and Reinheimer2008) and the suitability of spray drying in skim milk for the dehydration of commercial probiotic cultures (Páez et al. Reference Páez, Lavari, Vinderola, Audero, Cuatrin, Zariztky and Reinheimer2012). Additionally, an enhanced functional capacity of spray dried cultures, compared with fresh ones, was observed in an in vivo model of gut mucosal immunostimulation in mice (Páez et al. Reference Páez, Lavari, Audero, Cuatrin, Zariztky, Reinheimer and Vinderola2013). The aim of this work was to study the suitability of dairy by-products (cheese and ricotta whey and whey permeate) for the biomass production of autochthonous LAB with probiotic potential and the capacity of cheese whey to be used as thermoprotectant for spray drying.
Materials and methods
Strains and culture conditions
Lb. paracasei JP1, Lb. rhamnosus 64 and Lb. gasseri 37 were used in this study. Strains were isolated in our laboratory from faeces of neonates (Vinderola et al. Reference Vinderola, Capellini, Villarreal, Suárez, Quiberoni and Reinheimer2008) and were shown to display probiotic properties related to the ability to promote gut defences mediated by IgA in mice (Gregoret et al. Reference Gregoret, Perezlindo, Vinderola, Reinheimer and Binetti2013). The strains are kept in the culture collection of the INLAIN (UNL-CONICET, Santa Fe, Argentina). When needed, overnight cultures (16 h, 37 °C) were obtained in MRS (de Man, Rogosa and Sharpe) broth (Biokar, Beauvais, France), after three transfers from frozen (−70 °C) stocks maintained in MRS added with 18% (w/t) glycerol (Ciccarelli, Santa Fe, Argentina).
Growth in in-house formulated broth and in dairy media
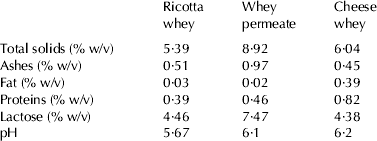
Different simplified versions of commercial MRS were prepared (Table 1), named in-house formulated broth, using ingredients from local providers (casein and meat peptone and yeast extract from Microquin S.A., Santa Fe, Argentina and the rest of the components were obtained from Cicarelli, Reagents S.A., Santa Fe, Argentina). In-house formulated broths were autoclaved at 121 °C for 15 min. The aim of assessing formulated growth media using ingredients from local providers (less expensive than imported commercial media) was to determine their capacity to support the growth of the strains under study in case a cost reduction is needed when scaling-up the biomass production in the future. The variants studied included a reduced amount of nutrients compared with MRS, glucose as sole carbon source, enhanced glucose or reduced in casein peptone, meat peptone or yeast extract (Table 1). Dairy by-products were used as well to assess the growth capacity of probiotic lactobacilli and their potential for biomass production. Liquid cheese whey, ricotta whey and whey permeate were obtained fresh from local dairy industries. The physicochemical characteristics are displayed in Table 2 and were determined by standard procedures: fat, International Dairy Federation (IDF, 1987a); total protein (IDF, 2001); total solids (IDF, 1987b); ash (AOAC, 1995) and lactose by difference. Ricotta whey and whey permeate were autoclaved at 121 °C for 15 min. To avoid protein precipitation by autoclaving at 121 °C for 15 min, cheese whey was treated at 100 °C for 30 min. Routine microbiological assessment was performed in order to confirm microbial inactivation after heat treatment. Overnight fresh cultures of the strains under study (MRS, 37 °C, 16 h, aerobic incubation) were centrifuged (6000 g, 15 min, 5 °C), washed twice with and resuspended in PBS (phosphate-buffered saline) solution (pH 7·4). Cell suspensions were inoculated (1% v/v) in in-house formulated broth and in dairy media and incubated overnight (37 °C, 16 h, aerobic incubation). Cell counts were performed in MRS agar. Biological Oxygen Demand (BOD) was assessed in cheese whey, before and after growth of Lb. rhamnosus 64, by standard methods (APHA, 1998) used for the examination of water and wastewater by the Chemistry Laboratory of the Universidad Tecnológica Nacional, Facultad Regional Rafaela, Rafaela, Santa Fe, Argentina.
Table 1. Composition (g/100 ml) of in-house formulated culture broth used for biomass production of probiotic lactobacilli

† MRS composition (g/100 ml): polypeptone (1), meat extract (1), yeast extract (0·5), glucose (2), Tween 80 (108 μl), K2HPO4 (0·2), sodium acetate (0·5), ammonium citrate (0·2), magnesium sulphate (0·02), manganese sulphate (0·005)
Table 2. Composition (g/100 ml) and pH of dairy by-products used in this study
Optimisation of spray drying of cheese whey
Powdered cheese whey was obtained from a local dairy plant. A suspension of 10 g of cheese whey and 10 g of starch (Glutal S.A., Buenos Aires, Argentina) in 200 ml of water was prepared and autoclaved at 121 °C for 15 min. Cheese whey-starch suspension was spray dried in a laboratory scale spray dryer (Buchi mini spray dryer model B290, Flawil, Switzerland). The spray draying parameters were optimised by Response Surface Methodology (RSM), using a Central Composite design (CCD) (Table 3). The CCD used has two levels, and needs fewer experiments than other designs. The independent variables used were air flux and inlet temperature, using two levels for each of them. The outcome variables considered for optimisation were moisture (less than 5%) and outlet temperature. Three repetitions for the centre points were carried out.
Table 3. Central compose design used to optimise the spray drying of cheese whey-starch solution using surface response methodology

† mm the column of the equipment used, correspond to 601 l/h (50 mm), 473 l/h (40 mm) and 357 l/h (30 mm)
Spray drying of probiotic lactobacilli in cheese whey
Overnight cultures of the three strains obtained in in-house formulated culture media were harvested (6000 g, 15 min, 5 °C), washed twice with PBS and re-suspended, 10× concentrated, in 200 ml water with 10 g cheese whey- 10 g starch or in 200 ml water with 10 g skim milk- 10 g starch. According to previous results, drying in skim milk-starch was done as a reference condition (Páez et al. Reference Páez, Lavari, Vinderola, Audero, Cuatrin, Zariztky and Reinheimer2012). The addition of starch enhances physical properties of the powders obtained (removal from cyclone and manipulation) and cell viability after drying and resistance to simulated gastrointestinal digestion, according to previous findings (Páez et al. Reference Páez, Lavari, Vinderola, Audero, Cuatrin, Zariztky and Reinheimer2012). Cell suspensions were spray dried in the laboratory scale spray dryer (Buchi B290). A constant inlet air temperature of 170 °C, an outlet temperature of 85 °C and an air flux of 600 l/h were used for skim milk-starch suspensions and an inlet temperature of 150 °C, an outlet temperature of 80 °C and 357 l/h of air flux were used for cheese whey-starch suspensions (see Results and Discussion section). Cell suspensions were atomised and sprayed into the drying chamber by using a two-fluid nozzle. The product dried almost instantaneously and the residence time was negligible. Three independent replicates were performed for each strain. Spray dried powders were vacuum sealed in individual samples of 10 g and stored for 6 months at 5 °C. Residual moisture (% w/w) was determined in triplicate at 101±1 °C overnight (IDF, 1993). Cell counts of lactobacilli were performed on MRS agar (37 °C, 48 h, aerobic incubation) before and after spray drying and every two months during storage.
Statistical analysis
The results of cell counts were transformed to log10 CFU/ml and expressed as mean±sd or log difference of at least three independent experiments in each trial. The data of growth in the culture media and the data of survival to spray drying and during storage were analysed statistically using a completely randomised design with a factorial arrangement of treatments to determine differences in the growth of the strains in the culture media or in their survival to drying and storage. Strains (3 levels) and the culture media (7 levels for in-house formulated broth and 4 levels for dairy media including MRS as reference) were considered as factors in the first design whereas strains (3 levels) and the carriers (2 levels) were considered as factors in the second design. Analysis of variance and the least significant difference (LSD) were used to test significant factors and to compare treatment means, respectively, with an error level of 5%. The statistical program used was SAS® 2002–2003 through the MIXED procedure.
Results and discussion
Growth in in-house formulated and dairy media
The increasing demand for probiotics and the new foods into which they are incorporated, challenge the industry to produce high amounts of viable cultures in stable forms (Muller et al. Reference Muller, Ross, Fitzgerald, Stanton, Charalampopoulos and Rastall2009). A combination of lower cost culture media and lower-operating cost and widely available drying technologies, such as spray drying, is likely to be welcome by the food industry for the diversification of the market of probiotics and for local developments based on autochthonous strains.
Depending on the culture medium considered, the behaviour of the strains was different. This means that the interaction strain-culture medium was statistically significant (P<0·05). We determined for which strain-culture medium combinations there were statistical differences. The culture media formulated (Table 1) were a simplified version of the commercial culture medium MRS, having in mind the possibility of industrial bulk production of these strains in the future. The six different variants were satisfactory for the growth of Lb. paracasei JP1, as no significant differences (P>0·05) were observed in cell counts when compared with those achieved in MRS (Table 4). The cost analysis (data not shown) of the in-house formulated culture media showed that their costs were around a third of MRS, paving the way for their use at a higher scale. For Lb. rhamnosus 64, M0 and M2 were as effective as MRS for biomass production. However, Lb. gasseri 37 showed significant differences (P<0·05) in all in-house formulated media compared with MRS. This strain seemed to be more nutrient demanding and the formulated culture medium had to be reinforced with nutrients (composition shown at the bottom of Table 4) to achieve a growth (9·16±0·21 log CFU/ml) comparable with that obtained in MRS (9·23±0·21 log CFU/ml).
Table 4. Cell counts of overnight cultures (37 °C, 16 h, aerobic incubation) of probiotic lactobacilli in commercial MRS broth compared with in-house formulated broths
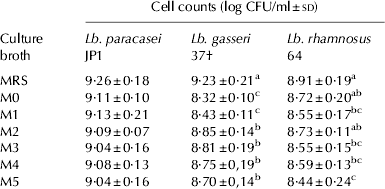
a,b,cValues in columns with different superscripts are significantly different (P<0·05).
† Composition of enhanced in-house formulated broth for adequate biomass production of this strain: (g/100 ml): polypeptone (0·75), meat extract (0·75), yeast extract (0·75), glucose (1), Tween 80 (50 μl), K2HPO4 (0·2), sodium acetate (0·5), ammonium citrate (0·2), magnesium sulphate (0·02), manganese sulphate (0·005).
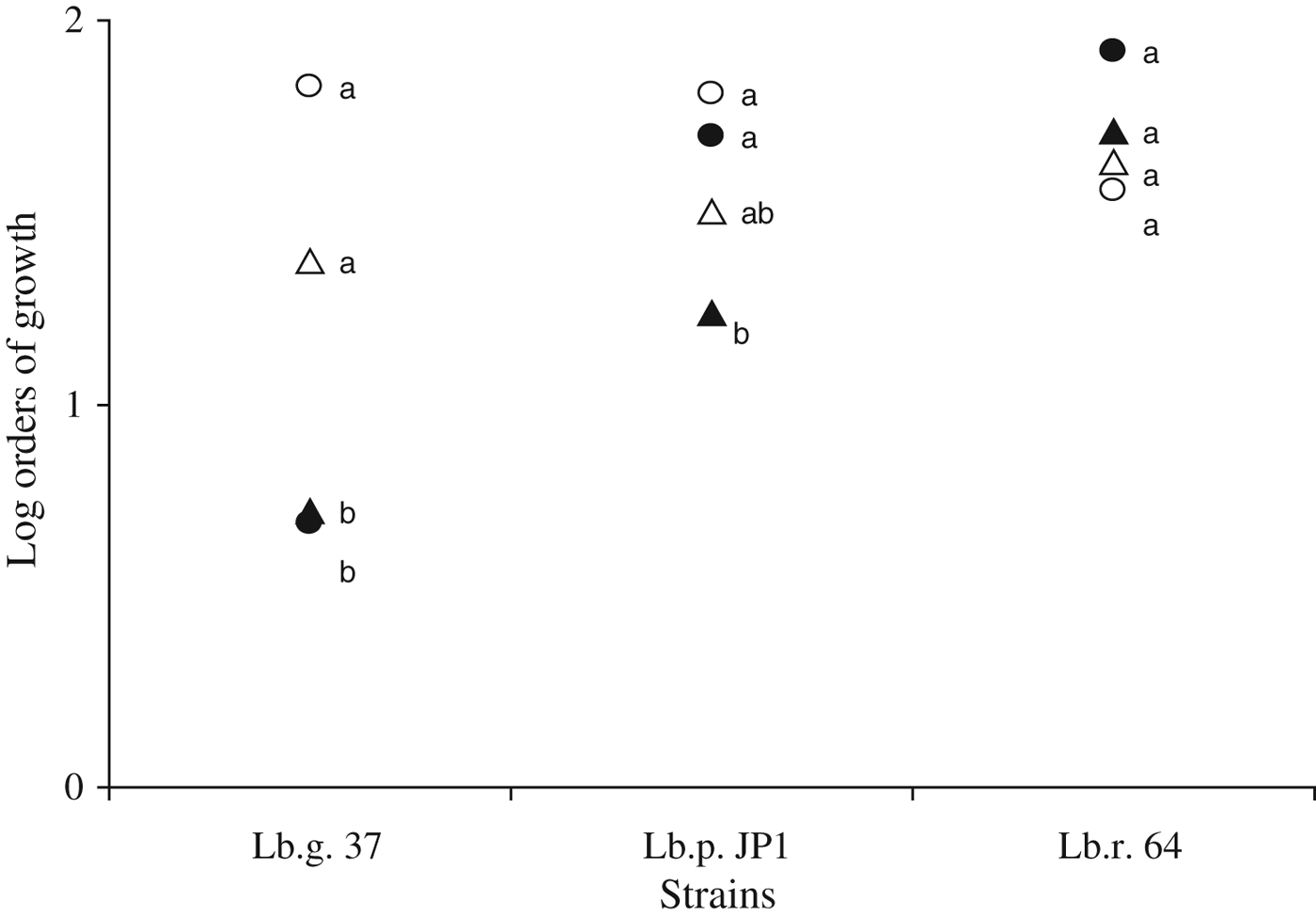
The growth capacity of the strains was also assessed in dairy by-products (Fig. 1). Growth of Lb. gasseri 37 in MRS and cheese whey was not different. However the strain achieved a significant lower growth (less than 1 log order) in ricotta whey and whey permeate compared with MRS. For Lb. paracasei JP1 growth was not satisfactory in whey permeate only. In fact, whey permeate alone was reported to be rather poor to support the growth of LAB (Parente & Zottola, Reference Parente and Zottola1991). However, for Lb. rhamnosus 64 no differences were observed among culture media assessed, showing an interesting technological versatility of this strain from an industrial point of view for biomass production. The addition of glucose and/or yeast extract had no impact on the growth of any of the strains under study in all dairy media assessed (data not shown). Most of the work conducted on dairy based media as growth substrate for probiotic bacteria was performed in cheese whey (Pérez Guerra et al. Reference Pérez Guerra, Fajardo Bernárdez, Méndez, Cachaldor and Pastrana Castro2007; Burns et al. Reference Burns, Molinari, Vinderola and Reinheimer2008; Fajardo Bernárdez et al. Reference Fajardo Bernárdez, Rodríguez Amado, Pastrana Castro and Pérez Guerra2008; Aguirre-Ezkauriatza et al. Reference Aguirre-Ezkauriatza, Aguilar Yáñez, Ramírez Medrano and Alvarez2010; Brusch Brinques et al. Reference Brusch Brinques, Do Carmo Peralba and Záchia Ayub2010), compared with the few reports about the exploitation of ricotta whey (Maragkoudakis et al. Reference Maragkoudakis, Nardi, Bovo, Corich and Giacomini2010) or whey permeate (Parente & Zottola, Reference Parente and Zottola1991). As regards the biological oxygen demand (BOD) of cheese whey, the growth of Lb. rhamnosus 64 reduced this value from 65 500±3540 to 33 062±4860 ppm. This ca. 50% reduction in BOD contributes to a significant reduction of the cheese whey polluting load. This is also an important issue as the cheese whey used in this study presented an initial BOD higher than the one commonly reported as usual for this product (30 000–50 000 ppm, according to Siso, Reference Siso1996). Considering that cheese whey is the main by-product of the dairy industry (De Castro Cislaghi et al. Reference De Castro Cislaghi, Dos Reis E Silva, Fritzen Freire, Goulart Lorenz and Sant'Anna2012) and that it satisfactorily supported the growth of the strains, it was chosen for further studies about its role as thermoprotectant for spray drying.

Fig. 1. Log orders of growth of Lb. gasseri 37 (Lb.g. 37), Lb. paracasei JP1 (Lb.p. JP1) and Lb. rhamnosus 64 (Lb.r. 64) in MRS (○), cheese permeate (●) cheese-whey (△), or ricotta whey (▲). Values were calculated as the difference in cell counts (CFU/ml) after and before growth (37 °C, 18 h aerobic incubation) in the corresponding medium. Values of log orders of growth, for the same strain, with different letter are significantly different (P<0·05).
Optimisation of spray drying of cheese whey-starch solution
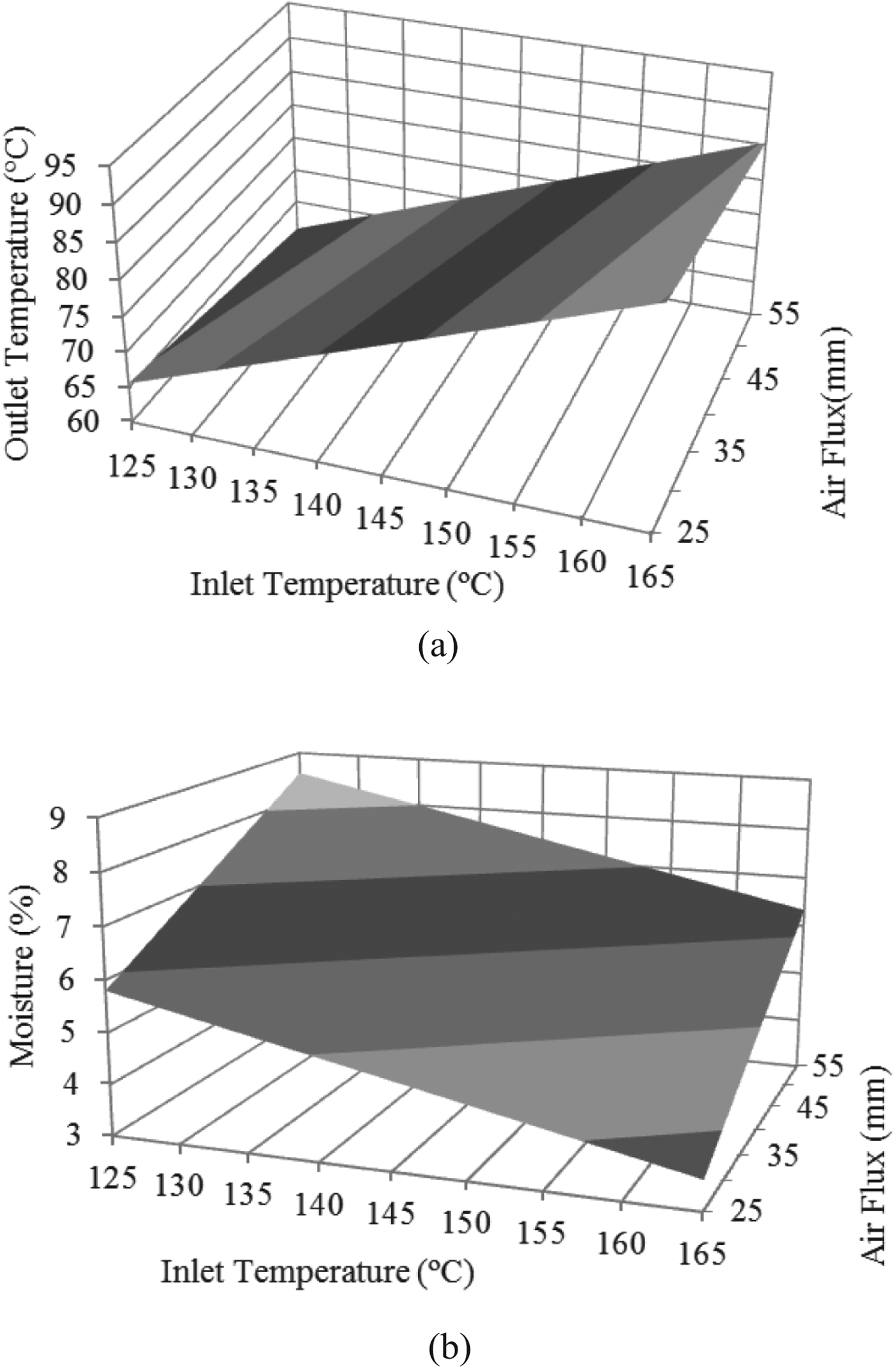
Few studies were devoted to the optimisation of the dehydration of the carrier matrix before adding viable cell cultures and there are no reports on the optimisation of the spray drying of cheese whey using Response Surface Methodology. Cheese whey was not spray dried alone but in the presence of 50% (w/w) of starch, as it was reported that it enhances the drying process, powder recovery and viability during storage (O'Riordan et al. Reference O'Riordan, Andrews, Buckle and Conway2001; Gharsallaoui et al. Reference Gharsallaoui, Roudaut, Chambin, Voilley and Saurel2007; Páez et al. Reference Páez, Lavari, Vinderola, Audero, Cuatrin, Zariztky and Reinheimer2012). The income variables used were air flux and inlet temperature whereas the outcome variables were moisture and outlet temperature. A maximum admissible value of moisture of less than 5% was set, as insufficient or too extensive dehydration (moisture>5% (w/w) or <2·8% (w/w), respectively) causes bacterial inactivation (Zayed & Roos, Reference Zayed and Roos2004). A maximal admissible outlet temperature of 85 °C was also set for adequate cell viability, according to previous findings (Peighambardoust et al. Reference Peighambardoust, Tafti and Hesari2011). The adjusted model obtained for moisture was: M (% w/t)=11·02−0·05×IT+0·08×AF (P=0·07), where M, IT and AF are moisture, inlet temperature and air flux respectively. In Fig. 2, moisture decreased as inlet temperature increased, whereas moisture increased with air flux. The adjusted model obtained for outlet temperature (OT) is: OT=−4·22+0·63×IT−0·28×AF (P<0·05). According to the model applied, spray drying of cheese whey-starch can be carried out with an IT of 145–155 °C and an AF of 30 mm (357 l/h), achieving an OT of less than 85 °C and obtaining a powder with moisture of 4–5% (w/w).

Fig. 2. Surface response curves obtained for the optimisation of spray-drying of cheese-whey-starch solution. (a) Air flux and inlet temperatures were used as independent variables and (b) moisture and outlet temperature (O.T.) as outcome variables.
In the present setting of the equipment used, if air flux is below 30 mm (357 l/h), the product cannot be dried whereas an inlet temperature between 145 and 155 °C resulted in adequate moisture (4–5% w/w). Increasing outlet temperature (Gardiner et al. Reference Gardiner, O'Sullivan, Kelly, Auty, Fitzgerald, Collins, Ross and Stanton2000) and moisture (Jantzen et al. Reference Jantzen, Göpel and Beermann2013) was related with lower survival rates. In relation to yield, approximately 4–5 g every 100 ml of solution (ca. 25% of the initial total solids) were recovered from the cyclone. This yield might seem low, however it must be taken into account that the equipment used operates in a batch mode, whereas spray drying at larger scale operates in a continuous fashion. Then, the stickiness of the powder due to the lactose content determines a dead volume, or dead amount of dried powder, that might be significant when drying small volumes in a batch mode (as it was this case), but that will gradually lose importance when drying higher volumes of solution in a continuous way.
Spray drying of probiotic lactobacilli in cheese whey-starch solution
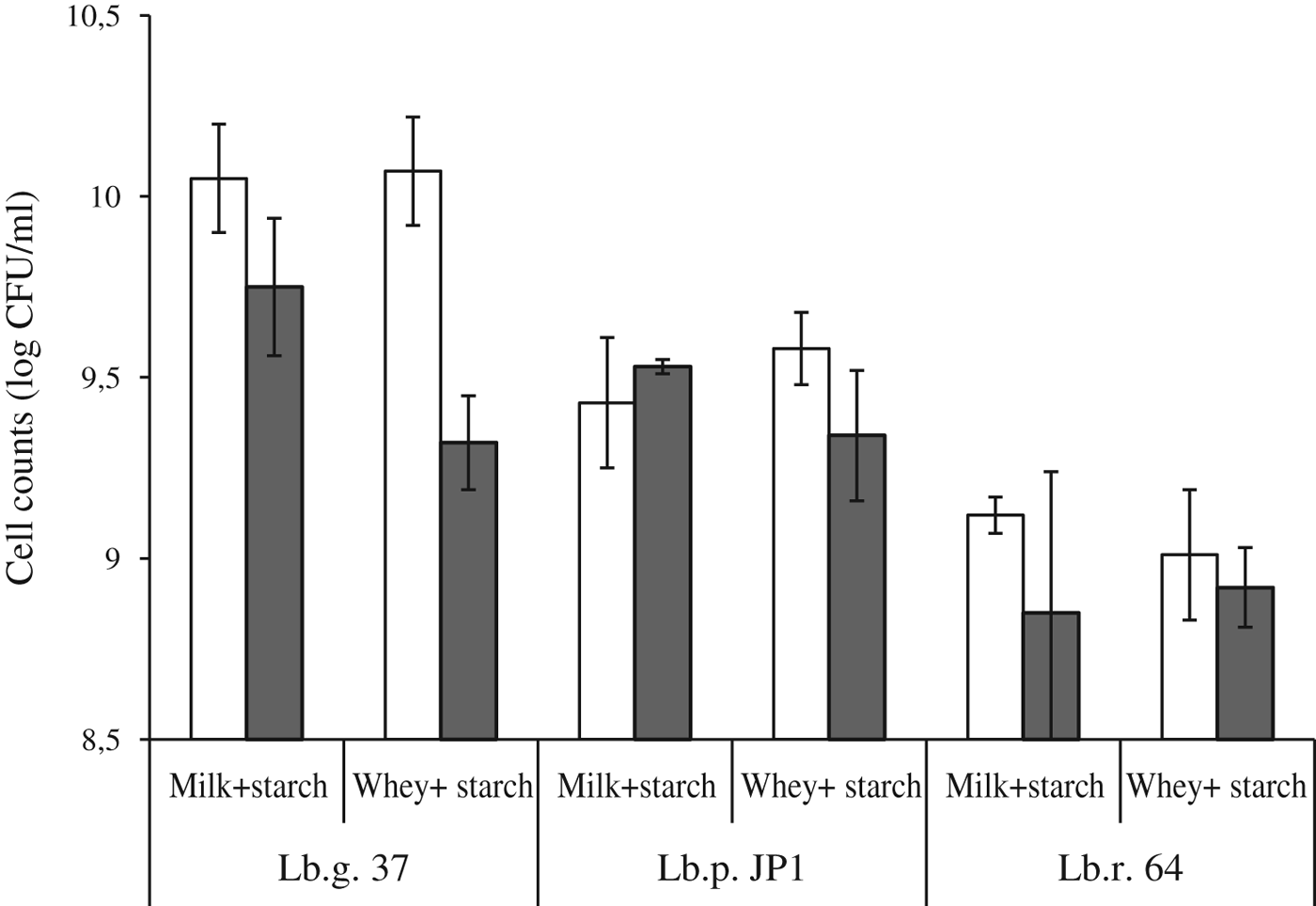
Considering the area defined as optimal for spray drying of cheese whey-starch solution, the strains under study were spray dried using an air flux of 30 mm (357 l/h) and an inlet temperature of 150 °C in cheese whey-starch solution and in skim milk-starch solution (different conditions of drying were used for skim milk-starch solution, according to previous reports, see Materials and Methods), the latter being a reference thermoprotectant according to Páez et al. (Reference Páez, Lavari, Vinderola, Audero, Cuatrin, Zariztky and Reinheimer2012, Reference Páez, Lavari, Audero, Cuatrin, Zariztky, Reinheimer and Vinderola2013). Significant reductions in cell counts, before and after spray drying, were observed for Lb. gasseri 37 in skim milk-starch (P=0·0013) and in whey-starch (P<0·0001). For Lb. paracasei JP1, no differences in counts were observed in skim milk-starch but in cheese whey-starch counts were significantly lower after drying (P=0·0231). Finally, counts of Lb. rhamnosus 64 were significantly lower (P=0·0167) after drying in skim milk-starch, but no differences were observed when dried in cheese whey-starch (Fig. 3).

Fig. 3. Cell counts of Lb. gasseri 37 (Lb.g. 37), Lb. paracasei JP1 (Lb.p. JP1) and Lb. rhamnosus 64 (Lb.r. 64) before (□) and after (![]() ) spray-drying in 200 ml of water with 10 g cheese whey- 10 g starch or in 200 ml water with 10 g skim milk-10 g starch. Counts after spray-drying were expressed in CFU/ml because powders obtained were reconstituted to the original volumes taking into account the amount of water removed during drying.
) spray-drying in 200 ml of water with 10 g cheese whey- 10 g starch or in 200 ml water with 10 g skim milk-10 g starch. Counts after spray-drying were expressed in CFU/ml because powders obtained were reconstituted to the original volumes taking into account the amount of water removed during drying.
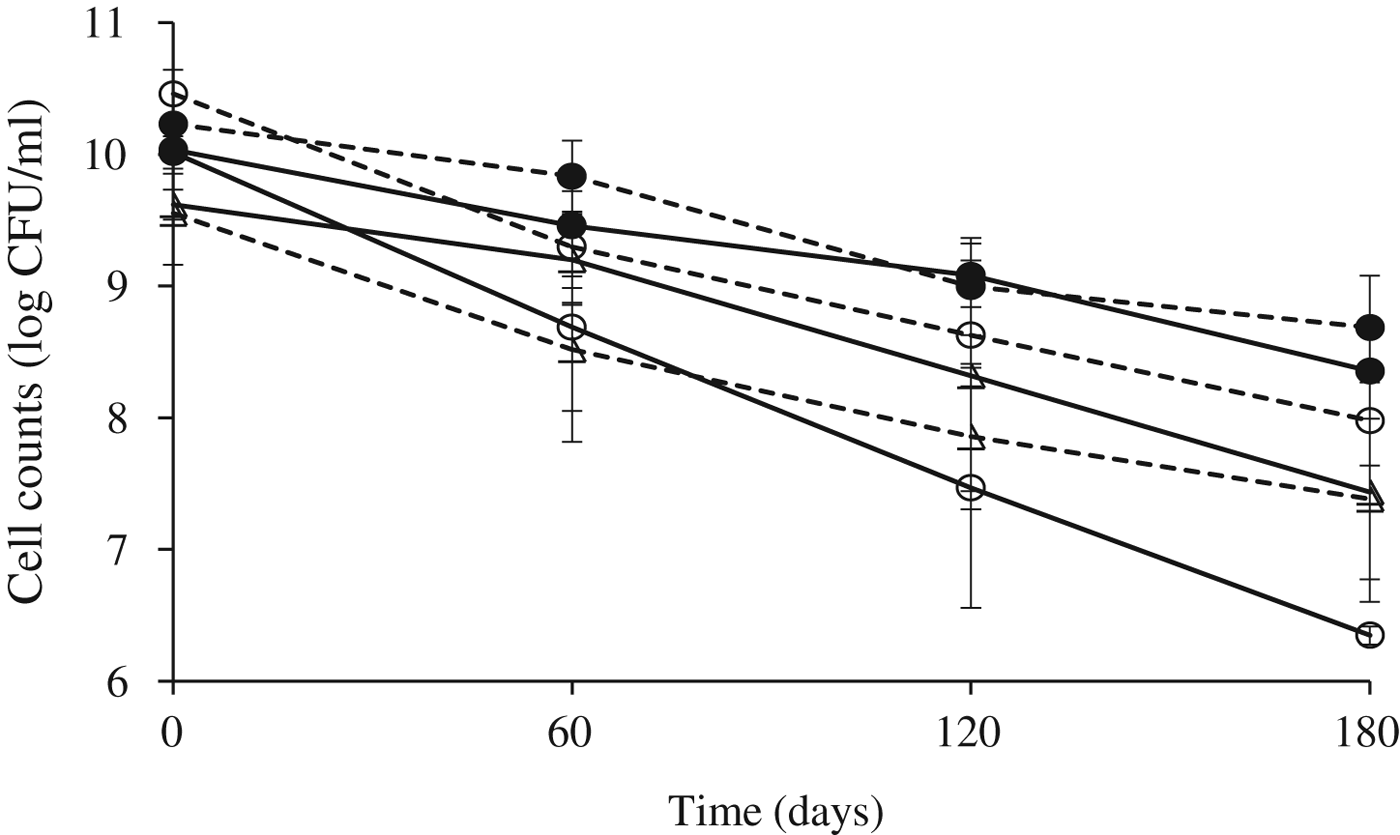
During storage of powders at 5 °C, survival was dependent on the strain and on the carrier used (Fig. 4). By the second month of storage, a lost in cell viability of 1·2–1·3 and of 0·4–0·6 log orders was observed for Lb. gasseri 37 and Lb. paracasei JP1, respectively, without significant differences with respect to the carrier considered for Lb. paracasei JP1, but for Lb. gasseri 37 counts in skim milk-starch were significantly higher (P=0·0457) than those in cheese whey-starch. For Lb. rhamnosus 64 cell reduction in skim milk was of ca. 1 log order whereas in cheese whey it was of less than 0·5 log order. By 6 months of storage, reductions in log orders were of 2·2 and 1·6 for Lb. rhamnosus 64 and Lb. paracasei JP1, respectively, irrespective of the carrier used. For Lb. gasseri 37 losses in log orders of viable cells were of 2·5 and 3·7 in skim milk and cheese whey, respectively, being statistically different (P=0·0006). No significant differences in counts between skim milk-starch and cheese whey-starch were observed during storage for Lb. rhamnosus 64 and Lb. paracasei JP1. However, for Lb. gasseri 37 counts in skim milk-starch were higher than those in cheese whey-starch by month 2 (P=0·0457), 4 (P=0·0003) and 6 (P=0·0006) of storage.

Fig. 4. Cell counts of Lb. gasseri 37 (○), Lb. paracasei JP1 (●) and Lb. rhamnosus 64 (△) in spray-dried skim milk-starch (dashed lines) or in cheese whey- starch (full lines) during the storage (days) at 5 °C. Counts after spray-drying were expressed in CFU/ml because powders obtained were reconstituted to the original volumes taking into account the amount of water removed during drying.
Survival during storage was strain and carrier-dependent, as previously reported by Lian et al. (Reference Lian, Hsiao and Chou2002) for different strains of bifidobacteria spray dried in 10% (w/w) gelatin, gum arabic and soluble starch. Most of the work about microencapsulation of probiotic bifidobacteria and lactic acid bacteria in dairy-based carriers was conducted using skim milk as the main component of the thermoprotectant medium (Teixeira et al. Reference Teixeira, Castro, Malcata and Kirby1995; Gardiner et al. Reference Gardiner, O'Sullivan, Kelly, Auty, Fitzgerald, Collins, Ross and Stanton2000, Reference Gardiner, Bouchier, O'Sullivan, Kelly, Collins, Fitzgerald, Ross and Stanton2002; Desmond et al. Reference Desmond, Stanton, Fitzgerald, Collins and Ross2002; Lian et al. Reference Lian, Hsiao and Chou2002; Corcoran et al. Reference Corcoran, Ross, Fitzgerald and Stanton2004; Ananta et al. Reference Ananta, Volkert and Knorr2005; Simpson et al. Reference Simpson, Stanton, Fitzgerald and Ross2005; Golowczyc et al. Reference Golowczyc, Silva, Teixeira, De Antoni and Abraham2011; Fritzen Freire et al. Reference Fritzen Freire, Prudêncio, Ambonia, Pinto, Negrão Murakami and Murakami2012). Other coating materials used for spray drying of probiotics were starch (O'Riordan et al. Reference O'Riordan, Andrews, Buckle and Conway2001), cellulose acetate phthalate (Fávaro Trindade & Grosso, Reference Fávaro Trindade and Grosso2002), buttermilk (Curda et al. Reference Curda, Rudolfová, Stetina and Dryák2006) and cocoa powder (Ricci et al. Reference Ricci, Borgo, Ferrario and Fortina2011). Fewer and more recent reports can be found where cheese whey itself (De Castro Cislaghi et al. Reference De Castro Cislaghi, Dos Reis E Silva, Fritzen Freire, Goulart Lorenz and Sant'Anna2012; Jantzen et al. Reference Jantzen, Göpel and Beermann2013) or whey protein concentrates (Rodrigues et al. Reference Rodrigues, Sousa, Rocha Santos, Silva, Sousa Lobo, Costa, Amaral, Pintado, Gomes, Malcata and Freitas2011; Ying et al. Reference Ying, Sun, Sanguansri, Weerakkody and Augustin2012; Soukoulis et al. Reference Soukoulis, Behboudi-Jobbehdar, Yonekura, Parmenter and Fisk2013) were used as carriers, indicating then that these ingredients were not yet fully exploited in this regard. In particular, in our study Lb. rhamnosus 64 displayed a negligible lost in cell viability only during a short-term storage (2 months) at 5 °C. Except in the report of De Castro Cislaghi et al. (Reference De Castro Cislaghi, Dos Reis E Silva, Fritzen Freire, Goulart Lorenz and Sant'Anna2012), where no lost in cell viability was observed throughout 12 months of storage at 4 °C, in the rest of the works (Rodrigues et al. Reference Rodrigues, Sousa, Rocha Santos, Silva, Sousa Lobo, Costa, Amaral, Pintado, Gomes, Malcata and Freitas2011; Ying et al. Reference Ying, Sun, Sanguansri, Weerakkody and Augustin2012; Jantzen et al. Reference Jantzen, Göpel and Beermann2013; Soukoulis et al. Reference Soukoulis, Behboudi-Jobbehdar, Yonekura, Parmenter and Fisk2013) where whey or whey proteins were used as the sole or the main component of the drying carrier, 1–2 log orders of cell death were observed during the first two months of storage at 5 °C, except in the work of Ying et al. (Reference Ying, Sun, Sanguansri, Weerakkody and Augustin2012) where a storage temperature of 25 °C was used. It is interesting to note that in the present work and in the reports of Rodrigues et al. (Reference Rodrigues, Sousa, Rocha Santos, Silva, Sousa Lobo, Costa, Amaral, Pintado, Gomes, Malcata and Freitas2011), Ying et al. (Reference Ying, Sun, Sanguansri, Weerakkody and Augustin2012) and Soukoulis et al. (Reference Soukoulis, Behboudi-Jobbehdar, Yonekura, Parmenter and Fisk2013), an outlet temperature higher than 75 °C was used, whereas in the work of De Castro Cislaghi et al. (Reference De Castro Cislaghi, Dos Reis E Silva, Fritzen Freire, Goulart Lorenz and Sant'Anna2012) and Jantzen et al. (Reference Jantzen, Göpel and Beermann2013) outlet temperature below 65 °C was employed. Outlet temperature might be one of the main factors to consider when using whey or whey components for drying probiotic cultures. However, the lower the outlet temperature the higher the moisture content (Jantzen et al. Reference Jantzen, Göpel and Beermann2013) and this may also make the drying process too slow. Other factors, beyond outlet temperature, that may affect cell viability to spray drying and through storage are the intrinsic resistance of the strain to heat, the carrier, the presence (or not) of glass transition state, the water activity and residual moisture and the storage conditions (mainly low temperatures and absence of oxygen and light) (Chávez & Ledeboer, Reference Chávez and Ledeboer2007).
The double use of cheese whey (growth substrate and thermoprotectant) is an innovative technological option due to the possibility to give new uses to the large amounts of whey produced world-wide. In this regards, only one study was reported so far concerning the direct spray drying of probiotic Lb. reuteri from slurry fermentation with whey (Jantzen et al. Reference Jantzen, Göpel and Beermann2013).
Considering the growth capacity in in-house formulated culture media and in dairy media, the resistance to spray drying in cheese whey-starch solution and the negligible lost in cell viability during a short-term storage (2 months), Lb. rhamnosus 64 seems to be promissory for further technological studies. However, some strategies must be applied in order to enhance survival during storage, such as sublethal thermal stress applied to cell suspensions before spray drying, which proved to be successful for enhanced survival during storage in previous works (Desmond et al. Reference Desmond, Stanton, Fitzgerald, Collins and Ross2002; Páez et al. Reference Páez, Lavari, Vinderola, Audero, Cuatrin, Zariztky and Reinheimer2012). Another possible strategy that will be explored for enhanced biomass production is the use of fed-batch biomass production in biofermentor (Aguirre-Ezkauriatza et al. Reference Aguirre-Ezkauriatza, Aguilar Yáñez, Ramírez Medrano and Alvarez2010) or the gradual addition of possible limiting nutrients.
This work was supported by the following projects: ‘Nuevas estrategias para aumentar la funcionalidad de alimentos y cepas probióticas’, PICT Bicentenario 2010 No 0161, ANPCyT, 2011–2013 and ‘Ecosuero con valor agregado’, Proyecto FONARSEC Convocatoria FITS Agroindustria 2010-Lactosueros, 2011- 2014. Luisina Lavari is doctoral fellow of CONICET (Argentina).