Staphylococcus aureus is one of the most important pathogens associated with bovine intramammary infections. However, as most infections are subclinical, they are often undetected. Furthermore, the efficacy of control programmes differs broadly among herds (Zecconi et al. Reference Zecconi, Piccinini and Fox2003; Moret-Stalder et al. Reference Moret-Stalder, Fournier, Miserez, Albini, Doherr, Reist, Schaeren, Kirchhofer, Graber, Steiner and Kaufmann2009) probably as a consequence of the multifactorial pathogenesis of Staph. aureus mastitis. The microorganism enters the mammary gland via the teat canal, multiplying within the cistern, where adhesion to mammary epithelial cells represents an important step in the process of infection (Dego et al. Reference Dego, Van Dijk and Nederbragt2002).
Many studies performed in vitro have demonstrated the ability of the bacteria to penetrate and divide into various human, bovine, chicken and mouse cells, including neutrophils (Chandler et al. Reference Chandler, Reid, Harrison and France1974; Anderson & Chandler, Reference Anderson and Chandler1975; Ogawa et al. Reference Ogawa, Yurberg, Hatcher, Levitt and Lowy1985; Almeida et al. Reference Almeida, Matthews, Cifrian, Guidry and Oliver1996; Lammers et al. Reference Lammers, Nujiten and Smith1999; Lowy, Reference Lowy2000). In experimental infections, Staph. aureus have been shown within the alveoli and lactiferous ducts (Nickerson & Heald, Reference Nickerson and Heald1981; Gudding et al. Reference Gudding, McDonald and Cheville1984) and inside mammary epithelial cells (Hensen et al. Reference Hensen, Pavicic, Lohuis, de Hoog and Poutrel2000). Intramammary challenge using a high inoculum density has caused severe acute mastitis, characterized by cell necrosis and a wide distribution of bacteria in the gland (Chandler et al. Reference Chandler, Reid, Harrison and France1974). In chronically infected cows, viable Staph. aureus have been demonstrated in macrophages and in some alveolar cells isolated from milk (Hebert et al. Reference Hebert, Sayasit, Senechal, Dubreuil and Lagace2000). Staph. aureus produces several virulence factors, such as proteins promoting adhesion to mammary tissues, toxins that can damage the parenchyma and enterotoxins that interfere with the immune response of the udder, being superantigens.
Recent studies have shown that Staph. aureus strains may differ in virulence (Tollersrud et al. Reference Tollersrud, Kenny, Caugant and Lund2000; Zecconi et al. Reference Zecconi, Cesaris, Liandris, Daprà and Piccinini2006; Fournier et al. Reference Fournier, Kuhnert, Frey, Miserez, Kirchhofer, Kaufmann, Steiner and Graber2008; Piccinini et al. Reference Piccinini, Cesaris, Daprà, Borromeo, Picozzi, Secchi and Zecconi2009). Furthermore, the interactions between microorganism, host immune system and environmental factors can modulate the outcome of the disease, but the role of each factor in the pathogenesis of mastitis has not been investigated in commercial dairy herds, since most in-vivo studies are experimental challenges. Therefore, the goals of this study were to determine whether (a) the ability of Staph. aureus isolates to colonize mammary tissue differed among cows and herds in naturally infected animals and (b) such differences could be related to molecular characteristics of isolates.
Materials and Methods
Animals
Twenty-two Holstein cows from two dairy herds (herd sizes 100 and 180 lactating cows respectively), characterized by a high prevalence of Staph. aureus subclinical mastitis, were sampled at slaughter, according to the European regulation CE 1774/2002. Out of them, 6 cows from herd A and 13 from herd B had been diagnosed as infected by Staph. aureus during lactation, 3–4 months before slaughter, and showed somatic cell counts (SCC) continuously higher than 5×105/ml of milk. The remaining 3 animals were chosen as negative controls, because all the bacteriological analysis performed during lactation were negative for Staph. aureus. All cows were free of clinical infections.
Bacteriological analysis
Just before culling, quarter milk samples were taken from each animal in accordance with National Mastitis Council (NMC) guidelines (NMC, 1999). Then one section of parenchymal tissue and one section of cisternal tissue were collected from each mammary quarter for bacteriological investigation. A total of 28 quarters were examined from herd A and 60 from herd B. All samples were kept at 4°C until bacteriological analysis of milk and tissues was performed. Cisternal and parenchymal sections were sampled with a sterile swab that was rotated inside a 2-cm long cut, over a surface of 1 cm2, and then plated on a blood agar plate. Ten μl of quarter milk were plated on blood agar plate (5% bovine blood; Oxoid, Cambridge, UK) and incubated overnight at 37°C. Colonies of growth were isolated; the large and haemolytic ones that were catalase and coagulase positive were identified as Staph. aureus following Hogan et al. (Reference Hogan, Gonzales, Harmon, Nickerson, Oliver, Pankey and Smith1999) and thereafter were confirmed by API ID32 Staph. (Biomerieux, F).
The antibiotic susceptibility of all isolates to the drugs mostly used in mastitis therapy (penicillin, oxacillin, first generation cephalosporins, tylosin, streptomycin, rifaximin) was tested by the Minimal Inhibitory Concentration (MIC) method in microplate, following Clinical and Laboratory Standards Institute (2008). The MIC value is defined as the lowest concentration that completely inhibits microbial growth. Finally, the production of α-, β- and δ-haemolysin was determined by cross-streaking the isolates perpendicularly to an only β-haemolytic Staph. aureus on a blood agar plate, following Traber et al. (Reference Traber, Lee, Benson, Corrigan, Cantera, Shopsin and Novick2008). Subsequently, isolates were frozen in Brain Heart Infusion Broth (Oxoid) with 15% glycerol (v/v) until further molecular analyses were performed.
Molecular analysis of Staph. aureus isolates
Immediately after thawing, Staph. aureus isolates were subcultured on blood agar plate (5% bovine blood; Oxoid) and processed by PFGE, Multilocus Sequence Typing (MLST) and a Staph. aureus array.
PFGE analysis
Total DNA preparation, digestion with the SmaI restriction enzyme (MBI Fermentas, Vilnius, Lithuania) and PFGE were performed as described by Chung et al. (Reference Chung, de Lencastre, Matthews, Tomasz, Adamsson, Aires de Sousa, Camou, Cocuzza, Corso, Couto, Dominguez, Gniadkowski, Goering, Gomes, Kikuchi, Marchese, Mato, Melter, Oliveira, Palacio, Sa-Leao, Santos Sanches, Song, Tassios and Villari2000). To determine strain relatedness, DNA band patterns were analysed with Molecular Analyst software, version 1.6 (Bio-Rad Laboratories). A band position tolerance of 4% was used for comparison of DNA patterns.
DNA extraction
From each isolate, one colony was cultured in Nutrient Broth (Oxoid) at 37°C overnight. The broth was then centrifuged at 15 000 g for 2 min, the pellet was resuspended in 50 mm EDTA and lysostaphin (5 mg/ml; Sigma-Aldrich, St. Louis MO, USA) was added for bacterial lysis. DNA was extracted using DNeasy kit (QIAgen, Hilden, Germany); DNA amount and purity were tested with a ND-100 Spectrophotometer (NanoDrop Technologies Inc., Wilmington DC, USA), while DNA integrity was checked by electrophoresis on 0·8% agarose gel containing ethidium bromide (0·5 μg/ml) in TAE buffer.
MLST analysis
MLST was performed on two representative isolates from each herd. DNA extracted as indicated above, was processed following Bonura et al. (Reference Bonura, Plano, Di Carlo, Calà, Cipolla, Corsello and Mammina2010).
Staph. aureus array analysis and PCR
DNA microarray (Alere Technologies, Jena, Germany) was used to genotype Staph. aureus isolates. A set of probes covering antibiotic resistance determinants, different toxins (enterotoxins, leucotoxins, haemolysins) and the allelic variants of the agr gene are included in the chip. The arrays were processed as previously described (Monecke & Ehricht, Reference Monecke and Ehricht2005). Briefly, genomic DNA was employed for linear amplification and labelling of target genes with biotin-16-dUTP (Roche Diagnostics, Mannheim, Germany). The amplified products were added to the array tubes and any hybridization detected using streptavidin-horseradish-peroxidase and a chromogenic substrate (Seramun, Wolzig, Germany). The reaction was read on an array tube reader ATR01 and the image interpreted with the Iconoclust software package (Alere Technologies, Jena, Germany). Genes showing a signal intensity above 0·4 were considered to be present, those below 0·3 were considered absent and those between 0·3 and 0·4 were considered ambiguous. To confirm the presence of lukD, a PCR for detection of the whole gene was also designed. Each reaction mixture (25 μl) contained 1 μl of the purified genomic DNA, 2 U of Taq DNA polymerase (Promega, Madison WI, USA), 200 μm dNTPs (Promega, Madison WI, USA), 2·5 mm MgCl2, and a 0·1 μm concentration of each of the following primers: fw (5′–3′) ATGAAAATTGAAAAATTAGGCAAATCATCA and rv (5′–3′) TTATACTCCAGGATTAGTTTCTTTAGAATC. Amplicon size was 984 bp. Amplifications were carried out in a thermocycler (Mastercycler gradient, Eppendorf, Hamburg, Germany) through the following temperature program: 95 °C for 5 min; 40 cycles including 95°C for 30 s and 50°C for 30 s; 72°C for 1 min. The products were electrophoresed in a 0·8% agarose gel containing ethidium bromide and visualized by transillumination under u.v. Finally, the amplicons were submitted to sequencing, that was performed on an Applied Biosystems 3730 DNA Analyzer, following the manufacturer's instructions (Applied Biosystems, Forster City CA, USA).
Statistical analysis
Both Fisher's exact test and Chi-square test of association were carried out to compare both the molecular data and the number of quarters infected in the cattle. The genetic markers were tabulated by the number of samples categorized as present or absent for both A or B groups. The Fisher's exact test compared the number of present genes with absent or ambiguous genes, between the two groups and calculated the odds ratio with an associated 95% exact confidence interval (Fleiss, Reference Fleiss1981). The infected quarters data were tested using the same methodology.
Results
Bacteriological analysis
Overall 88 quarter milk samples, 88 samples of quarter parenchyma and 88 samples of cisternal tissues were analysed. Bacteriologic results confirmed that the 19 cows culled because of Staph. aureus mastitis were infected, while the 3 negative controls were free from contagious microorganisms (Table 1). Infection was defined as positive counts of Staph. aureus, expressed as colony-forming units (CFU) per ml of milk, and CFU in 1 cm2 of parenchyma. All cisternal samples showed the same results as the milk (data not shown).
Table 1. Bacterial isolation of Staph. aureus from milk and parenchyma samples from mammary quarters. The number of cow and bacterial isolate from each herd is also given

† Colony forming units (CFU)/ml
‡ Negative control cow (culled for reasons other than chronic Staph. aureus infection)
There were milk or tissue infections in 16 out of 24 quarters in herd A and 29 of 52 quarters in herd B, and both milk and tissue infection in 10 of 24 quarters in herd A, and 12 of 52 in herd B. Twenty Staph. aureus isolates, 6 in herd A and 14 in herd B, were identified. Five isolates infected only the milk (n. 2145, 2146, 2148, 2160) or only the parenchyma (n. 2164) in herd B, a factor not seen for any of the isolates in herd A, all of which infected both milk and parenchyma. Interestingly, two Staph. aureus isolates, with different status for harbouring the collagen-binding adhesin gene (data not shown), were recovered from cow B-11 but from different quarters (one from front right and rear left quarters, the other from front left and rear right quarters) (Table 1).
Although differences were observed in the rates of infection between the two herds, the statistical analysis could not demonstrate this (P>0·1). However, an association was found between the spread of the infection from a single quarter to multiple quarters, and the infection being located in both the tissue and the milk, compared with a single location (P=0·0369). Furthermore, evidence of this association can still be seen after accounting for herd differences (Table 2, P=0·07). Therefore it was more likely for a cow from herd A to have both milk and tissue infection in multiple quarters compared with a cow from herd B. From the microarray data it was found that many markers were associated with isolates gathered from either herd A or B (see next section).
Table 2. Differences in Staph. aureus infection between herds A and B

False negative results of milk microbiological analysis occurred in both herds: in herd A, 1 out of 16 infected quarters was Staph. aureus positive only in the tissue (6·25%) while in herd B, 3 of 29 (10·3%) infected quarters were Staph. aureus positive only in the tissue i.e. milk false negative.
All 20 Staph. aureus isolates were tested for their susceptibility to a number of antimicrobial compounds that are commonly used in the treatment of mastitis. The results showed that the isolates were highly susceptible to most antibiotics tested, although less so to streptomycin (Table 3).
Table 3. Minimal inhibitory concentration (MIC) of Staph. aureus isolates to a number for antimicrobial agents commonly used for treatment of mastitis

† MIC is defined as the lowest concentration that completely inhibits microbial growth and is expressed as μg/ml
Molecular analysis of Staph. aureus isolates
The molecular differences between the 20 Staph. aureus isolates from herds A and B were characterized by PFGE, MLST and microarray. Major results from array analysis are summarized in Table 4. Presence or absence of lukD gene was further confirmed by PCR set up for the whole gene, since a product was obtained only in the strains from herd A. All amplicon sequences matched with GenBank accession number Y13225.1 with 98% homology. Inter-herd differences were evidenced by PFGE and MLST patterns, as well as by array profiles. PFGE revealed two patterns differing in 5 bands, which could be considered as distinct, in accordance with Tenover et al. (Reference Tenover, Arbeit, Goering, Mickelsen, Murray, Persing and Swaminathan1995). One pattern was exclusively displayed by all Staph. aureus isolates in herd A, and the other was characteristic of all herd B isolates (data not shown). Also, a single profile was identified by MLST in herd A and belonged to ST8, while the isolates from herd B were characterized by ST20.
Table 4. Major results of PCR and microarray analysis of Staph. aureus isolates. Unless indicated otherwise, (+) denotes presence, whilst (−) denotes absence in all isolates within each herd. The values from the Fisher's exact test are also given where applicable
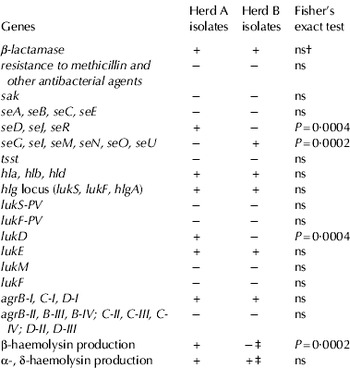
† Not significant
‡ Isolate 2163 produced β-haemolysin, but neither α- nor δ-haemolysin
Analogously, the presence of enterotoxin and leukocidin genes also differentiated the herds. In fact using the Fisher's exact test on our array data, we found strongly significant genetic differences between herds A and B (Table 4). The genes seD, seJ, seR, and lukD were more likely to be present in herd A [P=0·0004; 95% exact CI (4·3, Inf*)] whereas seG, seI, seN, seM, seO, and seU were more likely to be present in herd B [P=0·0002; 95% exact CI (5·0, Inf*)]; (*Inf, because in cases where no present strains were found in either group an upper 95% exact CI cannot be calculated). In contrast, the allelic variants of agr genes BCD group-I, were identified in all the isolates from both herds, while group-II, III, IV variants were overall absent. All four haemolysin genes (hla, hlb, hld and hlgA) were carried by all strains. Despite their presence, production of β-haemolysin was highly specific to herd A (P=0·0002; 95% exact CI [5·0, Inf1], but not to herd B, where β-haemolysin was never demonstrated except in one isolate (Table 4).
Discussion and Conclusion
To our knowledge, the present study represents the first investigation involving 22 udders collected at slaughter from two herds. Nineteen of the 22 udders were from infected glands of dairy cows culled because of subclinical Staph. aureus mastitis, whilst 3 were negative controls. The results confirm that different Staph. aureus strains can be isolated from cattle suffering chronic subclinical mastitis in different herds, as previously described (Zecconi et al. Reference Zecconi, Cesaris, Liandris, Daprà and Piccinini2006; Fournier et al. Reference Fournier, Kuhnert, Frey, Miserez, Kirchhofer, Kaufmann, Steiner and Graber2008; Graber et al. Reference Graber, Naskova, Studer, Kaufmann, Kirchhofer, Brechbühl, Schaeren, Steiner and Fournier2009; Piccinini et al. Reference Piccinini, Borromeo and Zecconi2010). Furthermore, we documented in the field the ability of Staph. aureus to infect mammary parenchyma and contaminate milk. Analogous results had been shown by Hebert et al. (Reference Hebert, Sayasit, Senechal, Dubreuil and Lagace2000) who isolated the bacteria from milk macrophages and alveolar cells of chronically infected cows. An interesting finding from our results was the difference in the rate of infection between herds A and B, and a statistically significant association being found of Staph. aureus present in milk and tissue from multiple quarters in cattle from herd A compared with herd B. This result could have important consequences in the field, as in multiple-quarter infections the cure rate is lower at the cow level than for single-quarter infections (Osteras et al. Reference Osteras, Edge and Martin1999).
It is worth noting that this study found ∼6–10% of instances with false negative results of milk bacteriological analysis, where Staph. aureus was isolated from the parenchyma of infected quarters but the microorganism was not shed in the milk. Also, bacterial counts in the parenchyma appeared always at a much lower level than in the milk, supporting the results of numerous histopathological studies of the gland, which barely demonstrated the presence of Staph. aureus in infected udder tissues (Chandler & Reid, Reference Chandler and Reid1973; Sordillo et al. Reference Sordillo, Nickerson and Akers1989).
Molecular analysis of Staph. aureus isolates from herds A and B showed differences in their profile which may account for the differences seen in colonization between the herds. Firstly, both PFGE and MLST profile of isolates from herd A were distinct from herd B although the intra-herd PFGE isolate profile was identical, suggesting infection and proliferation with a different clonal type for each herd. Secondly, the virulence pattern of the isolates from each herd was virtually identical and statistically significant differences in carriage of several virulence determinants were evidenced between the herds. These differences included the enterotoxin and leukocidin genes. The seD, seJ, and seR enterotoxin genes and both lukD and lukE leukocidin genes were identified in all isolates from herd A, whilst herd B isolates harboured seG, seI, seN, seM, seO and seU enterotoxin genes and only the lukE gene. The seDJR genes are encoded on a large penicillinase plasmid (Osteras et al. Reference Osteras, Edge and Martin1999) while the seGINMOU genes are commonly found in the enterotoxin gene cluster (egc) on a genomic island (Lindsay & Holden, Reference Lindsay and Holden2006). The differences between isolates from each herd were probably reflected in the PFGE and MLST profiles. Interestingly, all herd A isolates carrying enterotoxin genes on plasmids, lukD/E and only agrI, belonged to ST8 and shared the genetic background of MRSA lineage as reported by Argudin et al. (Reference Argudín, Mendoza, Méndez, Martín, Guerra and Rodicio2011). On the contrary, herd B isolates belonged to ST20, which is rarely detected in the dairy cow (Monecke et al. Reference Monecke, Kuhnert, Hotzel, Slickers and Ehricht2007; Bystron et al. Reference Bystron, Podkowik, Korzekwa, Lis, Molenda and Bania2010). In accordance with the literature, all ST20 isolates detected in herd B carried egc and β-lactamase (blaZ) gene. Both MLST profiles identified could be grouped with human clones, demonstrating that host-association is not absolute, as reported by Holmes & Zadoks (Reference Holmes and Zadoks2011).
Even though lukE was carried by all the strains, the active toxin could be produced only in herd A, since the D component of lukD/E gene was demonstrated only in that group. Owing to the possibility of a sequence variant undetectable by array, the results were further confirmed by PCR and sequence of the amplicons.
It could be hypothesized that the statistically significant greater ability of Staph. aureus isolates from herd A to infect multiple quarters of cows was related to the presence of lukD/E and seDJR genes for three reasons: firstly, this leukocidin is known to strongly interfere with mammary response by damaging the leukocytes (Rainard et al. Reference Rainard, Corrales, Barrio, Cochard and Poutrel2003; Younis et al. Reference Younis, Krifucks, Fleminger, Heller, Gollop, Saran and Leitner2005); secondly, seDJR genes have been related to higher Staph. aureus prevalence in infected herds (Fournier et al. Reference Fournier, Kuhnert, Frey, Miserez, Kirchhofer, Kaufmann, Steiner and Graber2008; Piccinini et al. Reference Piccinini, Borromeo and Zecconi2010); thirdly, the egc superantigens are significantly more frequent in commensal strains than in invasive isolates, probably because the concentration of these toxins does not increase with increasing bacterial counts. In contrast, plasmid toxins are able to orchestrate an efficient adaptive immune response (Grumann et al. Reference Grumann, Scharf, Holtfreter, Kohler, Steil, Engelmann, Hecker, Völker and Bröker2008).
Moreover expression of haemolysins was characteristic of each herd. The array data showed that the genes for the different haemolysins were present in all the Staph. aureus isolates considered, as would be expected from previous reports (Aarestrup et al. Reference Aarestrup, Larsen, Eriksen and Elsberg1999; Haveri et al. Reference Haveri, Roslof, Rantala and Pyorala2007). Nevertheless, in contrast to Aarestrup et al. (Reference Aarestrup, Larsen, Eriksen and Elsberg1999), 95% of our isolates expressed α-haemolysin, but only 35% expressed β-haemolysin. It is noteworthy that 86% of the isolates expressing β-haemolysin were from herd A. As suggested by Larsen et al. (Reference Larsen, Aarestrup and Jensen2002), β-haemolysin may be an active factor in the pathogenesis of bovine mastitis, contributing to the highest invasiveness of herd A isolates. Such differences in expression are probably due to the hlb gene becoming inactivated by integration of lysogenic phages, as reported by van Wamel et al. (Reference Van Wamel, Rooijakkers, Ruyken, van Kessel and van Strijp2006), but this requires further investigation.
Finally, the same allelic variants of the agr group were present in all the isolates. The agr locus is important for virulence in a variety of animal models of infection and it regulates the production of both α- and δ-haemolysin (Traber et al. Reference Traber, Lee, Benson, Corrigan, Cantera, Shopsin and Novick2008), which were used in this study as a surrogate to determine agr expression (Shopsin et al. Reference Shopsin, Eaton, Wasserman, Mathema, Adhikari, Agolory, Altman, Holzman, Kreiswirth and Novick2010). The results indicated that 95% of the isolates expressed both haemolysins and hence will be more likely to express plasmid enterotoxins, which are under control of the sar and agr response regulators (Cheung et al. Reference Cheung, Bayer, Zhang, Gresham and Xiong2004). The only isolate with an anomalous result in haemolysin production was strain 2163, which was one of two different isolates recovered from two quarters of the same cow in herd B.
The carriage of genes encoding antibiotic resistance was rare amongst these isolates, as previously reported (Monecke et al. Reference Monecke, Kuhnert, Hotzel, Slickers and Ehricht2007) and was reflected in the very low MIC values. This result was similar to that reported by Rubin et al. (Reference Rubin, Ball and Chirino-Trejo2011) who detected resistance only to trimethoprim/sulphamethoxazole. Minor differences were observed in herd A for oxacillin, tylosin and streptomycin, while all herd B isolates demonstrated identical susceptibility. A clear difference between herds was shown for penicillin, since MIC values in herd B were 5-times higher than in herd A, even though blaZ was carried by all isolates. The sensitivity of herd B strains was consistent with the literature (Haveri et al. Reference Haveri, Suominen, Rantala, Honkanen-Buzalski and Pyorala2005; Kaase et al. Reference Kaase, Lenga, Friedrich, Szabados, Sakinc, Kleine and Gatermann2008) reporting MIC values as low as 0·06 μg/ml for isolates proved to possess blaZ. To the contrary, lower values were always related to the absence of the gene. The MIC of 0·05 μg/ml observed in the present study could be considered as a borderline value, still consistent with blaZ carriage.
In conclusion, our data indicate that Staph. aureus isolates have different abilities to colonize mammary tissue and disseminate across mammary quarters. These differences are likely to be due to virulence factors which influence the genetic characteristics and probably the diffusiveness of the different strains. Further studies are required to determine whether the virulence factors identified in this study are the only or even the key components involved in infection and dissemination across herds or whether they are part of a complex interaction of various factors involved in pathogenesis of mastitis in naturally infected animals.
We thank Dr C Bonura of the Department of Health Promotion Sciences, ‘G. D'Alessandro’, University of Palermo, Italy, for MLST of Staph. aureus isolates collected in the study.






