Organic milk in the Nordic countries is in most cases not homogenized, in order to maintain a more original milk. This milk is, therefore, more susceptible to creaming due to larger fat globules compared to homogenized milk. Additionally, the scarcely studied phenomenon, called ‘cold agglutination’, plays a key role in the mechanism and the resulting consumer acceptance of non-homogenized milks.
Natural creaming of stored raw milk is a well-known phenomenon. It occurs because milk fat globules possess lower density than milk serum and are therefore disposed to rise upwards forming a creaming layer on top of the remaining skim milk (Fredrick et al. Reference Fredrick, Walstra and Dewettinck2009). This process is accelerated at low temperatures due to ‘cold agglutination’. Current consensus is that immunoglobulins (Ig's) adhere to fat globules and somehow agglutinate these together in large floccules at reduced temperatures. The floccules have lower density than individual milk fat globules, thus they rise more rapidly upwards. On their way up they overtake small and single fat globules causing increased flocculation and rising. The result is a rapidly formed and harder cream layer on top of the milk (Payens et al. Reference Payens, Koops and Kerkhof Mogot1965; Huppertz & Kelly, Reference Huppertz, Kelly, Fox and Sweeney2006), which can be manually shaken apart by consumers. Even so, the majority of conventional, commercial milk is homogenized in order to meet consumer demands for a stable product. In addition to reducing milk fat globule size, homogenization inactivates the agglutination complex (Frenyo et al. Reference Frenyo, Butler and Guidry1986), probably through denaturation of Ig's (Huppertz & Kelly, Reference Huppertz, Kelly, Fox and Sweeney2006). Due to the lack of homogenization of most organic Nordic milk, the agglutination mechanism is retained as long as temperature during the pasteurization process is not too high. However, the complex formation has been shown to be inactivated at temperatures between 62 and 81 °C (Caplan et al. Reference Caplan, Melilli and Barbano2013; Mainer et al. Reference Mainer, Sánchez, Ena and Calvo1997). Heat-induced deactivation of the agglutination mechanism causes the milk fat globules to interact with each other directly through milk fat globule membrane (MFGM) proteins or through heat-denatured casein micelles (Ye et al. Reference Ye, Singh, Taylor and Anema2004). As these interactions are stronger than the agglutinin-mediated mechanism, a significantly harder cream layer is formed. This layer does not dissolve upon shaking in contrast to the agglutinin-mediated soft cream layer. Rather, solid fat lumps are formed, resulting in consumer rejection. Therefore, even a few degrees of variation during pasteurization can mean the difference between consumer acceptance (a soft, re-dissolvable cream layer formed by the retained agglutination process) and consumer rejection (insoluble fat layer caused by heat-inactivated agglutination process).
The agglutinins responsible for creaming in milk are not fully described, and research in this area is limited. In addition to milk fat globules the complex is assumed to consist of immunoglobulin M (IgM) and lipoproteins of skim milk membranes (Huppertz et al. Reference Huppertz, Kelly, Fox and Tamime2009), also known as extracellular vesicles (Blans et al. Reference Blans, Hansen, Sørensen, Hvam, Howard, Möller, Wiking, Larsen and Rasmussen2017).
In general, it is assumed that IgM acts as a cryoagglutinin; i.e. at cold temperatures it forms an interaction between milk fat globule membrane proteins and lipoproteins of the milk serum through specific carbohydrate moieties (Huppertz & Kelly, Reference Huppertz, Kelly, Fox and Sweeney2006). For example, Euber et al. (Reference Euber, Brunner, Nilsson, Mattsson and Singher1984) showed that IgM played a key role in promoting clustering of milk fat globules at cold temperatures. In addition to IgM, immunoglobulin A (IgA) has also been shown to associate with milk fat globules and enhance the creaming activity (Honkanen-Buzalski & Sandholm, Reference Honkanen-Buzalski and Sandholm1981). A third component of the immune system, the poly immunoglobulin receptor (pIgR), may play a supportive role in agglutination, since IgA and IgM are transported into milk by pIgR (Stadtmueller et al. Reference Stadtmueller, Huey-Tubman, López, Yang, Hubbell and Bjorkman2016). pIgR binds polymeric IgA and IgM on the basolateral membrane of the mammary gland epithelial cells and transports them across the cell to the apical membrane, where the Ig's are ultimately secreted into the milk (Honkanen-Buzalski & Sandholm, Reference Honkanen-Buzalski and Sandholm1981; Stadtmueller et al. Reference Stadtmueller, Huey-Tubman, López, Yang, Hubbell and Bjorkman2016).
Despite efforts to describe agglutination, the phenomenon has so far only been observed macroscopically, e.g. by measuring degree of creaming (Caplan et al. Reference Caplan, Melilli and Barbano2013; Geer & Barbano, Reference Geer and Barbano2014) or adsorption of IgM to milk fat (Honkanen-Buzalski & Sandholm, Reference Honkanen-Buzalski and Sandholm1981). IgM has been visualized by confocal laser scanning microscopy (CLSM) using a fluorescent antibody in other research fields (human blood lymphocytes; Miyazaki et al. Reference Miyazaki, Nishimoto, Sasaki and Sugahara1998; neurons; Lombardi et al. Reference Lombardi, Erne, Lauria, Pareyson, Borgna, Morbin, Arnold, Czaplinski, Fuhr and Schaeren-Wiemers2005), however, the process of agglutination has not been investigated at a molecular level. All in all, there is a need for more research into the details of agglutination of milk. A deeper understanding of the agglutination process would aid Nordic organic dairies in deciding the right pasteurization time and temperature without compromising microbial safety.
The aim of the present study was to deploy a novel visualization technique for the process of agglutination, including the role of IgM. Using confocal laser scanning microscopy (CLSM) supported by 2-D sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of milk fractions, the effect of storage temperature (two h at 5, 15, 20 or 37 °C) on agglutination in raw milk was studied. Finally, the inactivation of the agglutinin complex with increasing pasteurization temperatures (20 s at 69, 71 or 73 °C) was examined.
Materials and methods
Materials
Raw bulk milk was obtained from the university herd of Danish Holstein dairy cows at Research Centre Foulum (Tjele, Denmark). Fluoresceinisothiocyanat (FITC)-conjugated bovine IgM antibody from Bethyl Laboratories (Montgomery, TX, USA) and rhodamine from Avanti Polar Lipids (Alabaster, AL, USA) were used as fluorescence stain agents with green and red colors, respectively. All other chemicals were of analytical grade and supplied by Sigma-Aldrich (St. Louis, MO, USA).
Heat treatment of milk
Five ml of milk was transferred to pre-warmed glass tubes and immediately placed in a water bath at 69, 71 or 73 °C, respectively. The holding time was 20 s after reaching pasteurization temperature. The time for obtaining the terminal temperatures were 2 min 30 s for 69 °C, 3 min 10 s for 71 °C and 3 min 20 s for 73 °C. After pasteurization samples were immediately cooled in an ice bath.
Visualizing agglutinins in milk by confocal laser scanning microscope (CLSM)
The attachment of IgM to the MFGM was analysed by an inverted CLSM microscope (Nikon C2, Nikon Instrument Inc, Tokyo, Japan). Raw milk was heated to 37 °C and 250 µl was mixed with an equal volume of 50 mm phosphate buffer (pH 6.8) and 80 µl of FITC-conjugated bovine IgM antibody. Rhodamine was dissolved in chloroform to a final concentration of 0.01%. The dye was added onto a concave glass slide, and upon solvent evaporation the milk solution was deposited. The glass slides were incubated for two h at 5, 15, 20 or 37 °C before images were captured. The pasteurized milk samples were analysed a 4 °C. Argon 488 nm and HeNE 561 nm laser were used for excitation to induce fluorescence emission. A 60× water-immersion objective lens was used.
Washing of milk fat globules and preparation of MFGM
Upon heat treatment samples were stored overnight at 4 °C and washed in 50 mm phosphate buffer (pH 6.8) at two different temperatures, 4 °C (‘cold’) or 25 °C (‘warm’). The washing was done in order to remove skim milk proteins not attached to the MFGM. After the washing step samples were centrifuged at 2500 g for 20 min at the appropriate temperature (4 or 25 °C) followed by removal of the cream layer. One g of cream was subsequently heated to 45 °C for 30 min and finally churned by centrifugation at 17 900 g for 10 min at 40 °C. MFGM fragments were extracted from the churned material by mixing with 300 µl methanol and 600 µl chloroform followed by centrifugation at 10 600 g.
Two-dimensional gel electrophoresis
Proteins were separated in two dimensions as described by Dickow et al. (Reference Dickow, Larsen, Hammershøj and Wiking2011). Briefly, 160 µg of protein was loaded onto pI strips (Bio-Rad Laboratories, Hercules, CA, USA) and separated in the pH range of 5 to 8 in the first dimension. Next, the pI strip was set on top of the second dimension polyacrylamide gel (8–16% gradient, Bio-Rad Laboratories) and the loaded proteins were separated according to molecular weight. Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS) was carried out as described by Larsen et al. (Reference Larsen, Wedholm-Pallas, Lindmark-Månsson and Andrén2010) to identify individual protein spots. All experiments were conducted in triplicate.
Results and discussion
Visualizing agglutinins in milk by confocal laser scanning microscope
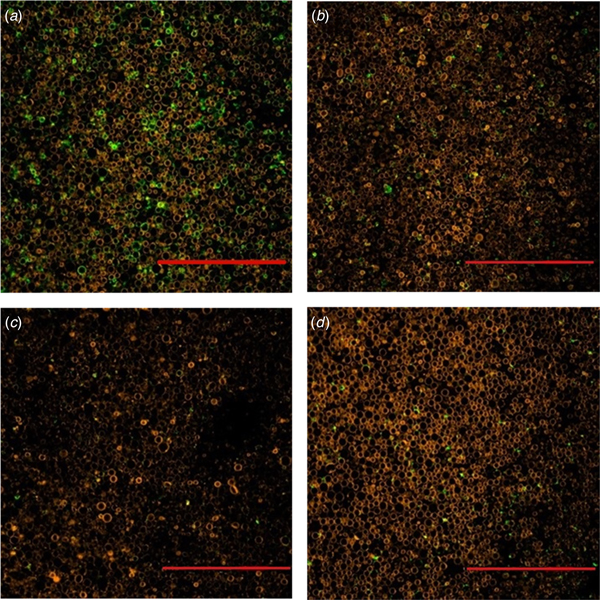
To illustrate the effect of storage temperature on the agglutination mechanism, raw milk samples were incubated at 5, 15, 20 or 37 °C for 2 h after addition of staining agents specific for MFGM and IgM, respectively. Figure 1 presents CLSM images, where presence of IgM is marked by green colour, whilst phospholipids of the MFGM are visualized by red. Clear differences in the association between IgM and fat globules were observed for the different temperatures. Surrounding most milk fat globules at 5 °C, IgM was omnipresent and formed agglutination between individual milk fat globules. Already at 15 °C, the extent of IgM around the milk fat globule was markedly reduced and only sporadic agglutinins were observed. This reduction in green colour meant that IgM molecules were not complexed to the fat globules, since the resolution of CLSM does not allow detection of individual IgM molecules, but merely its complexation to the fat globules. The absence of agglutinin complexes was even more pronounced at 20 and 37 °C, where only little IgM is visible. Figure 1 visually documents the process of cold agglutination by the milk fat globule-IgM-complexes formed only at cold temperatures. These novel observations confirm the agglutinin model put forward by Euber et al. (Reference Euber, Brunner, Nilsson, Mattsson and Singher1984). This model describes the agglutinin complexes as being ‘glued’ together by IgM through interactions with carbohydrate moieties in the lipoproteins of MFGM and in lipoproteins present in skim milk membranes. In this way, milk fat globules are clustered together in a tighter structure, than if partial coalescence was the only phenomenon occurring (Fredrick et al. Reference Fredrick, Walstra and Dewettinck2009).

Fig. 1. CLSM images of raw milk incubated for two hours at (a) 5 °C, (b) 15 °C, (c) 20 °C, (d) 37 °C. Red colour is caused by rhodamine, which binds to PLs of the milk fat globule. Green colour origins from the FITC-conjugated IgM antibody binding to IgM's. Scale bar = 10 µm.
Figure 2 shows the thermal inactivation by pasteurizing for 20 s at 69, 71 or 73 °C on the milk fat globule-IgM-complexes. The non-pasteurized sample was characterized by the same pattern as described above, i.e. an omnipresent IgM, surrounding most milk fat globules and joining them in agglutination. However, pasteurization appeared to inactivate IgM, as the sample heated to 69 °C contained markedly less IgM than the unpasteurized sample. Moreover, further increasing the pasteurization temperature gave rise to a progressively stronger inactivation of IgM, evident from the gradual disappearance of IgM from the samples at 71 °C, which was even more pronounced at 73 °C. While agglutinins in general have been shown to be inactivated in a temperature range of 62 to 81 °C (Mainer et al. Reference Mainer, Sánchez, Ena and Calvo1997), IgM in particular is known to be heat-labile and is totally denatured after 20 min at 85 °C (Ustonol & Sypien, Reference Ustonol and Sypien1997). However, Mainer et al. (Reference Mainer, Sánchez, Ena and Calvo1997) showed that the overall quaternary structure of IgM was not denatured by a HTST (high temperature-short time; 72 °C, 15 s) treatment similar to that performed in the present study. Furthermore, Mainer et al. (Reference Mainer, Sánchez, Ena and Calvo1997) hypothesized that local domains of IgM directly involved in agglutination may become unfolded at lower temperatures than needed for unfolding of the entire molecule, thereby being partially inactivated with respect to agglutination behaviour. A more recent study showed absence of agglutination upon exposure to temperatures above 76.9 °C for 25 s (Caplan et al. Reference Caplan, Melilli and Barbano2013), which the authors ascribed to inactivation of the immunoglobulins involved in agglutination. Hence, preceding studies support the observed progressive decline in IgM activity with increasing temperatures. To sum up, particular attention should be paid especially to pasteurization temperature in the dairy-relevant range of 69–73 °C in order to avoid agglutinin components inactivation, in particular IgM.

Fig. 2. CLSM images captured at 4 °C from milk samples left untreated (a) or pasteurized at 69 (b), 71 (c) or 73 °C (c) for 20 s. Samples were coloured with rhodamine (red) and FITC-conjugated IgM antibody (green). Scale bar = 50 µm.
2D gel electrophoresis
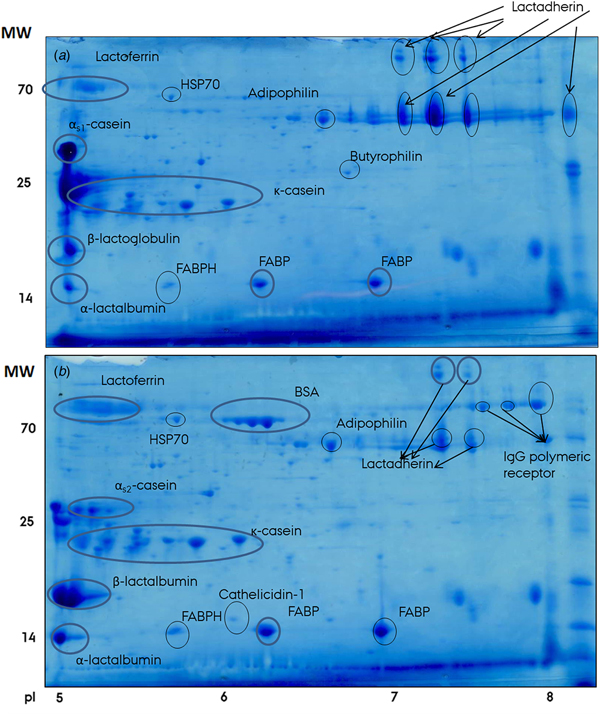
The effect of temperature on association of proteins to milk fat globules was studied by gel based proteomics of MFGM protein preparations isolated from milk at either cold (4 °C) or warm (25 °C) preceding storage, separated according to pI and molecular weight. Figure 3 shows the 2D SDS-PAGE gels of the two MFGM samples and also includes identified protein as determined by MALDI-TOF MS. It is evident that the composition of proteins associated with the MFGM was markedly different between cold and warm preparations. For instance, bovine serum albumin and lactoferrin were present in higher amounts in the ‘cold’ preparation compared to the ‘warm’, whilst the opposite was true for e.g. αs1-casein, β-lactoglobulin, lactadherin and α-lactalbumin. These observations are well supported by a former study from our lab (Dickow et al. Reference Dickow, Larsen, Hammershøj and Wiking2011), which demonstrated a change in distribution of proteins associated with the MFGM between the milk serum and cream phases upon cooling.

Fig. 3. Two-dimensional SDS-PAGE gels of MFGM samples from milk washed in warm (25 °C); (a) or cold (4 °C); (b) phosphate buffer. Proteins were separated according to pI 5–8 (horizontal dimension) and molecular weight (vertical dimension) and stained with Coomassie Brilliant Blue. Individual protein identities as determined by MALDI-TOF MS are denoted on the figure. BSA, bovine serum albumin; FABP, fatty acid-binding protein; FABPH, fatty acid-binding protein, heart; HSP70, 70 kDa heat shock protein; IgG, immunoglobulin G.
Of significance for understanding the nature of agglutination is the fact that polymeric immunoglobulin receptor (pIgR) was identified in cold, but not warm, MFGM preparations (Fig. 3). pIgR is a key component of the mammalian immune defense. It is expressed in epithelial cells of mammary glands, where it transports polymeric immunoglobulins, including IgM and IgA, from the basolateral to the apical membrane, eventually secreting them into the milk to aid the offspring's emerging immune system (Hood et al. Reference Hood, Kronenberg and Hunkapiller1985; Stadtmueller et al. Reference Stadtmueller, Huey-Tubman, López, Yang, Hubbell and Bjorkman2016). This explains its presence in the MFGM, as the latter is directly derived from the apical surface of mammary epithelial cells during the apocrine secretion of milk fat (Heid & Keenan, Reference Heid and Keenan2005). Due to its possibility of binding both IgM and IgA molecules, the exact same immunoglobulins as involved in agglutination, pIgR could be a marker for the presence of IgM and/or IgA and, consequently, whether agglutination can occur or not. As such, the absence of pIgR in warm MFGM samples confirmed the CLSM studies described earlier, which showed little agglutination at room temperature (Fig. 1). The opposite is true at lower temperatures; where pIgR was detected in conjunction with the MFGM fraction, agglutination was clearly visible by CLSM.
Detection of IgG receptor in wash water
Both light and heavy chains of IgG receptor were detected in a 2D SDS-PAGE of wash water from the isolation procedure of milk fat globule proteins (Fig. 4). This indicated that IgG was associated to a lesser extent with the fat globules and, hence, not a part of the agglutinin complex. This finding is supported by earlier studies showing the role of IgA and IgM, but not IgG, in agglutination (Euber et al. Reference Euber, Brunner, Nilsson, Mattsson and Singher1984; Honkanen-Buzalski & Sandholm, Reference Honkanen-Buzalski and Sandholm1981). In addition to IgG, other proteins were observed in the wash water, including bovine serum album, αs1-casein, β-lactoglobulin, lactadherin and α-lactalbumin. This is in accordance with studies by Dickow et al. (Reference Dickow, Larsen, Hammershøj and Wiking2011), which showed that the same proteins had low association with milk fat globules upon storage at 31 °C.

Fig. 4. Two-dimensional SDS-PAGE gels of wash water from the isolation of MFGM. Proteins were separated according to pI 5–8 (horizontal dimension) and molecular weight (vertical dimension) and subsequently stained with Coomassie Brilliant Blue. Individual protein identities as determined by MALDI-TOF MS are denoted on the figure. BSA, bovine serum albumin; IgG, immunoglobulin G; HC, heavy chain; LC, light chain.
Our studies revealed a redistribution of both IgA- and IgM-related proteins, as well as other protein species upon cooling. The potential bacterial association to fat globules through IgA (Honkanen-Buzalski & Sandholm, Reference Honkanen-Buzalski and Sandholm1981) and other Ig's (Caplan et al. Reference Caplan, Melilli and Barbano2013) as a function of heat treatment was not assessed in this study. For future studies it would be of great interest to study a possible correlation between agglutination and bacteria-IgA association.
Conclusion
For the first time CLSM has been used to visualize the role of IgM in cold agglutination. It was confirmed that IgM is a cryoglobulin involved in the agglutination complex responsible for the clustering of milk fat globules during cold storage. Storage temperature had a pronounced effect on the extent of agglutination, which was most pronounced at 5 °C and decreased progressively with increasing storage temperature (15, 20 and 37 °C). This confirmed the nature of cold agglutination. The results were supported by proteomics, showing the presence of the generic IgG receptor only in cold-stored MFGM preparations. Additionally, the effect of pasteurization temperature on agglutination was demonstrated. Increasing pasteurization temperature from 69 to 71 and 73 °C (all 20 s treatments) lead to a progressively higher degree of deactivation of IgM as an agglutinin resulting in decreased agglutination, showing the pronounced effect of pasteurization temperature on this phenomenon. Particular caution should be paid to temperature when pasteurizing non-homogenized milk in order not to deactivate the agglutinin complex, thereby leading instead to formation of insoluble fat aggregates in the products.
Acknowledgements
The authors wish to acknowledge Rita Albrechtsen and Hanne S. Møller for excellent technical assistance.
Financial support
None.