Probiotic bacteria are considered ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’ (FAO/WHO, 2002). The consumer's desire to use natural methods for health maintenance and disease prevention rather than long-term chemotherapeutic agents (i.e. antibiotics), linked to their expectation that food becomes a source of prolonged well-being, have notably augmented the consumption of probiotic foods and stimulated innovation and product development (Ong et al. Reference Ong, Henriksson and Shah2007). Beyond the health promoting effects, the overall properties, including sensory profile and nutritional value, of fermented milks, are important to consumers (Ott et al. Reference Ott, Hugi, Baumgartner and Chaintreau2000). Fermented milks obtained using only probiotic strains, mainly belonging to Bifidobacterium spp and Lb. acidophilus, are often characterised by the lack of desirable sensory features, texture and body (Penna et al. Reference Penna, Gurram and Barbosa-Canovas2006), whereas the physical properties such as firmness and the ability to retain water are the major criteria for quality assessment (Hassan et al. Reference Hassan, Frank, Schmidt and Shalabi1996). In order to improve the texture and body, several strategies have been proposed, such as the use of strains able to produce exopolysaccharides – instead of the addition of chemical additives (Lucey, Reference Lucey2004) which can adversely affect the product taste, flavour, aroma and mouth-feel (De Ancos et al. Reference De Ancos, Cano and Gomez2000) – or the addition of milk solids and/or whey protein concentrate (Mistry & Hassan, Reference Mistry and Hassan1992). Improved sensory properties have also been obtained through modulation of some physico-chemical and technological processes (Gardini et al. Reference Gardini, Lanciotti, Guerzoni and Torriani1999; Patrignani et al. Reference Patrignani, Lanciotti, Maina Mathara, Guerzoni and Holzapfel2006, Reference Patrignani, Iucci, Lanciotti, Vallicelli, Maina Mathara, Holzapfel and Guerzoni2007). HPH treatment has potential for several applications such as cell disruption for the recovery of intracellular bio-products and enzymes (Kelly & Muske, Reference Kelly and Muske2004), control and enhancement of proteolytic and fermentative activities of some Lactobacillus species (Coskun, Reference Coskun2006; Lanciotti et al. Reference Lanciotti, Patrignani, Iucci, Saracino and Guerzoni2007), modification of the microstructure and rheology of food emulsions (Guerzoni et al. Reference Guerzoni, Lanciotti, Westall and Pittia1997; Floury et al. Reference Floury, Desrumaux and Lardières2000), increasing cheese yield (Lanciotti et al. Reference Lanciotti, Chaves-Lopez, Patrignani, Papparella, Guerzoni, Serio and Suzzi2004a; Sandra & Dalgleish, Reference Sandra and Dalgleish2005; Burns et al. Reference Burns, Patrignani, Serrazanetti, Vinderola, Reinheimer, Lanciotti and Guerzoni2008). HPH also has great potential for the development of new products, differentiated from traditional products by sensory and structural characteristics or functional properties (Guerzoni et al. Reference Guerzoni, Vannini, Chaves-López, Lanciotti, Suzzi and Gianotti1999; Lanciotti et al. Reference Lanciotti, Vannini, Pittia and Guerzoni2004b; Madadlou et al. Reference Madadlou, Khosrowshahi, Mousavi and Emamdjome2006). However, little information is available concerning the fate of probiotic bacteria in fermented milks produced from HPH-treated milk or concerning the sensorial features of these products. In this context, the aim of the present study was to evaluate the potential of milk HPH treatment for the production of desirable probiotic fermented milks.
Materials and Methods
Starter and probiotic cultures
Streptococcus thermophilus Sty1 and Lactobacillus delbrueckii subsp. bulgaricus Lby1 (collection of the Dipartimento di Scienze degli Alimenti, University of Bologna, Italy), were used as fermentation starters for fermented milk production. Lb. acidophilus 08 and Lb. paracasei A13 (collection of the Instituto de Lactologia Industrial, UNL-CONICET, Santa Fe, Argentina), were used as probiotic adjunct cultures. The starter cultures were grown overnight (16 h) in Elliker and/or MRS broth (Biokar, Beauvais, France), respectively, at 37°C. These cultures were then transferred and maintained, as separate cultures, in reconstituted (100 g/l) skim milk powder (Oxoid, Basingstoke, UK). When needed, probiotic cultures were obtained in MRS broth (16 h, 37°C) and harvested by centrifugation (8000 g, 20 min, 4°C). Pellets were washed twice with 9 g NaCl/l distilled water and resuspended in sterile 100 g/l skim milk powder (Oxoid) before their addition to milk during the production of fermented milks.
Production of fermented milks
The production of fermented milks was carried out in a pilot-scale plant of a local dairy farm (Mambelli, Berinoro, Italy) using heat-treated (90°C, 20 min) reconstituted (100 g/l) skim milk powder (Oxoid). Half of the volume of heat-treated milk was further subjected to a HPH treatment. Heat-treated milk was homogenized at 60 MPa using a one-stage continual high pressure homogeniser PANDA (Niro Soavi, Parma, Italy) equipped with a PNSA valve with a flow rate of 10 l/h. The milk inlet temperature was of ca. 5°C; the temperature increase during homogenizing was monitored at the outlet product point. The milk outlet temperature did not exceed 20±2°C. Milk was collected in sterile receiving containers.
Four types of experimental fermented milks were produced as follows: i) from heat treated milk and starter cultures (HT); ii) from heat treated milk with starter and probiotic cultures (HT-Pro); iii) from heat- and HPH-treated milk plus starter cultures (HPH) and iv) from heat- and HPH-treated milk plus starter and probiotic cultures (HPH-Pro).
Milk samples (1·5 l) were inoculated (1% v/v) with an overnight culture (in reconstituted skim milk powder) of the starter mix (Strep. thermophilus/Lb. delbrueckii subsp. bulgaricus, in a ratio 100:1). For the production of HT-Pro and HPH-Pro fermented milks, milk was additionally inoculated (1% v/v) with a washed overnight culture of Lb. acidophilus 08 and Lb. paracasei A13. After the inoculation, milk was fractioned in 100 ml sterile containers and incubated at 42°C in a water bath. When pH reached a value of 4·6, the fermented milks were immediately brought to 4°C and stored at that temperature. Duplicate microbiological and physico-chemical analyses of each fermented milk type were performed on samples produced in the same dairy plant on 3 consecutive days.
Microbiological analysis
Microbiological analyses were carried out at days 0, 7, 14, 21, 28 and 35 of refrigerated storage. For the enumeration of the starter and probiotic cultures and the total lactic microflora, 20 g fermented milk were placed in 180 ml 20 g sodium citrate/l sterile solution and homogenised in stomacher (Lab-blender 80, Pbi International, Milan, Italy) for 3 min. Decimal dilutions of the homogenates were made in 1 g peptone/l solution and 0·1 ml of appropriate dilutions were spread onto the surface of different agar media. Strep. thermophilus was counted on Elliker agar (Biokar, Beauvais, France) (37°C, 48 h), Lb. delbrueckii subsp. bulgaricus on MRS agar (Biokar, Beauvais, France) acidified with glacial acetic acid (Merck, Darmstadt, Germany) at pH 5·4 with anaerobic incubation (Oxoid BR38 kit) (42°C, 48 h). For the enumeration of Lb. paracasei and Lb. acidophilus, MRS-LP agar and MRS-bile agar (37°C, 48 h) (Vinderola & Reinheimer, Reference Vinderola and Reinheimer2000a), respectively, were used. The Total Lactic Microflora (TLM) was determined in Plate Count Agar (Oxoid, Basingstoke, UK) with 10% (w/v) skim milk powder (Oxoid) at 37°C for 48 h.
Texture analyses
After 12 h from coagulation, samples were analysed for their textural features. Firmness, consistency, cohesiveness and viscosity indexes were evaluated using a back extrusion cell (A/AB) on a Texture Analyser TA DHI (Stable Micro System, UK) according to the manufacturer's instructions. A solid rod (35 mm diameter) was thrust into a cylindrical container (48 mm diameter) holding 100 ml sample using a 5 kg load cell. Three independent measures were performed for each sample.
Aroma profiles
Volatile compounds were monitored at d 1 and 35 of refrigerated storage, using a GC-MS coupled with a solid phase microextraction (GC–MS-SPME) technique. Samples (5 g) were placed in 10 ml sterilized vials, sealed by PTFE/silicon septa and heated for 10 min at 45°C, after that a fused silica fibre covered by 75 μm Carboxen Polydimethyl Siloxane (CAR/PDMS) (Supelco, Steiheim, Germany) was introduced in the head-space for 50 min. Adsorbed molecules were desorbed in the gas-chromatograph for 5 min. For peak detection, an Agilent Hewlett–Packard 6890 GC gas-chromatograph equipped with a MS detector 5970 MSD (Hewlett–Packard, Geneva, Switzerland) and a Supelcovax 10 (60 m×0·32 i.d.) fused silica capillary column were used. The conditions were as follows: injection temperature, 250°C; detector temperature, 250°C; carrier gas (He) flow rate, 1 ml/min; splitting ratio, 1:20 (v/v). The oven temperature was programmed as follows: 50°C for 1 min; from 50°C to 65°C, at 4·5 deg C/min; from 60°C to 230°C, at 10 deg C/min, then holding for 25 min. Volatile peak identification was carried out by computer matching of mass spectral data with those of the compounds contained in the Agilent Hewlett–Packard NIST 98 and Wiley vers. 6 mass spectral database. The GC-SPME analyses were performed sampling three vials for each sample.
DNA extraction from pure cultures and total DNA extraction from fermented milk
For the pure culture genomic DNA and the total DNA from fermented milks, Insta Gene Matrix kit (BIO-RAD Laboratories Milano, Italy) and kit mo-bio (Cambrex Biosciences, Bergamo, Italy) were used, respectively.
DNA amplification
The PCR amplification of approximately 200 base pairs (bp) of the V2-V3 variable region of the 16S rRNA gene was obtained using the primers HDA1-GC (5′-AC TCC TCC TAC GGG AGG CAG CAG-3′; a 40-bp GC clamp was attached to the 5′ end of that primer) and HAD2 (5′-GTA TTA CCG CGG CTG CTG GCA-3′) (Walter et al. Reference Walter, Tannock, Tilsala-Timisjarvi, Rodtong, Loach, Munro and Alassatova2000). This PCR reaction and the amplification program were performed according to the methods proposed by Theunissen et al. (Reference Theunissen, Britza, Torriani and Witthuhn2005) and Fasoli et al. (Reference Fasoli, Marzotto, Rizzotti, Rossi, Dellaglio and Torriani2003), respectively. PCRs were performed with T 3000 Thermocycler (Biometra®, Göttingen, Germany).
Denaturing gradient gel electrophoresis
The PCR fragments were separated by denaturing gradient gel electrophoresis (DGGE) using the Bio-Rad DCodeTM Universal Mutation Detection System (Bio-Rad Laboratories, Milano, Italy). Separation of the PCR amplicons was obtained by the direct application of 35 ml PCR products onto 80 g/l polyacrylamide gels in 0·5 X TAE buffer containing a linear denaturant gradient of from 30 to 70% (v/v). The 100% denaturing solution contained 40% (v/v) formamide and 7·9 m-urea. Gels were electrophoresed as described by Theunissen et al. (Reference Theunissen, Britza, Torriani and Witthuhn2005). As markers, equal amounts of amplicons obtained from Lb. delbrueckii subsp. bulgaricus, Strep. thermophilus, Lb. paracasei and Lb. acidophilus were separately used.
Proteolysis
The proteolysis of different fermented milks was evaluated by SDS-PAGE electrophoresis after 12 h from coagulation. A Vertical System Hoefer SE 600 SERIES (Amersham Pharmacia Biotech, UK) was used. Sample loading volume and concentrations of separating and stacking gels were 20 μl, 15% (v/v) and 5% (v/v) acrylamide-bisacrylamide, respectively. Protein and peptide extracts were prepared homogenising 5 g cheese with 20 ml water for 3 min at 20°C and incubating for 1 h at pH 4·6 at 40°C according to the method of Kuchroo & Fox (Reference Kuchroo and Fox1982). Protein and large peptide solutions were prepared by heating 1 ml supernatants for 5 min at 95°C and adding 0·2 ml β-mercaptoethanol. Glycerol (0·2 ml) and 0·2 ml bromophenol (0·02% w/v) were added to the sample before loading on the gel. The standards used were made of SDS-PAGE Molecular Weight Standard Broad Range (BioRad Laboratories, Milano, Germany).
The gel was electrophoresed with constant voltage of 280 V and fixed and stained with Coomassie Blue G250 (Bio-Rad Laboratories, Milano, Italy) for 2 h and de-stained in a 5% (v/v) methanol and 7% (v/v) acetic acid solution for 2–4 h.
Sensory evaluation
The panel test of fermented milks was performed after 14 d refrigerated storage. Twenty-five trained evaluators tasted each sample served at 15°C under controlled conditions of environment and light according to Standard 8589 (ISO, 1988). The assessors were asked to evaluate colour, flavour, appearance, consistency, taste, acidity, aftertaste and overall acceptance attributing a score ranging between 0 (low or poor) to 10 (high or very excellent) point scale.
Statistical analysis of data
The microbiological, textural and panel assessor averaged data were analysed by one way analysis of variance (ANOVA) using the statistical package Statistica for Windows 6.1 (Statsoft Inc., Tulsa, OK). The ability of each descriptor to discriminate between samples was investigated using the post-hoc comparisons of the ANOVA.
Results
Viability of lactic acid bacteria during the storage period and pH
In Table 1, the viability of the total lactic microflora, starter cultures and probiotic bacteria during the storage at 4°C for 35 d of the fermented milks is reported. Strep. thermophilus and Lb. delbrueckii subsp. bulgaricus reached levels ranging from 8·7 to 9·1 log cfu/g and from 7·0 to 7·5 log cfu/g, respectively, in all samples at the end of the fermentation. After 35 d refrigerated storage, Strep. thermophilus maintained a higher viability in fermented milks made from HPH-milk than in those made from only pasteurized milk. The highest cell loss for Lb. delbrueckii subsp. bulgaricus was observed in HT-fermented milks, where its cell counts diminished to 5·0 log cfu/g, a significantly lower cell load than the ones observed in HPH-, HPH-Pro- or HT-Pro fermented milks. Lb. paracasei A13 survived over the entire storage period in both HT-Pro and HPH-Pro fermented milks, maintaining cell counts of at least 7·0 log cfu/g. A higher decrease in cell viability, with respect to initial counts, was evidenced for Lb. acidophilus 08, which attained final levels of approximately 5·0 log cfu/g, independently of the treatment of milk. The SDS-PAGE profiles of the different fermented milks manufactured presented a similar pattern (Fig. 1). The identity of the two probiotic strains, over the refrigerated refrigerated storage, was further confirmed by the molecular technique applied (Fig. 2). At the end of the storage, the bio-fermented milks were characterized by lower pH values (4·27±0·02, HPH-Pro and 4·28±0·03, HT-Pro) than those of fermented milks without probiotic cultures (4·45±0·03, HT and 4·55±0·02, HPH).

Fig. 1. SDS-PAGE profiles of the different fermented milks after 12 h from coagulation.

Fig. 2. Denaturating Gel Gradient Electrophoresysis (DGGE) profiles of amplicons from the different fermented milks over the refrigerated storage.
Table 1. Counts of viable bacteria (mean±sd log cfu/g) in the fermented milks produced

HPH: fermented milk from high pressure treated milk, HPH-Pro: fermented milk from high pressure treated milk inoculated with probiotic cultures, HT: fermented milk from heat treated milk, HT-Pro: fermented milk from heat treated milk inoculated with probiotic cultures
—: not added
For each column considered, 35 d values with the same superscript letter are not statistically different (P>0·05)
Texture analyses
Firmness, cohesiveness, consistency and viscosity index results are reported in Table 2. Generally, the coagula from HPH treated milk was significantly (P<0·05) more compacted (higher firmness) than the one obtained with only pasteurized milk, and it was characterized by the highest values of consistency, cohesiveness and viscosity indexes with respect to the fermented milks produced from milk without HPH treatment. In particular, in fermented milks from HPH treated milk, the consistency was significantly higher in the presence of probiotic cultures.
Table 2. Texture parameters (mean value±sd) for the different fermented milks after 24 h from coagulation

HPH: fermented milk from high pressure treated milk, HPH-Pro: fermented milk from high pressure treated milk inoculated with probiotic cultures, HT: fermented milk from heat treated milk, HT-Pro: fermented milk from heat treated milk inoculated with probiotic cultures
For each column considered, values with the same superscript letter are not statistically different (P>0·05)
Sensory analysis
After 14 d storage, no significant differences were observed for the descriptors considered, except for flavour and consistency attributes (Table 3). In fact, fermented milks produced from heat treated milk (HT) obtained the highest values for flavour, but they received the lowest scores for the attribute of consistency, in agreement with the texture analysis.
Table 3. Scores (mean value±sd) obtained for the different attributes in the sensory evaluation of the different fermented milks after 14 d of refrigerated storage
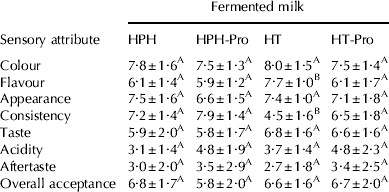
HPH: fermented milk from high pressure treated milk, HPH-Pro: fermented milk from high pressure treated milk inoculated with probiotic cultures, HT: fermented milk from heat treated milk, HT-Pro: fermented milk from heat treated milk inoculated with probiotic cultures
Values in the same row with the same superscript letter are not statistically different (P>0·05)
Profile of volatile compounds
In Table 4, the most important molecules, characterizing the aroma of fermented milks are reported. The highest amounts of acetone, 2-butanone and diacetyl, 24 h after coagulation, were detected in fermented milks made from HPH treated milk while a major quantity of acetaldehyde characterized the fermented milks obtained from heat treated milk containing probiotic cultures. Differently, acetic acid increased in both probiotic HT-Pro- and HPH-Pro-fermented milks. After 14 d storage, 2-butanone and diacetyl were higher in samples produced with the sole use of starter cultures (HPH- and HT-fermented milks) while acetaldehyde, acetone (2-propanone), acetic acid, 2-pentanone, hexanal, 2-methyl-3-pentanone, butanol, butanoic, hexanoic and octanoic acids were observed in higher concentrations in samples containing probiotic cultures (HPH-Pro- and HT-Pro-fermented milks). For acetoin, 3-decen-2-one and furfuryl alcohol, the highest amounts were determined in fermented milks elaborated from HPH treated milk.
Table 4. Volatile compounds (area×10−4) in fermented milks. The data reported are the means of 3 repetitions. The variability coefficient was always lower than 5%

HPH: fermented milk from high pressure treated milk, HPH-Pro: fermented milk from high pressure treated milk inoculated with probiotic cultures, HT: fermented milk from heat treated milk, HT-Pro: fermented milk from heat treated milk inoculated with probiotic cultures
n—: not detected
Discussion
Several strategies, including HPH, have been proposed to improve the sensorial features of probiotic fermented dairy products and/or to amplify the range of this sector (Patrignani et al. Reference Patrignani, Iucci, Lanciotti, Vallicelli, Maina Mathara, Holzapfel and Guerzoni2007). The data obtained in this experimentation show that the use of HPH treated milk favours the viability of starter cultures, particularly Strep. thermophilus, even at the end of the storage period without detrimental effect on the viability of probiotic bacteria. Higher levels of viable LAB at the end of the shelf life of HPH- or HPH-Pro-fermented milks is an interesting feature of the product due to the now recognized probiotic characteristics of yoghurt cultures (Guarner et al. Reference Guarner, Perdigon, Corthier, Salminen, Koletzko and Morelli2005). In agreement, Lanciotti et al. (Reference Lanciotti, Vannini, Pittia and Guerzoni2004b) evidenced that the HPH treatment of milk enhanced the viability of yoghurt starters during the refrigerated storage and favoured the growth of Strep. thermophilus with respect to Lb. delbrueckii subsp. bulgaricus, reducing the risks of post acidification.
In addition, Lb. acidophilus and Lb. paracasei attained values of ca. 5 and 7 log orders, respectively, at the end of the shelf life of the fermented milks. In this sense, fermented milks produced from HPH-treated milk were demonstrated to be as suitable as traditional yoghurts in relation to the viability of the strains of probiotic bacteria used in this study. Presumably, the use of more severe homogenizing treatments could have improved the viability also of the probiotic cultures in agreement with the findings of Burns et al (Reference Burns, Patrignani, Serrazanetti, Vinderola, Reinheimer, Lanciotti and Guerzoni2008). In fact, pressure levels of 100 MPa, applied to the milk for cheesemaking, were able to significantly increase the viability of the same probiotic cultures during the refrigerated storage of Crescenza cheese. The increased viability was attributed to the increased precocious availability of low molecular weight peptides and free fatty acids such as oleic acid, essential for the growth of many LAB (Guerzoni et al. Reference Guerzoni, Lanciotti and Cocconcelli2001). However, a marked increase of proteolytic and lipolytic patterns is regarded negatively for yoghurt and fermented milks. On the other hand, the electrophoretic profiles did not show significant differences among the samples, indicating that the chosen pressure level, able to modify the structure and organoleptic features, did not affect the hydrolytic patterns. Moreover, Patrignani et al. (Reference Patrignani, Iucci, Lanciotti, Vallicelli, Maina Mathara, Holzapfel and Guerzoni2007) reported levels ranging between 60–80 MPa as optimal both for the viability of probiotic and for the sensorial features of fermented milk obtained with the sole use of probiotic strains.
The higher sensitivity of Lb. acidophilus – compared with the Lb. casei group – to the stringent conditions of fermented milks has been reported. For instance, Nighswonger et al. (Reference Nighswonger, Brashears and Gilliland1996) found higher viability of Lb. casei compared with several strains of Lb. acidophilus in yoghurt and cultured buttermilk during refrigerated storage. Dave & Shah (Reference Dave and Shah1998) observed for Lb. acidophilus, incorporated into yoghurt, a 2 log order reduction whereas, depending on the type of yoghurt considered, Vinderola et al. (Reference Vinderola, Bailo and Reinheimer2000b) reported up to 4 log orders reduction in the counts of Lb. acidophilus. Moreover, in an earlier study (Gilliland & Speck, Reference Gilliland and Speck1977), Lb. acidophilus NCFM was not stable when added to yogurt made with Lb. delbrueckii subsp. bulgaricus and Strep. thermophilus. This fact was attributed to hydrogen peroxide formed by the Lactobacillus species and to the low pH reached in the product, which influenced the survival of the probiotic bacteria during the storage. However, probiotic properties have been still reported for dairy products containing non-viable bacteria (Ouwehand & Salminen, Reference Ouwehand and Salminen1998; Vinderola et al. Reference Vinderola, Duarte, Thangavel, Perdigon, Farnworth and Matar2005). Future in vivo studies shall determine whether fermented milks elaborated from HPH treated milk – containing lower levels of viable probiotic bacteria than at the beginning of the refrigerated storage – possess the functional characteristics of freshly-made products.
Acetaldehyde, ethanol, 2-propanone, diacetyl and 2-butanone are regarded as the main aroma compounds of yoghurt-type products (Ott et al. Reference Ott, Fay and Chaintreau1997) and they are required to give the characteristic flavour of those products (Tamime & Robinson, Reference Tamime, Robinson, Tamime and Robinson1999). In this study, all these compounds were detected both at the beginning and the end of the refrigerated storage. The loss of flavour in fermented milk products, due to the reduction of diacetyl, can be a problem (Obermanand & Libudzisz, Reference Oberman, Libudzisz and Wood1998). In our experimental conditions, the most relevant loss of diacetyl over the storage were observed in both fermented milks containing probiotic bacteria, that were characterized by the lowest pH values and the highest acetic acid peak area. Moreover, the panellists perceived as significantly different the flavour of the traditional yoghurt (heat-treated) with respect to the other products. However, all the samples received high scores for the descriptors considered, and were judged as presenting high quality. The lowest scores observed for HPH-Pro-fermented milks could be attributed to the different retention of flavour compounds dependent on the different gel network of proteins. In fact, HPH was reported to induce an increased exposure of hydrophobic regions of proteins (Guerzoni et al. Reference Guerzoni, Vannini, Chaves-López, Lanciotti, Suzzi and Gianotti1999). Moreover, the release of flavour compounds and their perception during consumption, which are a key quality parameter for foodstuff, is undoubtedly affected also by the food matrix and microstructure (Lanciotti et al. Reference Lanciotti, Vannini, Pittia and Guerzoni2004b). Although HPH is reported to affect the structure of basal milk, the entity of modifications induced on the food matrix depends on several factors including treatment severity (i.e. pressure level, number of pressure cycles), inlet and outlet temperatures of treated fluids as well as the physicochemical and compositional characteristics of raw material (Lanciotti et al. Reference Lanciotti, Gardini, Sinigaglia and Guerzoni1996; Vachon et al. Reference Vachon, Kheadr, Giasson, Paquin and Fliss2002; Patrignani et al. Reference Patrignani, Iucci, Lanciotti, Vallicelli, Maina Mathara, Holzapfel and Guerzoni2007). The manufactured yoghurts presented different rheological behaviours according to the initial milk treatment used and the presence of probiotic cultures. Generally the fermented milks manufactured from HPH milk were significantly (P<0·05) firmer, more viscous, more cohesive and more consistent than fermented milks produced from only heat treated milk. These results agree with the data of Penna et al. (Reference Penna, Gurram and Barbosa-Canovas2007a). The homogenization of milk is considered a common practise to improve yoghurt consistency and to produce firmer gels (Lucey & Singh, Reference Lucey and Singh1997; Lanciotti et al. Reference Lanciotti, Chaves-Lopez, Patrignani, Papparella, Guerzoni, Serio and Suzzi2004a, Reference Lanciotti, Vannini, Pittia and Guerzonib). The increase in homogenization pressure was reported to enhance viscosity of full fat yoghurts (Lanciotti, Reference Lanciotti, Vannini, Pittia and Guerzoni2004b). In fact, pressurization can modify the size of fat globules and casein micelles into smaller subunits with improved aggregating properties (Lopez-Fandiño et al. Reference Lopez-Fandiño, De la Fuente, Ramos and Olano1998; Lanciotti et al. Reference Lanciotti, Chaves-Lopez, Patrignani, Papparella, Guerzoni, Serio and Suzzi2004a, Reference Lanciotti, Vannini, Pittia and Guerzonib). The probiotic fermented milks elaborated in this study (HT-Pro- and HPH-Pro-fermented milks), if compared with their respective controls without probiotics, were characterized by the highest firmness and consistency values and lowest viscosity indexes. These results agree with those obtained by Penna et al. (Reference Penna, Rao-Gurram and Barbosa-Canovas2007b) who reported that probiotic strains combined with Strep. thermophilus and Lb. delbrueckii subsp. bulgaricus reduced viscosity compared with the yoghurt culture alone. Moreover, Shihata & Shah (Reference Shihata and Shah2002) and Hassan et al. (Reference Hassan, Frank, Schmidt and Shalabi1996) found that the indexes of viscosity and firmness were dependent of the type of cultures used as well as the final yoghurt pH. Generally, improvement of firmness is due to the attachment of mucogenic strains to the protein matrix via exopolysaccharides (Rawson & Marshall, Reference Rawson and Marshall1997) while the viscosity index seems to be affected by the presence of proteolytic strains able to hydrolyse proteins and reduce this parameter (Shihata & Shah, Reference Shihata and Shah2002). Thus, the apparent viscosity, as shown also in other studies (Faber et al. Reference Faber, Zoon, Kamerling and Vliegenthart1998) was not simply related to the concentration of exopolysaccharides in the fermented milks but dependent also of many other factors. In any case, the initial pressure treatment affected positively and significantly the coagulum texture parameters due to its ability to improve or modify textural and other organoleptical properties. Also the sensorial analysis data have confirmed the major consistency of the products from homogenized-heat treated milk.
Conclusions
The HPH treatment of milk can have a potential of diversifying the market of probiotic fermented milks, especially in terms of texture parameters. In fact, texture of fermented milks is an important criterion for the quality assessment and it plays an important role in consumers' acceptance. Moreover, the use of HPH applied to milk does not modify, with respect to the traditional products, the viability of the probiotic cultures whereas it increases the cell loads of the starter cultures. Although HPH can contribute to the production of new types of probiotic dairy products having different textures, changed lipid and protein exposures and hydrolytic breakdown patterns, then, detailed and further investigations are necessary to test better the product benefits claimed in vivo.
This research was partially supported by the Project of Scientific and Technological Cooperation SECYT-MAE (Argentina-Italy) IT/PA05-A/IX/010 “Potenzialità dell'applicazione delle alte pressioni di omogeneizzazione per la produzione di prodotti fermentati probiotici”. Jorge Reinheimer and Gabriel Vinderola are Main Researcher and Research Adjoint, respectively, from CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas). Patricia Burns is a doctoral fellow from CONICET.








